UVA, UVB Light Doses and Harvesting Time Differentially Tailor Glucosinolate and Phenolic Profiles in Broccoli Sprouts
Abstract
:1. Introduction
2. Results
2.1. Effect of UVA and UVB Light on the Accumulation of Glucosinolates
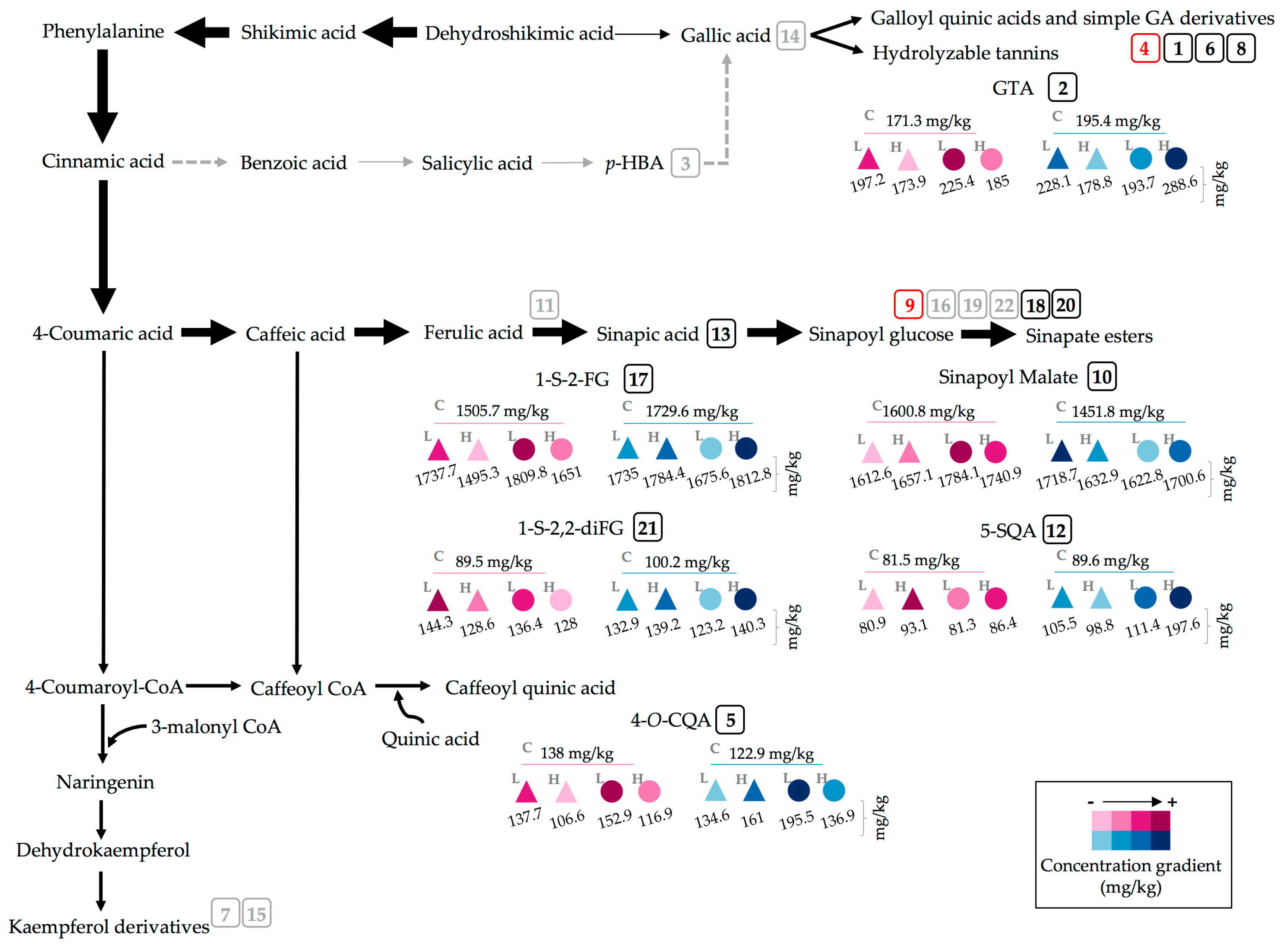
2.2. Effect of UVA and UVB Light on the Accumulation of Phenolic Compounds
3. Discussion
3.1. Effect of Extraction Solvent on Phytochemical Profiles
3.2. Effect of UVA and UVB Light on the Accumulation of Glucosinolates
3.3. Effect of UVA and UVB Light on the Accumulation of Phenolic Compounds
4. Materials and Methods
4.1. Chemical and Plant Material
4.2. Sprouting Method and UV Treatments
4.3. Phytochemical Analyses
4.3.1. Extraction of Phytochemicals
4.3.2. Analysis of Glucosinolates
Desulfation of Glucosinolates
Identification and Quantification of Desulfoglucosinolates by High-Performance Liquid Chromatography-Diode Array Detection (HPLC-DAD) and HPLC-Electrospray Ionization (ESI)-MSn
4.3.3. Analysis of Phenolic Compounds
Identification and Quantification of Phenolic Compounds by High-Performance Liquid Chromatography-Diode Array Detection (HPLC-DAD) and HPLC-Electrospray Ionization (ESI)-MSn
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martinez-Villaluenga, C.; Peñas, E.; Ciska, E.; Piskula, M.K.; Kozlowska, H.; Vidal-Valverde, C.; Frias, J. Time dependence of bioactive compounds and antioxidant capacity during germination of different cultivars of broccoli and radish seeds. Food Chem. 2010, 120, 710–716. [Google Scholar] [CrossRef]
- Maldini, M.; Baima, S.; Morelli, G.; Scaccini, C.; Natella, F. A liquid Chromatography-Mass Spectrometry approach to study “glucosinoloma” in broccoli sprouts. J. Mass Spectrom. 2012, 47, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Pająk, P.; Socha, R.; Gałkowska, D.; Rożnowski, J.; Fortuna, T. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Zevallos, L. The use of controlled postharvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. J. Food Sci. 2003, 68, 1560–1565. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Improving the phytochemical composition of broccoli sprouts by elicitation. Food Chem. 2011, 129, 35–44. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. An alternative use of horticultural crops: Stressed plants as biofactories of bioactive phenolic compounds. Agriculture 2012, 2, 259–271. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Stability of bioactive compounds in broccoli as affected by cutting styles and storage time. Molecules 2017, 22, 636. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yang, R.; Zhou, Y.; Gu, Z. Heat and hypoxia stresses enhance the accumulation of aliphatic glucosinolates and sulforaphane in broccoli sprouts. Eur. Food Res. Technol. 2015, 242, 107–116. [Google Scholar] [CrossRef]
- Mewis, I.; Schreiner, M.; Nguyen, C.N.; Krumbein, A.; Ulrichs, C.; Lohse, M.; Zrenner, R. UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: Induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol. 2012, 53, 1546–1560. [Google Scholar] [CrossRef] [PubMed]
- Barrientos Carvacho, H.; Pérez, C.; Zúñiga, G.; Mahn, A. Effect of methyl jasmonate, sodium selenate and chitosan as exogenous elicitors on the phenolic compounds profile of broccoli sprouts. J. Sci. Food Agric. 2014, 94, 2555–2561. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-García, D.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as biofactories: Postharvest stress-induced accumulation of phenolic compounds and glucosinolates in broccoli subjected to wounding stress and exogenous phytohormones. Front. Plant Sci. 2016, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Guo, L.; Jin, X.; Shen, C.; Zhou, Y.; Gu, Z. Enhancement of glucosinolate and sulforaphane formation of broccoli sprouts by zinc sulphate via its stress effect. J. Funct. Foods 2015, 13, 345–349. [Google Scholar] [CrossRef]
- Jenkins, G.I.; Brown, B.A. UV-B Perception and signal transduction. In Light and Plant Development; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 155–182. [Google Scholar]
- Solovchenko, A.E.; Merzlyak, M.N. Screening of visible and UV radiation as a photoprotective mechanism in plants. Russ. J. Plant Physiol. 2008, 55, 719–737. [Google Scholar] [CrossRef]
