Design, Synthesis and Evaluation of Hesperetin Derivatives as Potential Multifunctional Anti-Alzheimer Agents
Abstract
:1. Introduction
2. Results and Discussion
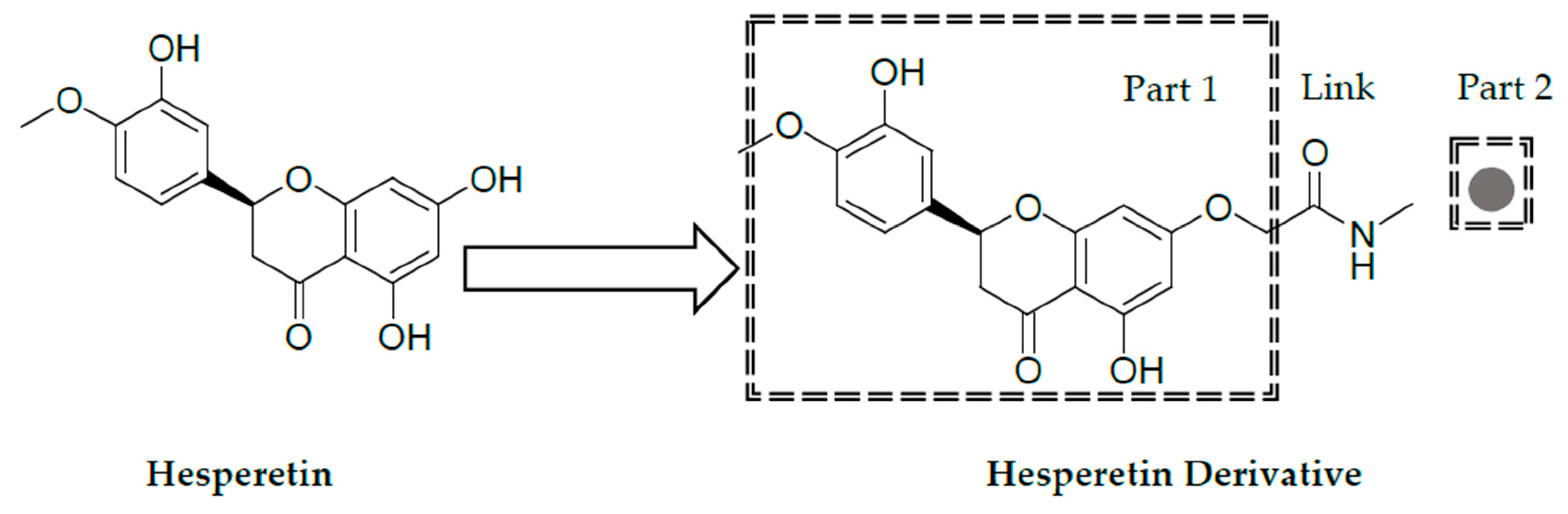
2.1. Chemistry
2.2. Inhibitory Study of Hesperetin Derivatives on AChE and BuChE
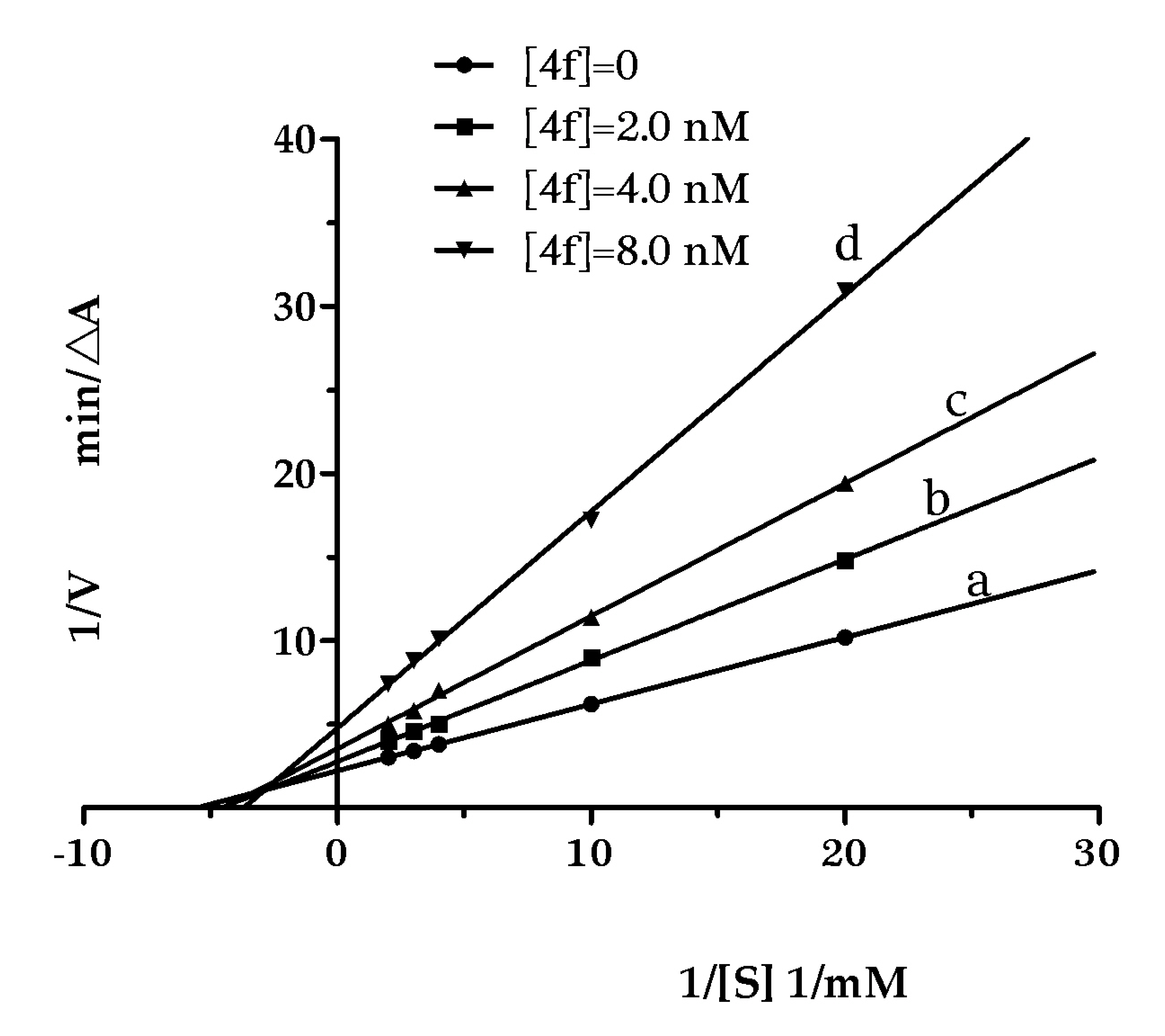
2.3. Kinetic Studies for the Inhibition of AChE
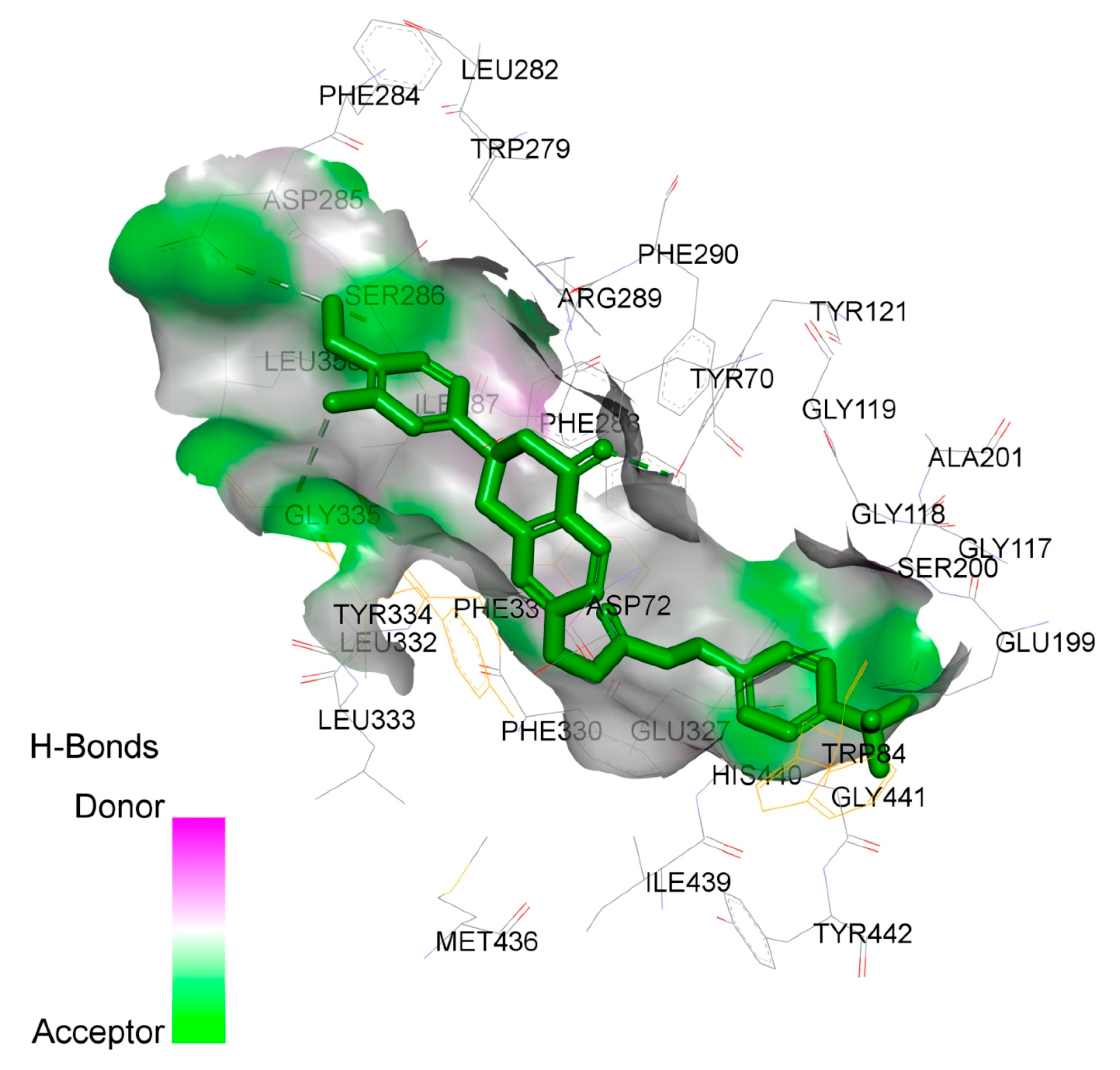
2.4. Study on the Interaction between 4f and AChE by Molecular Docking Method
2.5. Antioxidant Activity
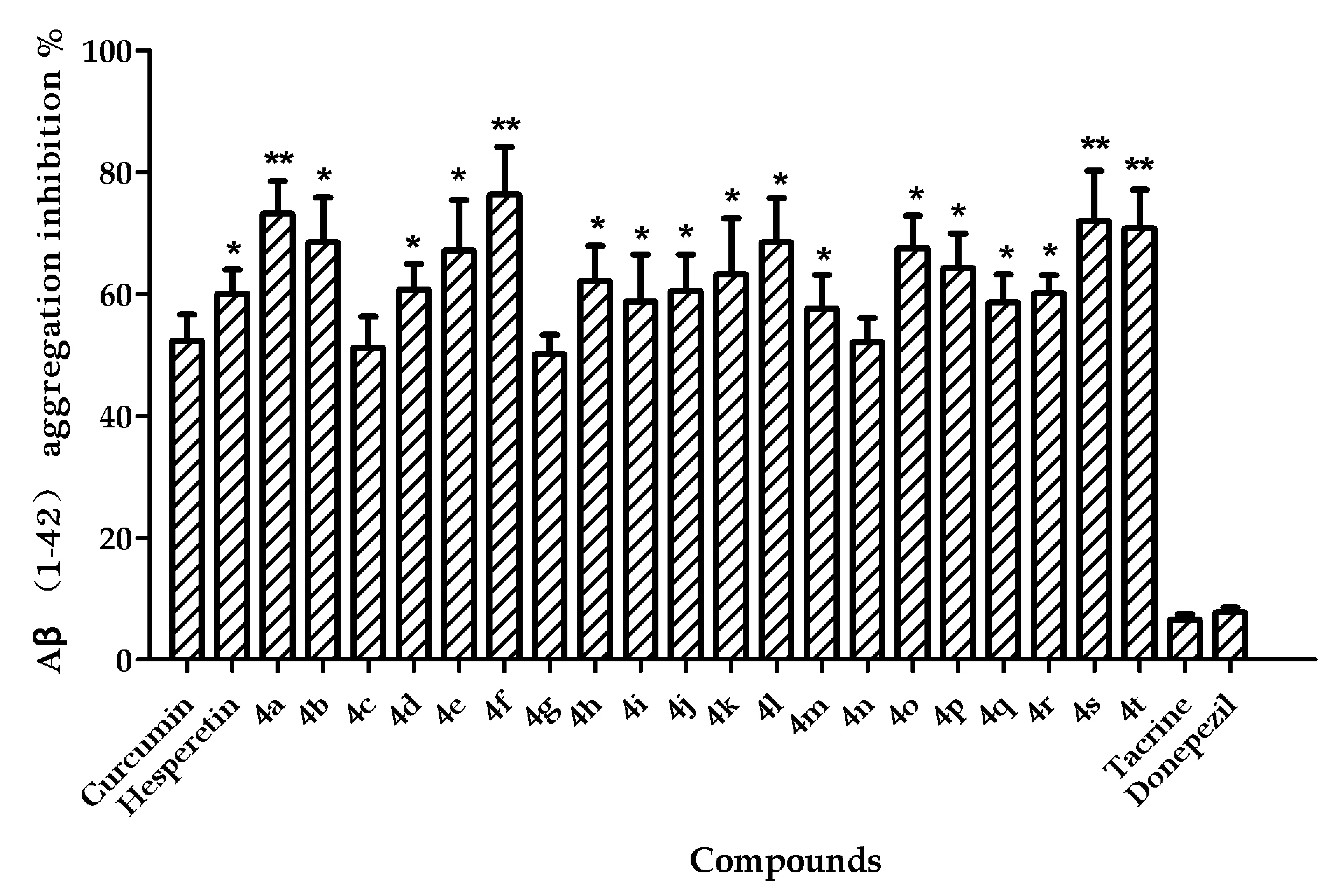
2.6. Inhibition of Self-Mediated Aβ (1–42) Aggregation
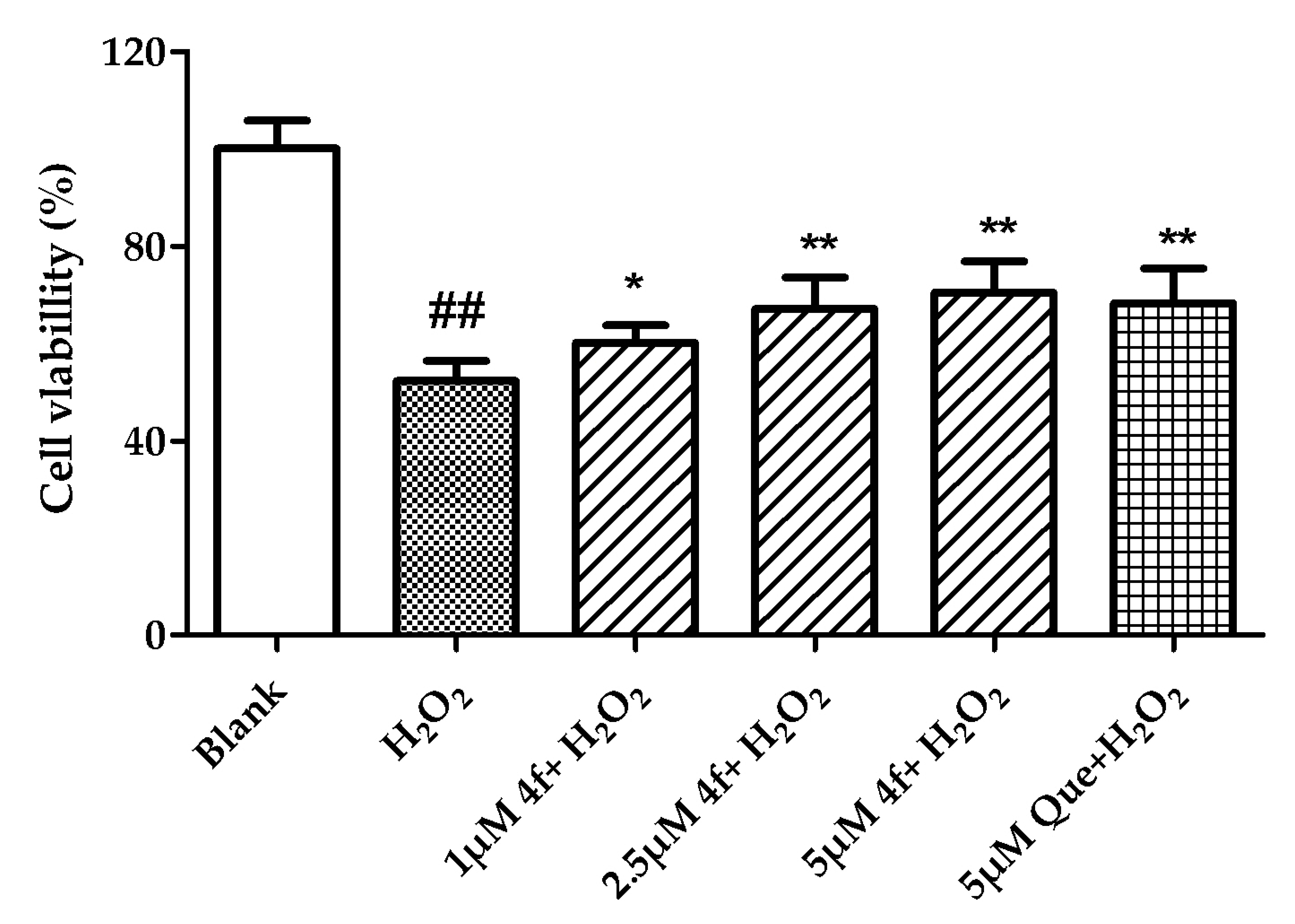
2.7. Neuroprotective Effect Against H2O2-Induced Cell Death in PC12 Neurons
2.8. Effects of Hesperetin Derivatives on Neurocyte Viability
3. Materials and Methods
3.1. General Information
3.2. Chemistry
3.2.1. Synthesis of Intermediate 2
3.2.2. Synthesis of Intermediate 3
3.2.3. General Procedures for the Preparation of Target Compounds 4a–4t
3.3. In Vitro Inhibition Studies on AChE and BuChE
3.4. Kinetic Characterization of AChE Inhibition
3.5. Molecular Modeling
3.6. Measurement of the Anti-Oxidation Activity
- (1)
- AUC = 1 + (F0 is the initial fluorescence at 0 min, and it is the fluorescence at time I);
- (2)
- The ORAC values were calculated as following: [(AUCsample − AUCblank)/(AUCTrolox − AUCblank)]/[(concentration of Trolox/concentration of sample)], and expressed as Trolox equivalents by using the standard curve calculated for each assay. Final result were in μM of Trolox equivalent/μM of pure compound.
