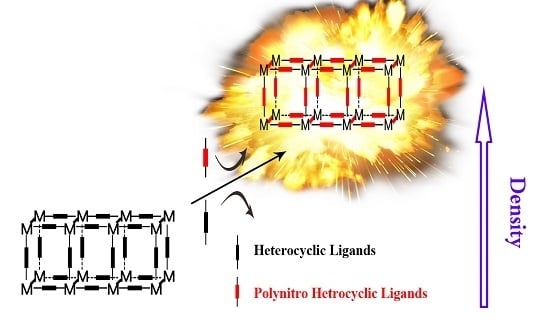
High-Density Energetic Metal–Organic Frameworks Based on the 5,5′-Dinitro-2H,2′H-3,3′-bi-1,2,4-triazole
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Energetic Complexes
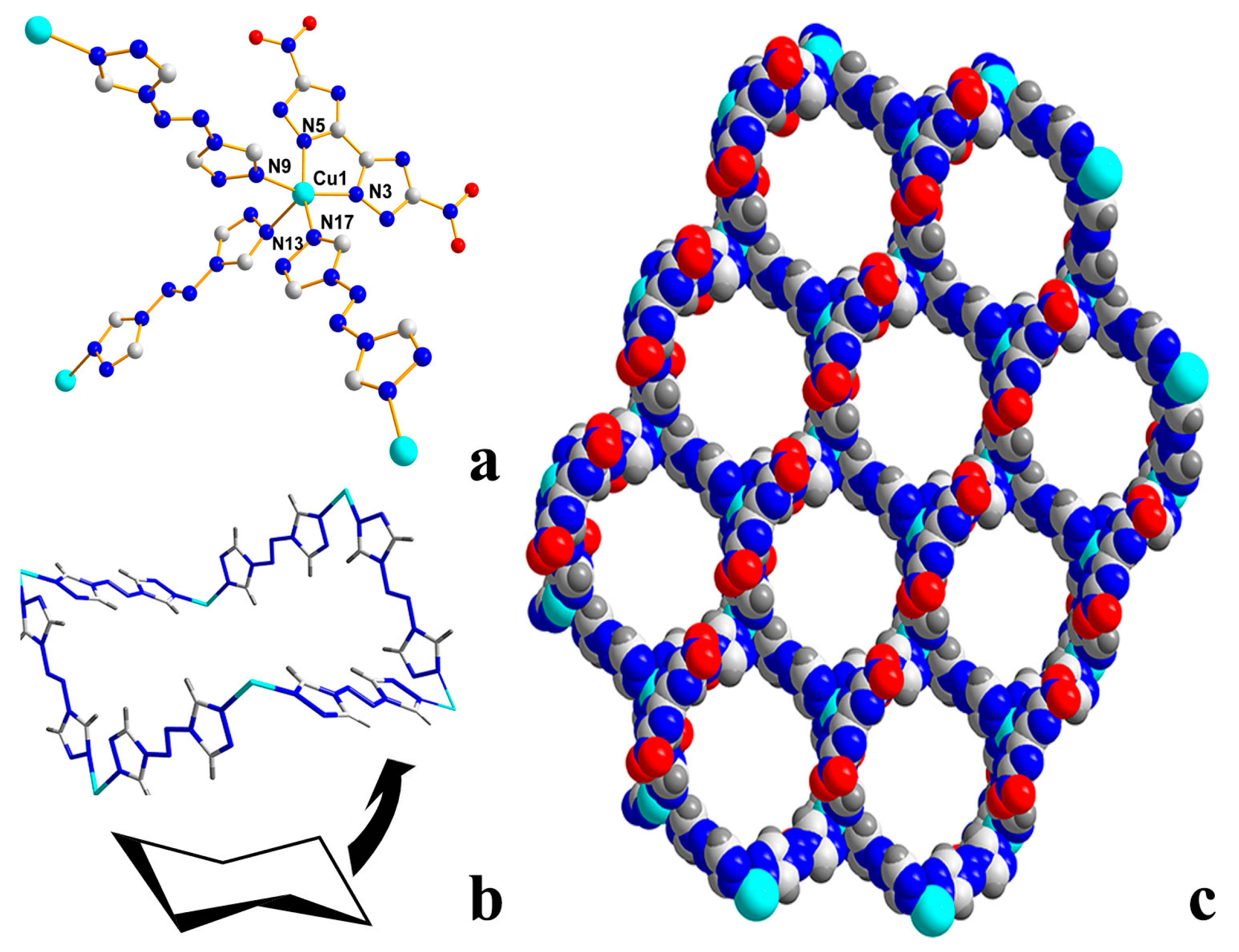