- Jansen, M.A.K.; Hectors, K.; O’Brien, N.M.; Guisez, Y.; Potters, G. Plant stress and human health: Do human consumers benefit from UV-B acclimated crops? Plant Sci. 2008, 175, 449–458. [Google Scholar] [CrossRef]
- Schreiner, M.; Mewis, I.; Neugart, S.; Zrenner, R.; Glaab, J.; Wiesner, M.; Jansen, M.A.K. UV-B Elicitation of Secondary Plant Metabolites. In III-Nitride Ultraviolet Emitters; Springer Series in Materials Science; Dreyer, C., Mildner, F., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 227, pp. 387–414. [Google Scholar]
- Kuhlmann, F.; Müller, C. Independent responses to ultraviolet radiation and herbivore attack in broccoli. J. Exp. Bot. 2009, 60, 3467–3475. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk-Plonska, A.; Hagen, S.F.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.K.; Wold, A.B. Glucosinolates in broccoli (Brassica oleracea L. var. italica) as affected by postharvest temperature and radiation treatments. Postharvest Biol. Technol. 2016, 116, 16–25. [Google Scholar] [CrossRef]
- Topcu, Y.; Dogan, A.; Kasimoglu, Z.; Sahin-Nadeem, H.; Polat, E.; Erkan, M. The Effects of UV radiation during the vegetative period on antioxidant compounds and postharvest quality of broccoli (Brassica oleracea L.). Plant Physiol. Biochem. 2015, 93, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S.A. Comparative study on the polyphenolic content, antibacterial activity and antioxidant capacity of different solvent extracts of Brassica oleracea vegetables. Int. J. Food Sci. Technol. 2012, 47, 223–231. [Google Scholar] [CrossRef]
- Gil-Chávez, J.G.; Villa, J.A.; Ayala-Zavala, F.J.; Heredia, B.J.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Torres-Carro, R.; Isla, M.I.; Ríos, J.L.; Giner, R.M.; Alberto, M.R. Anti-inflammatory properties of hydroalcoholic extracts of Argentine Puna Plants. Food Res. Int. 2015, 67, 230–237. [Google Scholar] [CrossRef]
- Villarreal-García, D.; Jacobo-Velázquez, D.A. Glucosinolates from broccoli: Nutraceutical properties and their purification. Curr. Trends Nutraceuticals 2016, 1, 1–6. [Google Scholar]
- Almeida, I.F.; Valentão, P.; Andrade, P.B.; Seabra, R.M.; Pereira, T.M.; Amaral, M.H.; Costa, P.C.; Bahia, M.F. Oak leaf extract as topical antioxidant: Free radical scavenging and iron chelating activities and in vivo skin irritation potential. BioFactors 2008, 33, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, F.; Tomás-Barberán, F.; García-Viguera, C. Health-promoting compounds in broccoli as influenced by refrigerated transport and retail sale period. J. Agric. Food Chem. 2003, 51, 3029–3034. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, M.; Krumbein, A.; Mewis, I.; Ulrichs, C.; Huyskens-Keil, S. Short-term and moderate UV-B radiation effects on secondary plant metabolism in different organs of nasturtium (Tropaeolum majus L.). Innov. Food Sci. Emerg. Technol. 2009, 10, 93–96. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, W.J.; Yan, X.F.; Wang, Y. Glucosinolate content and related gene expression in response to enhanced UV-B radiation in Arabidopsis. Afr. J. Biotechnol. 2011, 10, 6481–6491. [Google Scholar]
- Verdaguer, D.; Jansen, M.A.K.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Helsper, J.P.F.G.; de Vos, C.H.R.; Maas, F.M.; Jonker, H.H.; van den Broeck, H.C.; Jordi, W.; Pot, C.S.; Keizer, L.C.P.; Schapendonk, A.H.C.M. Response of Selected antioxidants and pigments in tissues of Rosa Hybrida and Fuchsia Hybrida to supplemental UV-A exposure. Physiol. Plant. 2003, 117, 171–178. [Google Scholar] [CrossRef]
- Jayakumar, M.; Amudha, P.; Kulandaivelu, G. Effect of low doses of UV-A and UV-B radiation on photosynthetic activities in Phaseolus Mungo L. J. Plant Biol. 2004, 47, 105–110. [Google Scholar] [CrossRef]
- Morales, L.O.; Tegelberg, R.; Brosché, M.; Keinänen, M.; Lindfors, A.; Aphalo, P.J. Effects of solar UV-A and UV-B radiation on gene expression and phenolic accumulation in Betula Pendula leaves. Tree Physiol. 2010, 30, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Sønderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of glucosinolates—Gene discovery and beyond. Trends Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Kunz, B.A.; Cahill, D.M.; Mohr, P.G.; Osmond, M.J.; Vonarx, E.J. Plant responses to UV radiation and links to pathogen resistance. Int. Rev. Cytol. 2006, 255, 1–40. [Google Scholar] [PubMed]