3.7. Inhibition Experiments of Self-Mediated Aβ (1–42) Aggregation
3.8. Protective Effect Against H2O2-Induced Cell Death in PC12Neurons
3.9. Cellular Toxicity
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shaik, J.B.; Palaka, B.K.; Penumala, M.; Kotapati, K.V.; Devineni, S.R.; Eadlapalli, S.; Darla, M.M.; Ampasala, D.R.; Vadde, R.; Amooru, G.D. Synthesis, pharmacological assessment, molecular modeling and in silico studies of fused tricyclic coumarin derivatives as a new family of multifunctional anti-Alzheimer agents. Eur. J. Med. Chem. 2016, 107, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Khunnawutmanotham, N.; Chimnoi, N.; Saparpakorn, P.; Techasakul, S. Synthesis and anti-acetylcholinesterase activity of scopoletin derivatives. Bioorg. Chem. 2016, 65, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-beta: A crucial factor in Alzheimer’ disease. Med. Princ. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Henderson, Z. Responses of basal forebrain cholinergic neurons to damage in the adult brain. Prog. Neurobiol. 1996, 48, 219–254. [Google Scholar] [CrossRef]
- Mecocci, P.; Cherubini, A.; Polidori, M.C.; Cecchetti, R.; Chionne, F.; Senin, U. Oxidative stress and dementia: New perspectives in AD pathogenesis. Aging (Milano) 1997, 9, 51–52. [Google Scholar] [CrossRef] [PubMed]
- Kandemirli, F.; Saracoglu, M.; Kovalishyn, V. Human acetylcholinesterase inhibitors: Electronic-topological and neural network approaches to the structure-activity relationships study. Mini. Rev. Med. Chem. 2005, 5, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Melchiorre, C. From