- Kusano, M.; Tohge, T.; Fukushima, A.; Kobayashi, M.; Hayashi, N.; Otsuki, H.; Kondou, Y.; Goto, H.; Kawashima, M.; Matsuda, F.; et al. Metabolomics reveals comprehensive reprogramming involving two independent metabolic responses of Arabidopsis to UV-B light. Plant J. 2011, 67, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Jenkins, S.N.; Fahey, J.W.; Ye, L.; Wehage, S.L.; Liby, K.T.; Stephenson, K.K.; Wade, K.L.; Talalay, P. Protection against UV-light-induced skin carcinogenesis in SKH-1 high-risk mice by sulforaphane-containing broccoli sprout extracts. Cancer Lett. 2006, 240, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Sousa, C.; Pereira, D.M.; Valentão, P.; Taveira, M.; Martins, A.; Pereira, J.A.; Seabra, R.M.; Andrade, P.B. Screening of antioxidant phenolic compounds produced by in vitro shoots of Brassica oleracea L. var. costata DC. Comb. Chem. High Throughput Screen. 2009, 12, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Nićiforović, N.; Abramovič, H. Sinapic acid and its derivatives: Natural sources and bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Siger, A.; Czubinski, J.; Dwiecki, K.; Kachlicki, P.; Nogala-Kalucka, M. Identification and antioxidant activity of sinapic acid derivatives in Brassica napus L. seed meal extracts. Eur. J. Lipid Sci. Technol. 2013, 115, 1130–1138. [Google Scholar]
- Sun, J.; Xiao, Z.; Lin, L.; Lester, G.E.; Wang, Q.; Harnly, J.M.; Chen, P. Profiling polyphenols in five Brassica species microgreens by UHPLC-PDA-ESI/HRMS. J. Agric. Food Chem. 2013, 61, 10960–10970. [Google Scholar] [CrossRef] [PubMed]
- Björkman, M.; Klingen, I.; Birch, A.N.E.; Bones, A.M.; Bruce, T.J.A.; Johansen, T.J.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in plant protection and human health—Influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.I. Signal transduction in responses to UV-B radiation. Annu. Rev. Plant Biol. 2009, 60, 407–431. [Google Scholar] [CrossRef] [PubMed]
- Caputo, C.; Rutitzky, M.; Ballaré, C.L. Solar ultraviolet-B radiation alters the attractiveness of Arabidopsis plants to diamondback moths (Plutella xylostella L.): Impacts on oviposition and involvement of the jasmonic acid pathway. Oecologia 2006, 149, 81. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Velázquez, D.A.; González-Agüero, M.; Cisneros-Zevallos, L. Cross-talk between signaling pathways: The link between plant secondary metabolite production and wounding stress response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.C.; Kusaka, R.; Walsh, P.S.; Allais, F.; Zwier, T.S. Plant sunscreens in the UV-B: Ultraviolet spectroscopy of jet-cooled sinapoyl malate, sinapic acid, and sinapate ester derivatives. J. Am. Chem. Soc. 2014, 136, 14780–14795. [Google Scholar] [CrossRef] [PubMed]
- Milkowski, C.; Baumert, A.; Schmidt, D.; Nehlin, L.; Strack, D. Molecular regulation of sinapate ester metabolism in Brassica napus: Expression of genes, properties of the encoded proteins and correlation of enzyme activities with metabolite accumulation. Plant J. 2004, 38, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, Y.; Yang, S.; Flourat, A.L.; Allais, F.; Han, K. Ultrafast barrierless photoisomerization and strong ultraviolet absorption of photoproducts in plant sunscreens. J. Phys. Chem. Lett. 2017, 8, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.A.; Cloix, C.; Jiang, G.H.; Kaiserli, E.; Herzyk, P.; Kliebenstein, D.J.; Jenkins, G.I. A UV-B-specific signaling component orchestrates plant UV protection. Proc. Natl. Acad. Sci. USA 2005, 102, 18225–18230. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Helliwell, K.; You, X. Analytical, Nutritional and clinical methods section isocratic elution system for the determination of catechins, caffeine and gallic acid in green tea using HPLC. Food Chem. 2000, 68, 115–121. [Google Scholar] [CrossRef]
- Tegelberg, R.; Julkunen-Tiitto, R.; Aphalo, P.J. Red: Far-red light ratio and UV-B radiation: Their effects on leaf phenolics and growth of silver birch seedlings. Plant Cell Environ. 2004, 27, 1005–1013. [Google Scholar] [CrossRef]
- Clé, C.; Hill, L.M.; Niggeweg, R.; Martin, C.R.; Guisez, Y.; Prinsen, E.; Jansen, M.A. Modulation of chlorogenic acid biosynthesis in Solanum lycopersicum; consequences for phenolic accumulation and UV-tolerance. Phytochemistry 2008, 69, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as biofactories: Stress-induced production of chlorogenic acid isomers in potato tubers as affected by wounding intensity and storage time. Ind. Crops Prod. 2014, 62, 61–66. [Google Scholar] [CrossRef]
- Ulm, R.; Baumann, A.; Oravecz, A.; Máté, Z.; Ádám, É.; Oakeley, E.J.; Schäfer, E.; Nagy, F. Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Simontacchi, M.; Galatro, A.; Ramos-Artuso, F.; Santa-María, G.E. Plant survival in a changing environment: The role of nitric oxide in plant responses to abiotic stress. Front. Plant Sci. 2015, 6, 977. [Google Scholar] [CrossRef] [PubMed]
- Tossi, V.; Amenta, M.; Lamattina, L.; Cassia, R. Nitric oxide enhances plant ultraviolet-B protection up-regulating gene expression of the phenylpropanoid biosynthetic pathway. Plant. Cell Environ. 2011, 34, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Surjadinata, B.B.; Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. UVA, UVB and UVC Light Enhances the Biosynthesis of Phenolic Antioxidants in Fresh-Cut Carrot through a Synergistic Effect with Wounding. Molecules 2017, 22, 668. [Google Scholar] [CrossRef] [PubMed]
- Nenadis, N.; Llorens, L.; Koufogianni, A.; Díaz, L.; Font, J.; Gonzalez, J.A.; Verdaguer, D. Interactive effects of UV radiation and reduced precipitation on the seasonal leaf phenolic content/composition and the antioxidant activity of naturally growing Arbutus unedo plants. J. Photochem. Photobiol. B 2015, 153, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Nardini, M.; Di Felice, M.; Scaccini, C. Benzoic and cinnamic acid derivatives as antioxidants: Structure-activity relation. J. Agric. Food Chem. 1999, 47, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, G.; Pernice, R.; Maggio, A.; de Pascale, S.; Fogliano, V. Glucosinolates profile of Brassica rapa L. subsp. Sylvestris L. Janch. var. esculenta Hort. Food Chem. 2008, 107, 1687–1691. [Google Scholar]
- Kusznierewicz, B.; Iori, R.; Piekarska, A.; Namieśnik, J.; Bartoszek, A. Convenient identification of desulfoglucosinolates on the basis of mass spectra obtained during Liquid Chromatography-Diode Array-Electrospray Ionisation Mass Spectrometry analysis: Method verification for sprouts of different Brassicaceae species extracts. J. Chromatogr. A 2013, 1278, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Madala, N.E. Surrogate standards: A cost-effective strategy for identification of phytochemicals. J. Agric. Food Chem. 2017, 65, 3589–3590. [Google Scholar] [CrossRef] [PubMed]
- Ramabulana, T.; Mavunda, R.D.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A.; Madala, N.E. Plant secondary metabolite perturbations in Phaseolus vulgaris leaves due to gamma radiation. Plant Physiol. Biochem. 2015, 97, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Ramabulana, T.; Mavunda, R.D.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A.; Madala, N.E. Perturbation of pharmacologically relevant polyphenolic compound in Moringa oleifera against photo-oxidative damages imposed by gamma radiation. J. Photochem. Photobiol. B 2016, 156, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; Barros, L.; Antonio, A.L.; Cabo Verde, S.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Rodrigues, P. Is gamma radiation suitable to preserve phenolic compounds and to decontaminate mycotoxins in aromatic plants? A case-study with Aloysia citrodora Paláu. Molecules 2017, 22, 347. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Kirkpatrick, J.; Kuhnert, N.; Roozendaal, H.; Salgado, P.R. LC-MSn analysis of cis isomers of chlorogenic acids. Food Chem. 2008, 106, 379–385. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
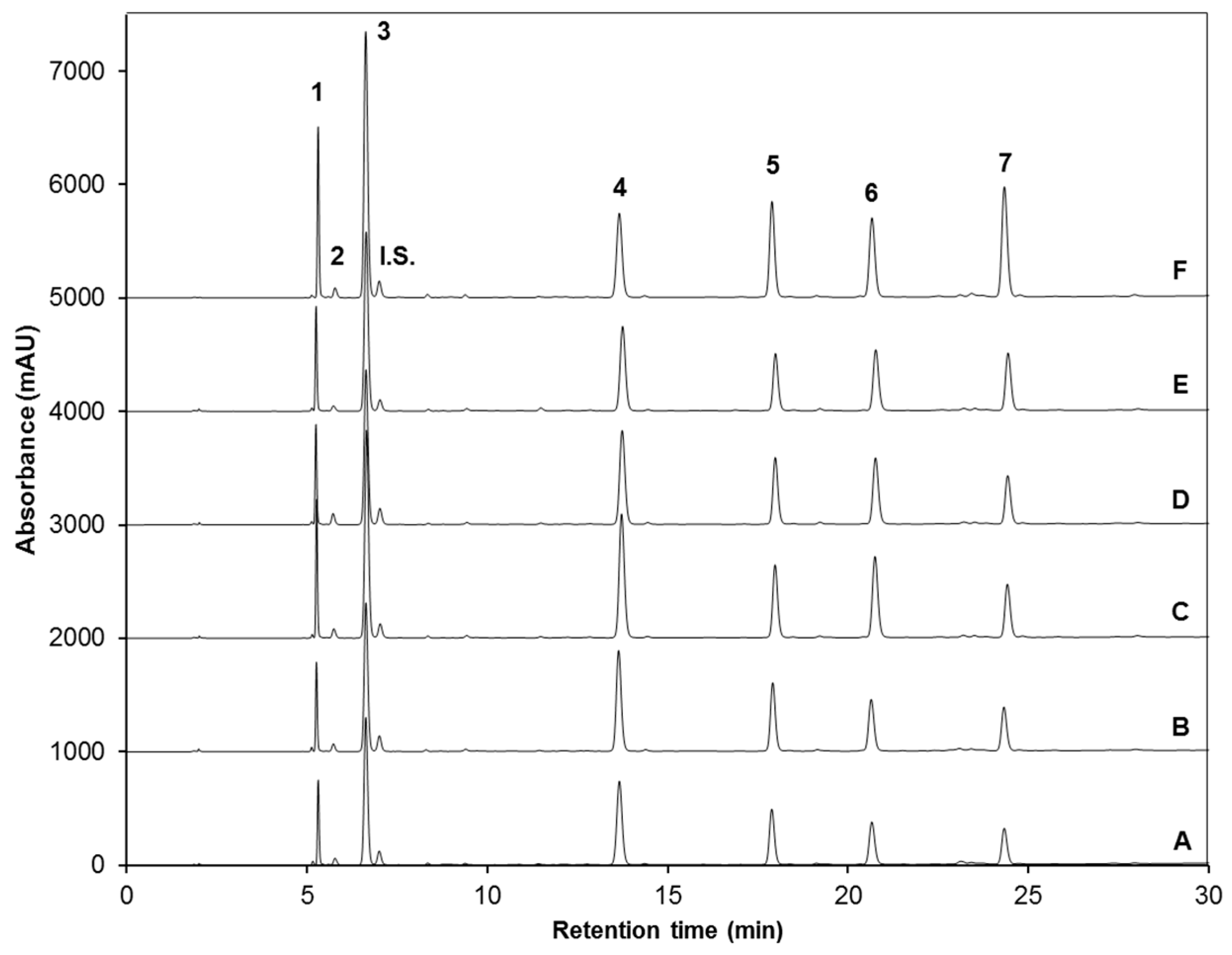
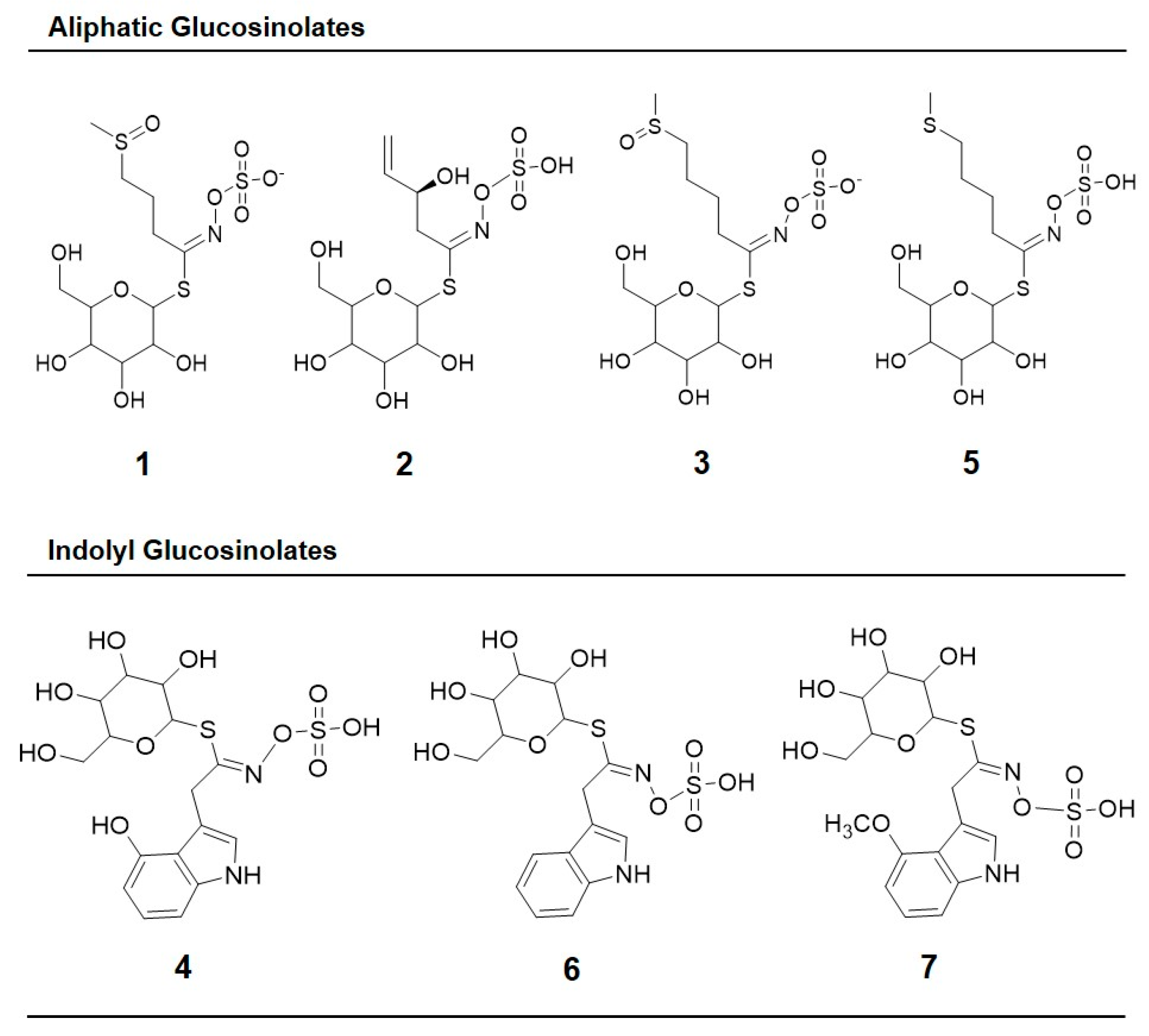
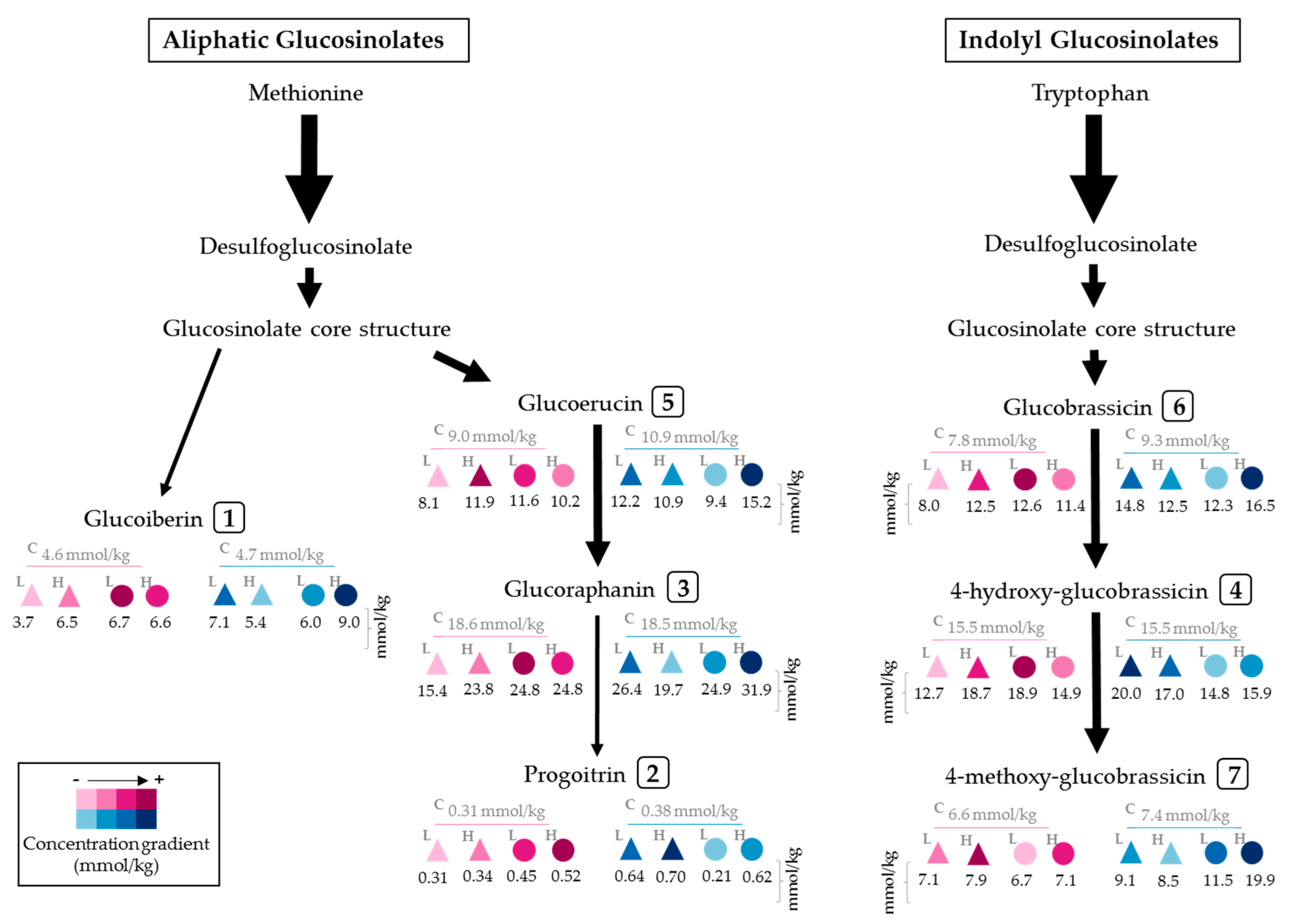
| Peak Number (Retention Time, min) | λmax (nm) | Identification | [M − H]− (m/z) | MS2 (m/z) a |
|---|---|---|---|---|
| 1 (5.3) | 222 | Glucoiberin-dsg | 342 | 179, 131 |
| 2 (5.8) | 224 | Progoitrin-dsg | 308 | 145, 129, 79 |
| 3 (6.6) | 222 | Glucoraphanin-dsg | 356 | 193 |
| 4 (13.6) | 221, 266 | 4-hydroxy-glucobrassicin-dsg | 383 | 221, 203, 153 |