dual binding site acetylcholinesterase inhibitors to multi-target-directed ligands (MTDLs): A step forward in the treatment of Alzheimer’s disease. Mini. Rev. Med. Chem. 2008, 8, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Rampa, A.; Belluti, F.; Gobbi, S.; Bisi, A. Hybrid-based multi-target ligands for the treatment of Alzheimer’s disease. Curr. Top. Med. Chem. 2011, 11, 2716–2730. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, G.; Greig, N.H.; Khan, J.A.; Kamal, M.A. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol. Disord. Drug Targets 2014, 13, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Doody, R.; Clark, C. Disease-modifying therapies for Alzheimer disease: Challenges to early intervention. Neurology 2007, 69, 1622–1634. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, G.; Palacios-Esquivel, R.; Moss, D.E. Cholinesterase inhibitors proposed for treating dementia in Alzheimer’s disease: Selectivity toward human brain acetylcholinesterase compared with butyrylcholinesterase. J. Pharmacol. Exp. Ther. 1995, 274, 767–770. [Google Scholar] [PubMed]
- Liston, D.R.; Nielsen, J.A.; Villalobos, A.; Chapin, D.; Jones, S.B.; Hubbard, S.T.; Shalaby, I.A.; Ramirez, A.; Nason, D.; White, W.F. Pharmacology of selective acetylcholinesterase inhibitors: Implications for use in Alzheimer’s disease. Eur. J. Pharmacol. 2004, 486, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, D.E.; Koeppe, R.A.; Snyder, S.E.; Minoshima, S.; Frey, K.A.; Kilbourn, M.R. In vivo butyrylcholinesterase activity is not increased in Alzheimer’s disease synapses. Ann. Neurol. 2006, 59, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Wilmsen, P.K.; Spada, D.S.; Salvador, M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J. Agric. Food Chem. 2005, 53, 4757–4761. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, H.; Yang, Z.; Liu, Z. Antioxidative effects of hesperetin against lead acetate-induced oxidative stress in rats. Indian J. Pharmacol. 2013, 45, 395–398. [Google Scholar] [PubMed]
- Crespo, M.E.; Galvez, J.; Cruz, T.; Ocete, M.A.; Zarzuelo, A. Anti-inflammatory activity of diosmin and hesperidin in rat colitis induced by TNBS. Planta Med. 1999, 65, 651–653. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Liu, K.C.; Chiou, Y.L. Melanogenesis of murine melanoma cells induced by hesperetin, a Citrus hydrolysate-derived flavonoid. Food Chem. Toxicol. 2012, 50, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Perez, M.; Ballester, A.R.; Gonzalez-Candelas, L. Identification and functional analysis of Penicillium digitatum genes putatively involved in virulence towards citrus fruit. Mol. Plant Pathol. 2015, 16, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, M.J.; Ha, E.; Chung, J.H. Apoptotic effect of hesperidin through caspase3 activation in human colon cancer cells, SNU-C4. Phytomedicine 2008, 15, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Elshazly, S.M.; Mahmoud, A.A. Antifibrotic activity of hesperidin against dimethylnitrosamine-induced liver fibrosis in rats. Naunyn. Schmiedebergs Arch. Pharmacol. 