| 5 (17.9) | 210 | Glucoerucin-dsg | 340 | 177, 160, 129, 113 |
| 6 (20.6) | 220, 280 | Glucobrassicin-dsg | 367 | 204, 187, 155, 129 |
| 7 (24.3) | 220, 268 | 4-methoxy-glucobrassicin-dsg | 397 | 234, 204, 154, 139 |
| Dose 4 | Solvent | Time of Harvest after Treatment 5 | Glucosinolate Concentration (mmol/kg DW) 1,2,3 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GIB | PRO | GRA | 4-HGBS | GER | GBS | 4-MGBS | Total | ||||||||||||
| Control | M | 2 h | 4.63 ± 0.2 | ij | 0.31 ± 0.03 | hi | 18.61 ± 0.4 | h | 15.52 ± 0.75 | d | 9.03 ± 0.41 | efgh | 7.79 ± 0.23 | g | 6.56 ± 0.54 | jk | 62.45 ± 1.44 | ij | |
| E | 5.09 ± 0.08 | ghi | 0.39 ± 0.06 | fgh | 22.1 ± 0.57 | efg | 12.16 ± 0.71 | ghi | 6.14 ± 0.68 | klm | 7.33 ± 0.2 | g | 5.69 ± 0.28 | l | 58.9 ± 1.71 | jk | |||
| M | 24 h | 4.71 ± 0.15 | hij | 0.38 ± 0.02 | fgh | 18.47 ± 0.3 | h | 15.49 ± 0.73 | de | 10.91 ± 0.14 | cd | 9.26 ± 0.08 | ef | 7.35 ± 0.08 | ghi | 66.56 ± 0.87 | hi | ||
| E | 5.32 ± 0.02 | fghi | 0.31 ± 0.04 | hi | 21.67 ± 0.43 | fg | 14.85 ± 0.43 | defg | 8.67 ± 0.43 | fghi | 9.51 ± 0.28 | def | 7.42 ± 0.19 | ghi | 67.76 ± 0.77 | ghi | |||
| UVA | UVAL | M | 2 h | 3.74 ± 0.03 | k | 0.31 ± 0.01 | hi | 15.42 ± 0.08 | i | 12.71 ± 0.94 | efghi | 8.05 ± 0.17 | ghij | 8.02 ± 0.24 | fg | 7.09 ± 0.24 | ij | 55.35 ± 1.52 | jk |
| E | 4.05 ± 0.03 | jk | 0.32 ± 0.02 | hi | 18.18 ± 0.5 | h | 10.09 ± 0.6 | i | 5.77 ± 0.2 | m | 7.38 ± 0.08 | fg | 6.79 ± 0.21 | ij | 52.59 ± 1.12 | k | |||
| M | 24 h | 7.06 ± 0.34 | d | 0.64 ± 0.07 | ab | 26.54 ± 1.2 | bc | 19.99 ± 1.2 | a | 12.19 ± 0.46 | bc | 14.77 ± 0.46 | a | 9.05 ± 0.23 | e | 90.24 ± 3.38 | c | ||
| E | 7.98 ± 0.15 | bc | 0.58 ± 0.01 | abcd | 31.8 ± 0.76 | a | 19.19 ± 1.65 | ab | 9.66 ± 0.85 | def | 14.52 ± 0.35 | a | 9.14 ± 0.29 | e | 92.89 ± 4.07 | bc | |||
| UVAH | M | 2 h | 6.54 ± 0.24 | de | 0.34 ± 0.01 | gh | 23.79 ± 1.2 | def | 18.72 ± 1.03 | abc | 11.93 ± 0.2 | bc | 12.52 ± 0.09 | b | 7.87 ± 0.13 | fg | 81.71 ± 1.38 | d | |
| E | 7.21 ± 0.12 | cd | 0.42 ± 0.02 | efgh | 28.16 ± 0.35 | b | 15.98 ± 1.1 | cd | 7.54 ± 0.68 | ijk | 11.25 ± 0.43 | bc | 7.37 ± 0.31 | ghi | 77.94 ± 2.46 | de | |||
| M | 24 h | 5.39 ± 0.09 | fgh | 0.7 ± 0.04 | a | 19.66 ± 0.47 | gh | 16.98 ± 0.05 | bcd | 10.93 ± 0.18 | bcd | 12.46 ± 0.07 | b | 8.53 ± 0.11 | ef | 74.66 ± 0.39 | defg | ||
| E | 5.39 ± 0.35 | fgh | 0.61 ± 0.03 | abc | 21.39 ± 1.58 | fg | 14.2 ± 0.47 | defgh | 7.62 ± 0.22 | hij | 11.63 ± 0.06 | bc | 7.83 ± 0.11 | fgh | 68.65 ± 2.3 | fghi | |||
| UVB | UVBL | M | 2 h | 6.74 ± 0.57 | d | 0.45 ± 0.07 | defg | 24.76 ± 1.52 | cde | 18.93 ± 1.17 | ab | 11.6 ± 0.55 | bc | 12.56 ± 0.19 | b | 6.7 ± 0.27 | ijk | 81.75 ± 3.97 | d |
| E | 7.07 ± 0.05 | d | 0.51 ± 0.06 | cde | 28.5 ± 0.32 | b | 14.96 ± 1.56 | def | 7.46 ± 0.54 | ijk | 11.06 ± 0.32 | bcd | 6.03 ± 0.24 | kl | 75.59 ± 2.69 | def | |||
| M | 24 h | 5.96 ± 0.43 | ef | 0.21 ± 0.04 | i | 24.87 ± 1.95 | cd | 14.8 ± 1.84 | defg | 9.41 ± 1.08 | efg | 12.34 ± 0.69 | b | 11.47 ± 0.48 | c | 79.05 ± 6.39 | d | ||
| E | 5.52 ± 0.06 | fg | 0.31 ± 0.06 | hi | 23.43 ± 0.43 | def | 10.5 ± 1.01 | i | 6.04 ± 0.35 | lm | 10.41 ± 0.35 | cde | 9.99 ± 0.22 | d | 66.19 ± 1.71 | hi | |||
| UVBH | M | 2 h | 6.59 ± 0.32 | de | 0.52 ± 0.03 | bcde | 24.75 ± 0.32 | cde | 14.92 ± 0.9 | defg | 10.18 ± 0.27 | de | 11.38 ± 0.26 | bc | 7.1 ± 0.1 | hij | 75.44 ± 1.93 | def | |
| E | 7.12 ± 0.02 | d | 0.47 ± 0.03 | def | 27.86 ± 0.23 | b | 11.66 ± 0.33 | hi | 7.24 ± 0.21 | jkl | 10.14 ± 0.07 | cde | 6.83 ± 0.14 | ij | 71.31 ± 0.38 | efgh | |||
| M | 24 h | 8.9 ± 0.48 | a | 0.62 ± 0.05 | abc | 31.86 ± 1.72 | a | 15.9 ± 0.91 | d | 15.2 ± 0.61 | a | 16.45 ± 1.56 | a | 19.86 ± 0.25 | a | 108.79 ± 2.07 | a | ||
| E | 8.72 ± 0.2 | ab | 0.51 ± 0.05 | bcdef | 33.33 ± 0.84 | a | 12.34 ± 0.89 | fghi | 12.52 ± 1.02 | b | 15.55 ± 3.01 | a | 18.2 ± 0.36 | b | 101.17 ± 2.4 | ab | |||
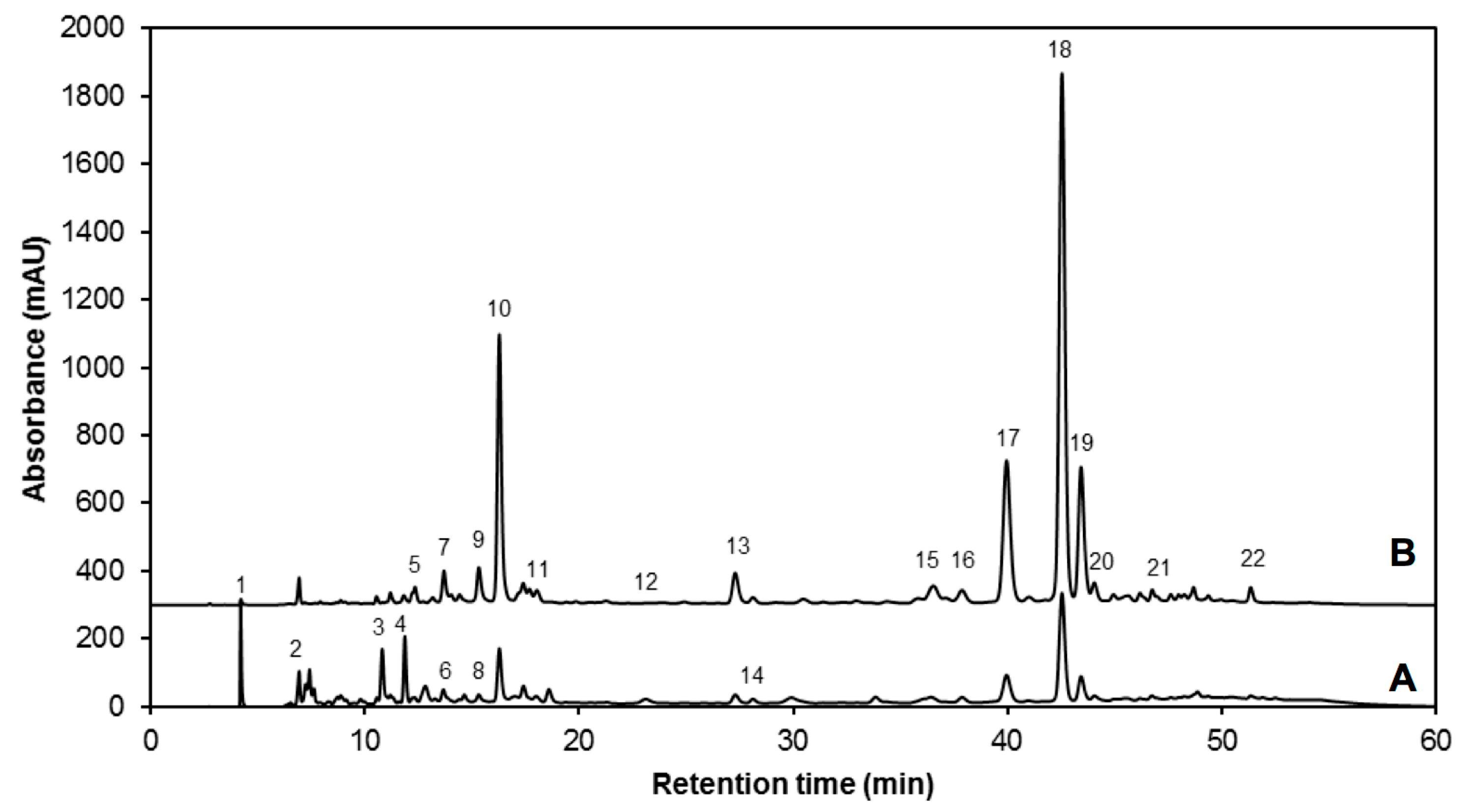
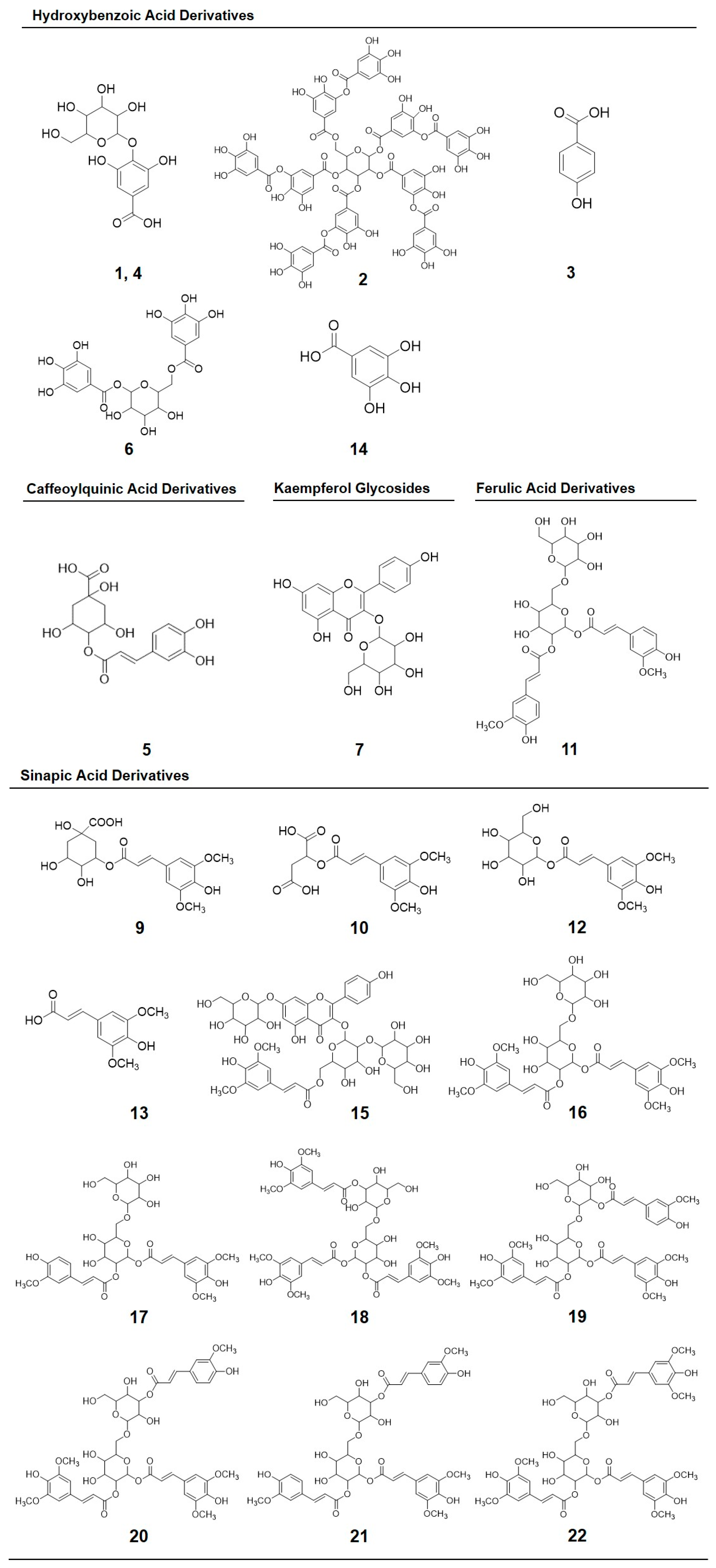
| Peak Number (Retention Time, min) | λmax (nm) | Identification | [M − H]− (m/z) | MS2 (m/z) a |
|---|---|---|---|---|
| 1 (4.2) | 262 | Gallic acid hexoside I | 331 | 162, 125 |
| 2 (6.9) | 210, 300 | Gallotannic acid | 1700 | 1530, 1378, 1225, 1091 |
| 3 (10.7) | 272 | p-Hydroxybenzoic acid | 137 | 122, 111, 107 |