2014, 387, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.C.; Kao, S.J.; Lin, C.C.; Wang, S.D.; Liu, C.J.; Kao, S.T. The immunomodulation of endotoxin-induced acute lung injury by hesperidin in vivo and in vitro. Life Sci. 2007, 80, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wang, T.; Hong, C.; Yang, Y.C.; Chen, Y.; Cen, J.; Xie, S.Q.; Wang, C.J. Design, synthesis and evaluation of 4-dimethylamine flavonoid derivatives as potential multifunctional anti-Alzheimer agents. Eur. J. Med. Chem. 2016, 122, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Wang, B.; Mai, Y.C.; Li, Y.; Hou, J.Q.; Huang, S.L.; Ou, T.M.; Tan, J.H.; An, L.K.; Li, D.; Gu, L.Q.; et al. Synthesis and evaluation of novel rutaecarpine derivatives and related alkaloids derivatives as selective acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2010, 45, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Rosini, M.; Simoni, E.; Milelli, A.; Minarini, A.; Melchiorre, C. Oxidative stress in Alzheimer’s disease: Are we connecting the dots? J. Med. Chem. 2014, 57, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Pratico, D.; Sung, S. Lipid peroxidation and oxidative imbalance: Early functional events in Alzheimer’s disease. J. Alzheimers Dis. 2004, 6, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Davalos, A.; Gomez-Cordoves, C.; Bartolome, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Hasegawa, K.; Yamada, M.; Naiki, H. Nicotine breaks down preformed Alzheimer’s beta-amyloid fibrils in vitro. Biol. Psychiatry 2002, 52, 880–886. [Google Scholar] [CrossRef]
- Smith, R.A.; Pontiggia, L.; Waterman, C.; Lichtenwalner, M.; Wasserman, J. Comparison of motility, recovery, and methyl-thiazolyl-tetrazolium reduction assays for use in screening plant products for anthelmintic activity. Parasitol. Res. 2009, 105, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.S.; Zhu, X.; Holloway, H.W.; Whittaker, N.F.; Brossi, A.; Greig, N.H. Anticholinesterase activity of compounds related to geneserine tautomers. N-Oxides and 1,2-oxazines. J. Med. Chem. 2002, 45, 3684–3691. [Google Scholar] [CrossRef] [PubMed]
- Qiang, X.; Sang, Z.; Yuan, W.; Li, Y.; Liu, Q.; Bai, P.; Shi, Y.; Ang, W.; Tan, Z.; Deng, Y. Design, synthesis and evaluation of genistein-O-alkylbenzylamines as potential multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014, 76, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, M.; Bertucci, C.; Bolognesi, M.L.; Cavalli, A.; Melchiorre, C.; Andrisano, V. Insight into the kinetic of amyloid β (1–42) peptide self-aggregation: Elucidation of inhibitors’ mechanism of action. Chembiochem 2007, 8, 2152–2161. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

| Compounds | AchE Inhibition a, (IC50) nM | BuChE Inhibition b, (IC50) nM | Selective Index c | ORAC d |
|---|---|---|---|---|
| Hesperetin | 1023 ± 37 | 2897 ± 67 | 3 | 5.1 |
| 4a | 25.51 ± 1.32 | 3400 ± 56 | 133 | 3.2 |
| 4b | 13.55 ± 0.78 | 3502 ± 66 | 258 | 2.7 |
| 4c | 12.44 ± 0.81 | 2078 ± 76 | 167 | 2.9 |
| 4d | 15.71 ± 0.95 | 2133 ± 64 | 136 | 2.6 |
| 4e | 17.88 ± 054 | 3208 ± 92 | 179 | 2.8 |
| 4f | 9.37 ± 0.87 | 2862 ± 45 | 305 | 3.0 |
| 4g | 14.03 ± 0.95 | 1634 ± 78 | 116 | 3.1 |
| 4h | 15.23 ± 2.03 | 2803 ± 39 | 184 | 2.5 |
| 4i | 19.23 ± 0.66 | 1985 ± 134 | 103 | 2.7 |