| 4 (11.8) | 218, 280 | Gallic acid hexoside II | 331 | 162, 125 |
| 5 (12.2) | 218 sh, 326 sh | 4-O-Caffeoylquinic acid | 353 | 191, 179, 173 |
| 6 (12.7) | 220, 268 | Digalloyl hexoside | 483 | 337, 169 |
| 7 (13.6) | 222, 265, 330 | 3-O-Hexoside kaempferol | 447 | 285 |
| 8 (14.6) | 220, 268 | Gallic acid derivative | - | - |
| 9 (15.3) | 240 sh, 328 | 1-O-Sinapoyl-β-d-glucose | 385 | 223, 205, 173, 145 |
| 10 (16.2) | 240 sh, 330 | Sinapoyl malate | 339 | 205.6, 173, 147, 132 |
| 11 (17.2) | 228, 330 | 1,2-Diferuloylgentiobiose | 693 | 499, 175 |
| 12 (22.5) | 220, 268 | 5-Sinapoylquinic acid | 397 | 222, 191 |
| 13 (27.1) | 235, 324 | Sinapic acid | 223 | 179, 163, 135, 119 |
| 14 (29.3) | 221, 290 | Gallic acid | 169 | 167, 141, 137, 125, 81 |
| 15 (36.2) | 238 sh, 270, 330 | Kaempferol 3-O-sinapoyl-sophoroside 7-O-glucoside | 977 | 771, 609, 429, 285 |
| 16 (37.6) | 240 sh, 268, 332 | 1,2-Disinapoylgentiobiose | 753 | 529, 223 |
| 17 (39.9) | 240 sh, 330 | 1-Sinapoyl-2′-ferulolylgentiobiose | 723 | 449, 223 |
| 18 (42.4) | 240 sh, 328 | 1,2,2′-Trisinapoylgentiobiose b | 959 | 735, 223 |
| 19 (43.2) | 240 sh, 331 | 1,2-Disinapoyl-1′-ferulolylgentiobiose | 929 | 705, 223 |
| 20 (43.9) | 220, 238, 328 | 1,2-Disinapoyl-2′-ferulolylgentiobiose | 929 | 705, 223 |
| 21 (46.6) | 242, 326 | 1-Sinapoyl-2,2′-diferuloylgentiobiose | 899 | 705, 223 |
| 22 (51.2) | 238 sh, 330 | 1,2,2′-Trisinapoylgentiobiose b | 959 | 735, 223 |
| Dose 5 | Solvent | Time of Harvest after Treatment 6 | Phenolic Concentration (mg/kg DW) 1,2,3,4 | ||||||||||||||||
| GAH I | GTA | p-HBA | GAH II | 4-O-CQA | diGH | 3-O-H-K | GAD | ||||||||||||
| Control | M | 2 h | 411.2 ± 8.5 | c | 171.3 ± 9.7 | d | 428.7 ± 18.8 | a | 423.5 ± 5.4 | a | 138 ± 8 | bcde | 202.6 ± 14.2 | bcde | 195.6 ± 1.8 | abcd | 67.7 ± 5.2 | ef | |
| E | 290.9 ± 0.7 | g | 98.5 ± 22.8 | e | 260.6 ± 21.3 | fghi | 322.1 ± 6.2 | bc | 86.2 ± 14.8 | f | 160.1 ± 10.3 | h | 136.3 ± 11.3 | hi | 74.8 ± 0.7 | de | |||
| M | 24 h | 439.6 ± 10.7 | b | 195.4 ± 3.3 | bcd | 424.4 ± 13.6 | a | 430.9 ± 9.5 | a | 122.9 ± 21.6 | bcdef | 221.2 ± 2.8 | bc | 200.9 ± 4.6 | abc | 70.6 ± 2.1 | ef | ||
| E | 324.7 ± 4.8 | f | 61.5 ± 9.3 | ef | 312.2 ± 10.4 | cde | 337.5 ± 1.7 | bc | 90.6 ± 1.1 | ef | 173.4 ± 5.5 | fgh | 145.9 ± 30.4 | gh | 75.9 ± 5.3 | cde | |||
| UVA | UVAL | M | 2 h | 469.2 ± 4.3 | a | 197.2 ± 8.6 | bcd | 354 ± 13.6 | bcd | 267 ± 7.6 | efg | 137.7 ± 16.5 | bcde | 155.7 ± 4.6 | h | 211.6 ± 12.7 | ab | 85.2 ± 0.7 | bcd |
| E | 357.1 ± 4.7 | de | 91.7 ± 29 | e | 251 ± 15.6 | ghi | 225 ± 4.4 | hij | 196.2 ± 2.2 | a | 120 ± 7 | i | 219.9 ± 3 | a | 100 ± 2.7 | a | |||
| M | 24 h | 339.2 ± 10.7 | ef | 228.1 ± 18.5 | b | 273.9 ± 21.3 | efghi | 250.2 ± 9.2 | fgh | 134.6 ± 33.3 | bcdef | 269.6 ± 13.7 | a | 162.7 ± 5.4 | efgh | 65.7 ± 2 | ef | ||
| E | 260.5 ± 4.2 | hi | 58.4 ± 13 | ef | 221.6 ± 4.6 | i | 199.5 ± 4.4 | jk | 169.7 ± 3.9 | abc | 195 ± 24.1 | cdef | 184.6 ± 2.1 | bcdef | 60.6 ± 0.2 | f | |||
| UVAH | M | 2 h | 346 ± 8.4 | ef | 173.9 ± 10.2 | d | 390.5 ± 27.9 | ab | 269.6 ± 5.1 | efg | 106.6 ± 17 | def | 220.3 ± 5.4 | bc | 158.5 ± 7.5 | fgh | 71.1 ± 1.8 | ef | |
| E | 261.1 ± 6.2 | hi | 66.9 ± 3.3 | ef | 246.6 ± 9.3 | hi | 221.6 ± 0.7 | hij | 134.4 ± 14.2 | bcdef | 156.9 ± 3.5 | h | 160.3 ± 12.3 | fgh | 71.2 ± 4 | ef | |||
| M | 24 h | 373.1 ± 20.4 | d | 178.8 ± 16.2 | d | 357.8 ± 25.8 | bc | 272.5 ± 7.6 | def | 161 ± 25.3 | abc | 222.5 ± 12.1 | b | 192.1 ± 4.6 | abcde | 67.5 ± 2 | ef | ||
| E | 291.6 ± 13 | g | 99.8 ± 28.2 | e | 284.9 ± 13.1 | efgh | 223 ± 10.9 | hij | 169.9 ± 7.9 | ab | 165.9 ± 7.4 | gh | 198.9 ± 4.6 | abc | 73.6 ± 7.5 | def | |||
| UVB | UVBL | M | 2 h | 410.6 ± 3.3 | c | 225.4 ± 6 | bc | 305.4 ± 20.8 | ef | 291.5 ± 5.5 | de | 152.9 ± 35.4 | abcd | 224.6 ± 4.1 | b | 211.3 ± 1.7 | ab | 94.1 ± 3.4 | ab |
| E | 328.7 ± 4.2 | f | 78.6 ± 10.6 | ef | 238.5 ± 7.8 | hi | 242.3 ± 5 | ghi | 164.9 ± 3 | abc | 188.8 ± 6.6 | defg | 212.1 ± 4.8 | ab | 93.4 ± 7.8 | ab | |||
| M | 24 h | 400.3 ± 3.9 | c | 193.7 ± 10.4 | bcd | 417.5 ± 12.1 | a | 350.2 ± 37 | b | 195.5 ± 7.3 | a | 187.2 ± 2.6 | efg | 188.3 ± 2 | bcdef | 85.1 ± 0.4 | bcd | ||
| E | 293.4 ± 4.5 | g | 102.5 ± 19.5 | e | 297.3 ± 10.2 | efg | 243.8 ± 1.1 | fghi | 165.5 ± 5.9 | abc | 130.2 ± 3.2 | i | 186 ± 2.4 | bcdef | 85.7 ± 9.4 | bcd | |||