| 4j | 22.33 ± 1.54 | 2456 ± 83 | 110 | 3.1 |
| 4k | 11.87 ± 1.12 | 3013 ± 74 | 254 | 2.7 |
| 4l | 19.52 ± 0.77 | 2790 ± 53 | 143 | 2.9 |
| 4m | 15.67 ± 0.85 | 3354 ± 66 | 214 | 2.6 |
| 4n | 17.22 ± 0.96 | 1986 ± 51 | 115 | 2.4 |
| 4o | 13.52 ± 1.44 | 2255 ± 81 | 167 | 3.1 |
| 4p | 17.22 ± 0.45 | 3469 ± 67 | 201 | 2.7 |
| 4q | 20.22 ± 1.88 | 2235 ± 77 | 111 | 2.5 |
| 4r | 16.11 ± 0.79 | 3025 ± 84 | 188 | 2.8 |
| 4s | 27.32 ± 0.99 | 1846 ± 55 | 68 | 2.4 |
| 4t | 30.65 ± 4.50 | 2355 ± 104 | 77 | 2.6 |
| Tacrine | 203.5 ± 3.25 | 30.50 ± 1.49 | 0.15 | <0.1 |
| Donepezil | 17.57 ± 1.01 | 5634 ± 76 | 320.7 | <0.1 |
| Curcumin | — | — | — | 2.7 |
| Compounds | Cell Viability a (%, 1 μM) | Cell Viability (%, 10 μM) | Cell Viability (%, 50 μM) | Cell Viability (%, 100 μM) |
|---|---|---|---|---|
| Hesperetin | 99.01 ± 5.87 | 93.03 ± 4.33 | 85.23 ± 3.43 | 67.34 ± 5.21 |
| 4a | 98.23 ± 8.02 | 91.56 ± 5.67 | 81.43 ± 5.23 | 63.21 ± 4.23 |
| 4b | 102.52 ± 4.56 | 95.87 ± 3.98 | 83.66 ± 4.12 | 65.41 ± 5.33 |
| 4c | 98.52 ± 4.98 | 97.54 ± 3.76 | 83.78 ± 2.32 | 58.75 ± 3.97 |
| 4d | 99.87 ± 5.76 | 99.45 ± 6.23 | 86.23 ± 5.14 | 70.02 ± 4.78 |
| 4e | 104.91 ± 3.87 | 98.32 ± 5.27 | 87.13 ± 6.37 | 66.54 ± 4.95 |
| 4f | 100.23 ± 4.76 | 95.77 ± 7.31 | 83.29 ± 5.44 | 62.32 ± 5.78 |
| 4g | 97.85 ± 6.83 | 95.65 ± 5.67 | 79.33 ± 4.33 | 70.81 ± 5.32 |
| 4h | 100.02 ± 6.52 | 98.75 ± 5.38 | 75.79 ± 3.83 | 60.65 ± 4.27 |
| 4i | 97.85 ± 6.32 | 96.65 ± 5.21 | 74.95 ± 4.12 | 55.05 ± 5.69 |
| 4j | 103.21 ± 6.47 | 98.21 ± 7.01 | 84.24 ± 3.20 | 70.80 ± 5.36 |
| 4k | 98.88 ± 6.30 | 96.55 ± 2.98 | 80.98 ± 3.22 | 68.54 ± 4.92 |
| 4l | 98.87 ± 4.07 | 94.54 ± 5.32 | 78.53 ± 3.21 | 63.21 ± 5.04 |
| 4m | 102.10 ± 6.65 | 97.31 ± 5.21 | 81.51 ± 4.01 | 64.52 ± 4.32 |
| 4n | 99.76 ± 3.89 | 96.54 ± 4.24 | 78.79 ± 5.21 | 55.61 ± 5.34 |
| 4o | 100.54 ± 6.31 | 97.23 ± 5.09 | 81.67 ± 3.52 | 59.67 ± 6.14 |
| 4p | 99.32 ± 4.34 | 94.44 ± 3.90 | 83.08 ± 4.27 | 64.45 ± 4.82 |
| 4q | 98.96 ± 5.28 | 92.01 ± 6.41 | 85.62 ± 3.95 | 66.54 ± 3.81 |
| 4r | 98.87 ± 5.2 | 95.81 ± 6.29 | 83.78 ± 4.24 | 62.19 ± 4.18 |
| 4s | 100.74 ± 6.17 | 97.54 ± 5.36 | 78.65 ± 3.46 | 59.64 ± 3.61 |
| 4t | 99.87 ± 4.52 | 97.54 ± 3.65 | 81.80 ± 4.06 | 61.23 ± 5.12 |
| Curcumin | 95.34 ± 5.21 | 90.87 ± 4.31 | 82.07 ± 3.07 | 71.83 ± 4.21 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Huang, A.-L.; Zhang, Y.-L.; Li, Z.; Ding, H.-W.; Huang, C.; Meng, X.-M.; Li, J. Design, Synthesis and Evaluation of Hesperetin Derivatives as Potential Multifunctional Anti-Alzheimer Agents. Molecules 2017, 22, 1067. https://doi.org/10.3390/molecules22071067
Li B, Huang A-L, Zhang Y-L, Li Z, Ding H-W, Huang C, Meng X-M, Li J. Design, Synthesis and Evaluation of Hesperetin Derivatives as Potential Multifunctional Anti-Alzheimer Agents. Molecules. 2017; 22(7):1067. https://doi.org/10.3390/molecules22071067
Chicago/Turabian StyleLi, Bo, Ai-Ling Huang, Yi-Long Zhang, Zeng Li, Hai-Wen Ding, Cheng Huang, Xiao-Ming Meng, and Jun Li. 2017. "Design, Synthesis and Evaluation of Hesperetin Derivatives as Potential Multifunctional Anti-Alzheimer Agents" Molecules 22, no. 7: 1067. https://doi.org/10.3390/molecules22071067