| UVBH | M | 2 h | 370.1 ± 1.8 | d | 185 ± 3.3 | cd | 399 ± 17.3 | ab | 218.7 ± 4.3 | ij | 116.9 ± 7.3 | cdef | 212.4 ± 9.8 | bcf | 170.1 ± 6.7 | cdefg | 96.6 ± 2.1 | ab | |
| E | 293.6 ± 1.1 | g | 47.2 ± 5 | f | 307.9 ± 3.3 | de | 188.1 ± 2.7 | k | 160.7 ± 6.9 | abc | 131.5 ± 5.2 | i | 162.1 ± 21.5 | efgh | 93.2 ± 6.8 | ab | |||
| M | 24 h | 335.4 ± 6.1 | ef | 288.6 ± 6.2 | a | 313.4 ± 21 | cde | 300.5 ± 6.6 | cd | 136.9 ± 28.2 | bcde | 218.7 ± 7.5 | bc | 166.8 ± 9.4 | defgh | 88.3 ± 1.1 | abc | ||
| E | 235.1 ± 0 | i | 38.4 ± 12.2 | f | 289.2 ± 19.7 | efgh | 221.9 ± 6.4 | hij | 119.7 ± 3.5 | bcdef | 160.6 ± 1.6 | h | 102.3 ± 19.9 | i | 75.8 ± 1.5 | cdef | |||
| Dose 5 | Solvent | Time of Harvest after Treatment 6 | Phenolic Concentration (mg/kg DW) 1,2,3,4 | ||||||||||||||||
| 1-O-S-β-d-g | Sinapoyl Malate | 1,2-diFG | 5-SQA | Sinapic Acid | Gallic Acid | K-3-O-S-so-7-O-g | 1,2-diSG | ||||||||||||
| Control | M | 2 h | 241.5 ± 4.9 | ab | 1600.8 ± 25 | efg | 152.2 ± 5.7 | abcd | 81.5 ± 5.5 | gh | 307.5 ± 9.2 | b | 168.8 ± 11.3 | abc | 276.8 ± 2.6 | cde | 178.3 ± 3.2 | abcd | |
| E | 181.9 ± 0.9 | fg | 1471.5 ± 5 | i | 121.7 ± 2.4 | def | 48.1 ± 4.1 | k | 245.6 ± 1.8 | cd | 136.2 ± 5.4 | fgh | 261.9 ± 26.1 | de | 139.8 ± 1.8 | g | |||
| M | 24 h | 251.6 ± 4.9 | a | 1451.8 ± 10.7 | i | 152.2 ± 8.7 | abcd | 89.6 ± 2.7 | efg | 300.8 ± 3.1 | b | 163.8 ± 5.1 | bcde | 359.2 ± 37.5 | abc | 194.4 ± 3.8 | a | ||
| E | 205.2 ± 4 | cd | 1320.6 ± 27.3 | j | 121.3 ± 22.2 | def | 64.9 ± 1.2 | j | 245.3 ± 5.4 | cd | 143.4 ± 15.5 | defgh | 296.5 ± 58.3 | bcde | 146.5 ± 15.4 | fg | |||
| UVA | UVAL | M | 2 h | 235.4 ± 3.4 | b | 1612.6 ± 20.4 | def | 175.7 ± 3.7 | a | 80.9 ± 1 | gh | 328.9 ± 11.5 | a | 167.2 ± 6 | abcd | 356.9 ± 6.7 | abc | 190.8 ± 2.7 | ab |
| E | 202.8 ± 4.9 | cde | 1565.8 ± 11.3 | fgh | 106.3 ± 17.3 | fg | 65.5 ± 1.9 | j | 293.7 ± 4.4 | b | 164.6 ± 9.7 | bcde | 338 ± 22.5 | abcd | 158.9 ± 2.3 | defg | |||
| M | 24 h | 169.6 ± 5.1 | hij | 1718.7 ± 63.8 | abc | 118 ± 16.1 | efg | 105.5 ± 6.4 | cd | 150.6 ± 5.1 | i | 156.4 ± 6.3 | bcdefg | 322.9 ± 45.7 | abcde | 166.1 ± 12.5 | cdef | ||
| E | 136.1 ± 0.5 | k | 1596.3 ± 37.1 | efg | 129.6 ± 1.2 | cdef | 66.6 ± 1.6 | ij | 119.9 ± 2.3 | j | 135.6 ± 3.3 | fghi | 397.5 ± 8.9 | a | 145.2 ± 3.9 | fg | |||
| UVAH | M | 2 h | 178.9 ± 2.6 | gh | 1657.1 ± 46.5 | bcde | 144.6 ± 2.5 | abcde | 93.1 ± 4.6 | ef | 218.2 ± 6 | ef | 191.8 ± 11.1 | a | 253.5 ± 4.3 | ef | 163.8 ± 5.3 | cdef | |
| E | 148.6 ± 3.7 | k | 1626.9 ± 29.8 | def | 119.3 ± 3.4 | defg | 59.2 ± 1.6 | j | 186.9 ± 1.4 | h | 145.8 ± 10 | cdefg | 177 ± 4.9 | f | 111 ± 1.5 | h | |||
| M | 24 h | 199.6 ± 5.6 | de | 1632.9 ± 24.6 | cdef | 128.7 ± 25.2 | cdef | 98.8 ± 6.6 | de | 207.4 ± 13.5 | fg | 179.5 ± 1.8 | ab | 330.1 ± 16 | abcde | 192.7 ± 5.4 | a | ||
| E | 171.3 ± 3.4 | ghi | 1583.5 ± 48.2 | efg | 60.8 ± 8.2 | h | 76.2 ± 1.5 | hi | 177.5 ± 10.3 | h | 160.1 ± 7.6 | bcdef | 333 ± 43.2 | abcde | 167 ± 4.9 | cdef | |||
| UVB | UVBL | M | 2 h | 230.8 ± 2.2 | b | 1784.1 ± 18.7 | a | 170.3 ± 2.5 | ab | 81.3 ± 1.8 | gh | 257.1 ± 1.4 | c | 152.5 ± 2.7 | cdefg | 308.7 ± 12.7 | abcde | 189.5 ± 11.9 | ab |
| E | 199.7 ± 8.3 | de | 1669.2 ± 45.5 | bcde | 136.5 ± 2.6 | bcdef | 60.2 ± 1.2 | j | 232.7 ± 5.2 | de | 133.5 ± 2.3 | ghi | 250.2 ± 28 | ef | 146.3 ± 13.4 | fg | |||
| M | 24 h | 210.9 ± 0.5 | c | 1622.8 ± 36.5 | def | 151 ± 7 | abcde | 111.4 ± 3.6 | c | 222.3 ± 3.2 | ef | 155.5 ± 4.5 | bcdefg | 282.2 ± 38.4 | bcde | 170.1 ± 7.8 | bcde | ||
| E | 162.9 ± 3.3 | j | 1491.9 ± 13.7 | hi | 123.4 ± 1 | def | 91.8 ± 1.8 | ef | 177.4 ± 10 | h | 140.5 ± 3.8 | efgh | 298.1 ± 31.1 | bcde | 145.2 ± 1.7 | fg | |||
| UVBH | M | 2 h | 192.4 ± 1.6 | ef | 1740.9 ± 6.3 | ab | 158 ± 2.5 | abc | 86.4 ± 1.9 | fg | 211 ± 3.8 | fg | 120.9 ± 9 | hi | 305.2 ± 20.7 | abcde | 177.9 ± 11.8 | abcd | |
| E | 160 ± 2 | j | 1636.7 ± 31.2 | cdef | 86.9 ± 16.6 | gh | 59.5 ± 2.4 | j | 196.1 ± 10 | gh | 110.7 ± 11.3 | i | 345 ± 14.6 | abcd | 153 ± 1 | efg | |||
| M | 24 h | 171.4 ± 2.6 | ghi | 1700.6 ± 25.1 | abcd | 137.8 ± 20.8 | bcdef | 197.6 ± 5.2 | a | 144.5 ± 3.6 | i | 164.7 ± 6.2 | bcde | 323.3 ± 43.2 | abcde | 181.4 ± 9.8 | abc | ||
| E | 121.1 ± 2.2 | l | 1502 ± 8.9 | ghi | 124.3 ± 2.2 | cdefg | 146.7 ± 3.9 | b | 90.4 ± 4.7 | k | 130.6 ± 26.5 | ghi | 371.5 ± 26.6 | ab | 154.6 ± 1.9 | defg | |||
| Dose 5 | Solvent | Time of Harvest after Treatment 6 | Phenolic Concentration (mg/kg DW) 1,2,3,4 | ||||||||||||||||
| 1-S-2-FG | 1,2,2-triSG 7 | 1,2-diS-1-FG | 1,2-diS-2-FG | 1-S-2,2-diFG | 1,2,2-triSG 7 | Total | |||||||||||||
| Control | M | 2 h | 1505.7 ± 24.7 | hij | 4394.1 ± 58.8 | cde | 1107.9 ± 38.5 | a | 162.6 ± 0.9 | bc | 89.5 ± 1 | h | 119.9 ± 2.2 | ef | 12,425.7 ± 180.6 | bc | |||
| E | 1374.7 ± 6.7 | k | 4069.6 ± 8.2 | gh | 1010.4 ± 14.8 | cd | 127.2 ± 1 | k | 55.9 ± 3.2 | i | 85.7 ± 1.3 | i | 10,759.8 ± 37.1 | ghi | |||||
| M | 24 h | 1729.6 ± 19.1 | abcd | 4793.6 ± 46.8 | a | 1134.9 ± 17.2 | a | 166.9 ± 2.2 | b | 100.2 ± 3.1 | gh | 139.2 ± 1.1 | abc | 13,133.6 ± 176.9 | a | ||||
| E | 1608.4 ± 24.9 | efg | 4561.7 ± 87 | bcd | 1089.2 ± 19.8 | ab | 134.6 ± 3.1 | hij | 68.8 ± 2.2 | i | 95.3 ± 12 | hi | 11,623.2 ± 264.3 | def | |||||
| UVA | UVAL | M | 2 h | 1737.7 ± 18.6 | abcd | 4261.3 ± 23.8 | efg | 840.7 ± 9.7 | gh | 162.3 ± 0.9 | bcd | 144.3 ± 2.4 | a | 126.3 ± 1.4 | de | 12,298.6 ± 123.3 | c | ||
| E | 1703.9 ± 17.6 | bcd | 4252 ± 60.4 | efg | 794.4 ± 11.3 | h | 137.2 ± 2.9 | hi | 111.2 ± 4.2 | efg | 98.4 ± 1.4 | h | 11,553.6 ± 130.3 | ef | |||||
| M | 24 h | 1735 ± 69 | abcd | 4240.1 ± 169.4 | efg | 892.1 ± 42.5 | efg | 163.8 ± 5.8 | bc | 132.9 ± 4.3 | abc | 130 ± 4.5 | cd | 11,925.8 ± 430.1 | cde | ||||
| E | 1645.5 ± 44.5 | defg | 4126 ± 63.1 | fgh | 835.2 ± 11.1 | gh | 130.5 ± 0 | ijk | 100.5 ± 7.1 | fgh | 103.1 ± 2.6 | gh | 11,017.6 ± 204.2 | fgh | |||||
| UVAH | M | 2 h | 1495.3 ± 48.6 | ij | 3807.9 ± 123.6 | i | 953.7 ± 22.6 | de | 145.8 ± 3.2 | fg | 128.6 ± 4.2 | bc | 115.2 ± 1.9 | f | 11,284 ± 297.4 | fg | |||
| E | 1450.5 ± 30.3 | jk | 3735.8 ± 76.8 | i | 919.9 ± 23.1 | ef | 114.4 ± 3.5 | m | 98.6 ± 6.8 | gh | 87.5 ± 2 | i | 10,300.4 ± 141.2 | i | |||||
| M | 24 h | 1784.4 ± 39.4 | ab | 4390 ± 102.5 | cde | 953.1 ± 15.9 | de | 155.3 ± 2.4 | de | 139.2 ± 1.9 | ab | 133.9 ± 2.2 | cd | 12,351 ± 247.6 | c | ||||
| E | 1759.8 ± 45.5 | abc | 4360.5 ± 118.5 | def | 950.1 ± 20.7 | de | 128.7 ± 2.9 | jk | 96.4 ± 2.6 | h | 111.4 ± 2.6 | fg | 11,643.7 ± 313.9 | def | |||||
| UVB | UVBL | M | 2 h | 1809.8 ± 23.6 | a | 4645.6 ± 75.9 | ab | 1126.3 ± 14.5 | a | 165.8 ± 2.7 | b | 136.4 ± 7.6 | abc | 136.6 ± 1.3 | bc | 13,110.7 ± 252.6 | a | ||
| E | 1760.6 ± 32.2 | abc | 4588.5 ± 100.7 | abc | 1099.6 ± 35.1 | a | 138.4 ± 1.7 | hi | 115.1 ± 1 | def | 112 ± 2.6 | fg | 12,189.7 ± 281.9 | cd | |||||
| M | 24 h | 1675.6 ± 16.6 | cde | 4350 ± 57.7 | def | 1027.5 ± 34.3 | bc | 158.6 ± 1.4 | cd | 123.2 ± 5.1 | cde | 145.7 ± 2.5 | ab | 12,424.7 ± 116.4 | bc | ||||
| E | 1587.6 ± 8.9 | fgh | 4091.1 ± 23.1 | gh | 951.2 ± 24.8 | de | 125.1 ± 1.2 | kl | 99.7 ± 6.5 | gh | 114.4 ± 2.8 | f | 11,104.7 ± 54.3 | fgh | |||||
| UVBH | M | 2 h | 1651 ± 22.2 | def | 3925 ± 16.2 | hi | 933.7 ± 17.3 | ef | 149.7 ± 0.7 | ef | 128 ± 6.4 | bcd | 114.5 ± 1.5 | f | 11,663.4 ± 95.4 | def | |||
| E | 1553 ± 19.1 | ghi | 3814.4 ± 71.8 | i | 873.8 ± 13.8 | fg | 119.1 ± 2.3 | lm | 91.5 ± 5.2 | h | 86 ± 0.4 | i | 10,669.9 ± 119.5 | hi | |||||
| M | 24 h | 1812.8 ± 14.2 | a | 4717.6 ± 43.9 | ab | 1087.7 ± 22 | ab | 176 ± 0.7 | a | 140.3 ± 6.1 | ab | 147.2 ± 1.7 | a | 12,951.5 ± 97.1 | ab | ||||
| E | 1654 ± 5.3 | def | 4351 ± 7.2 | cdef | 964.2 ± 3.8 | cde | 139.8 ± 0.4 | gh | 102 ± 7.5 | fgh | 114 ± 1.9 | fg | 11,209 ± 36.7 | fgh | |||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light Doses and Harvesting Time Differentially Tailor Glucosinolate and Phenolic Profiles in Broccoli Sprouts. Molecules 2017, 22, 1065. https://doi.org/10.3390/molecules22071065
Moreira-Rodríguez M, Nair V, Benavides J, Cisneros-Zevallos L, Jacobo-Velázquez DA. UVA, UVB Light Doses and Harvesting Time Differentially Tailor Glucosinolate and Phenolic Profiles in Broccoli Sprouts. Molecules. 2017; 22(7):1065. https://doi.org/10.3390/molecules22071065
Chicago/Turabian StyleMoreira-Rodríguez, Melissa, Vimal Nair, Jorge Benavides, Luis Cisneros-Zevallos, and Daniel A. Jacobo-Velázquez. 2017. "UVA, UVB Light Doses and Harvesting Time Differentially Tailor Glucosinolate and Phenolic Profiles in Broccoli Sprouts" Molecules 22, no. 7: 1065. https://doi.org/10.3390/molecules22071065