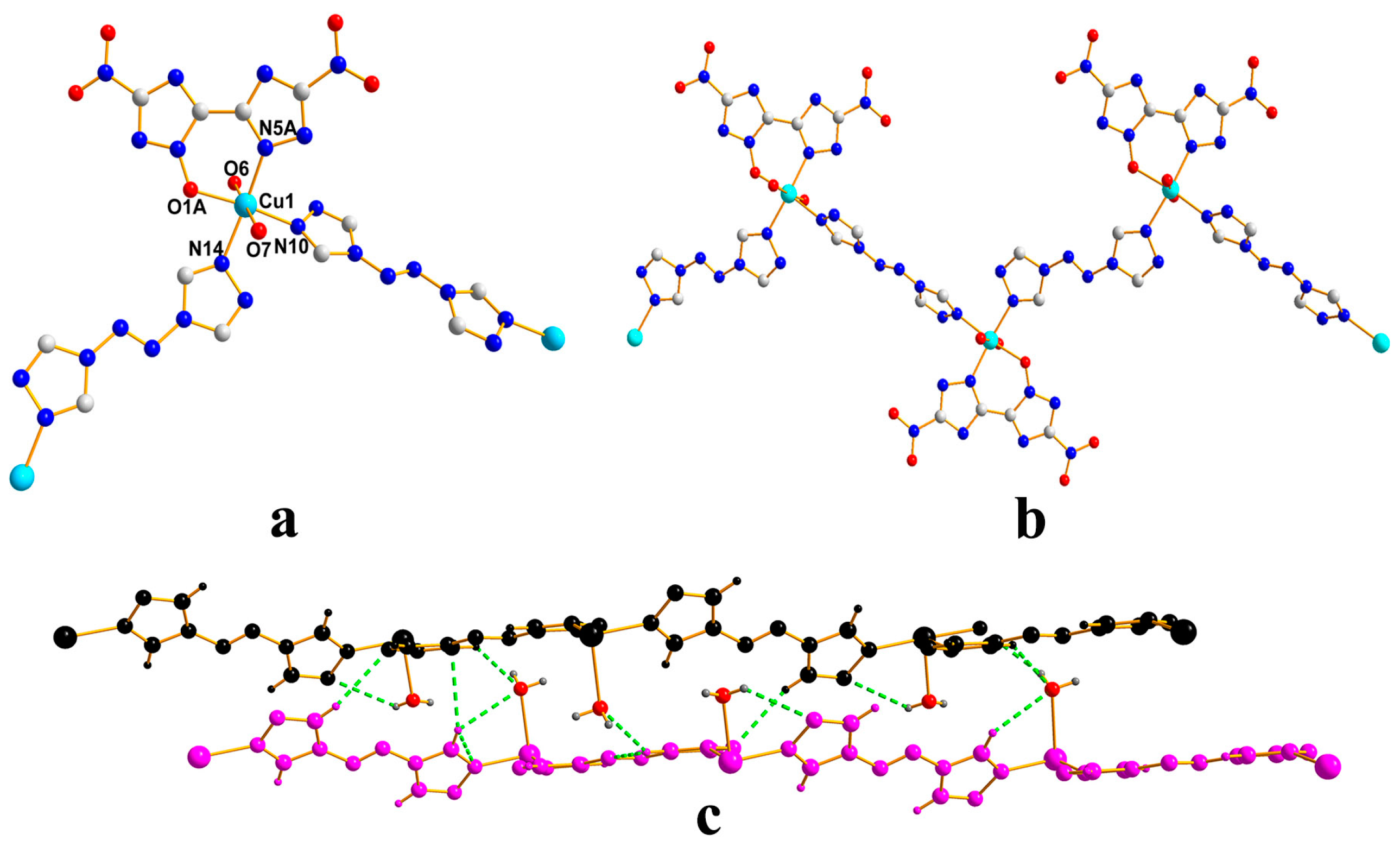
2.2. X-ray Crystallography
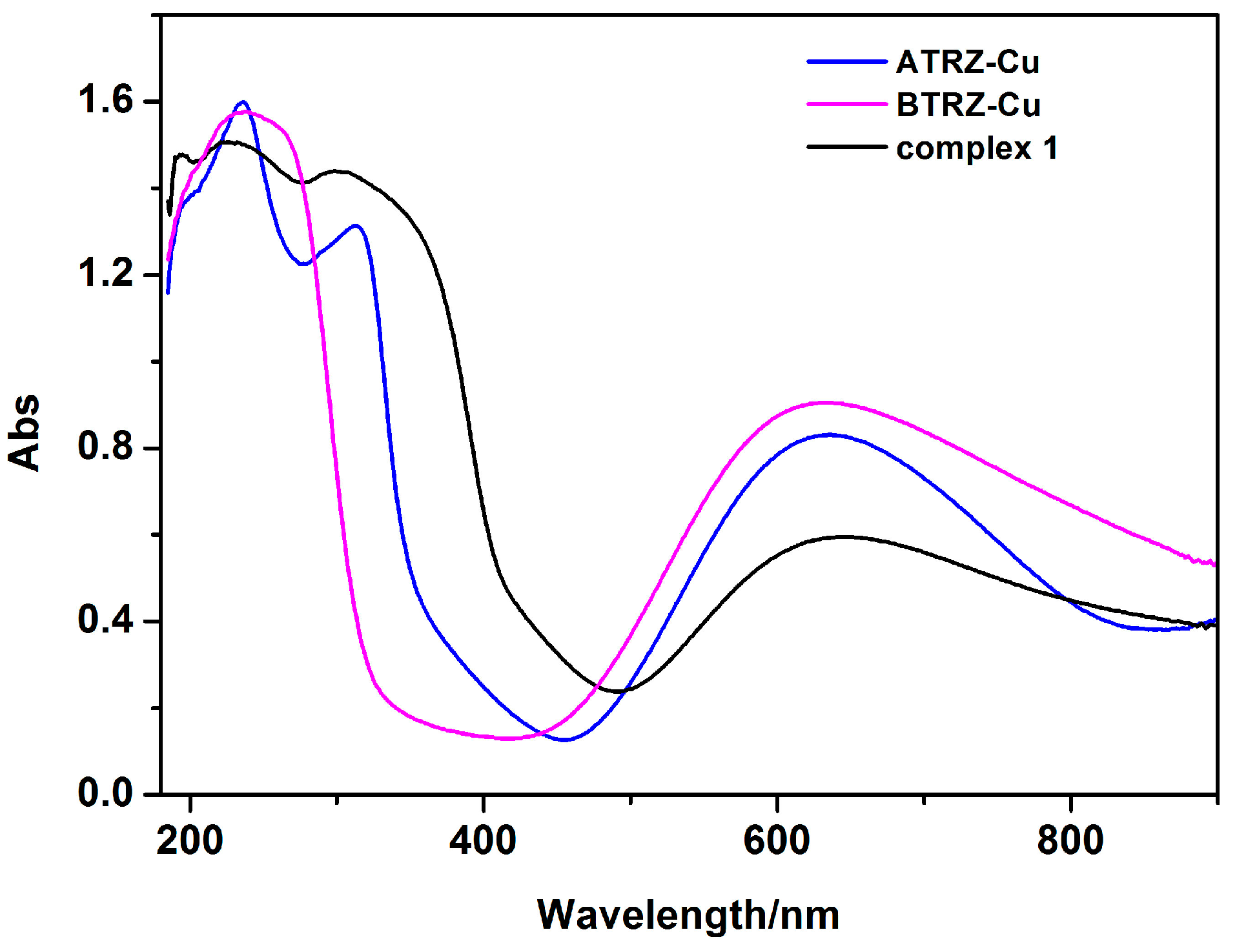
2.3. Ultraviolet-Visible (UV) Absorption
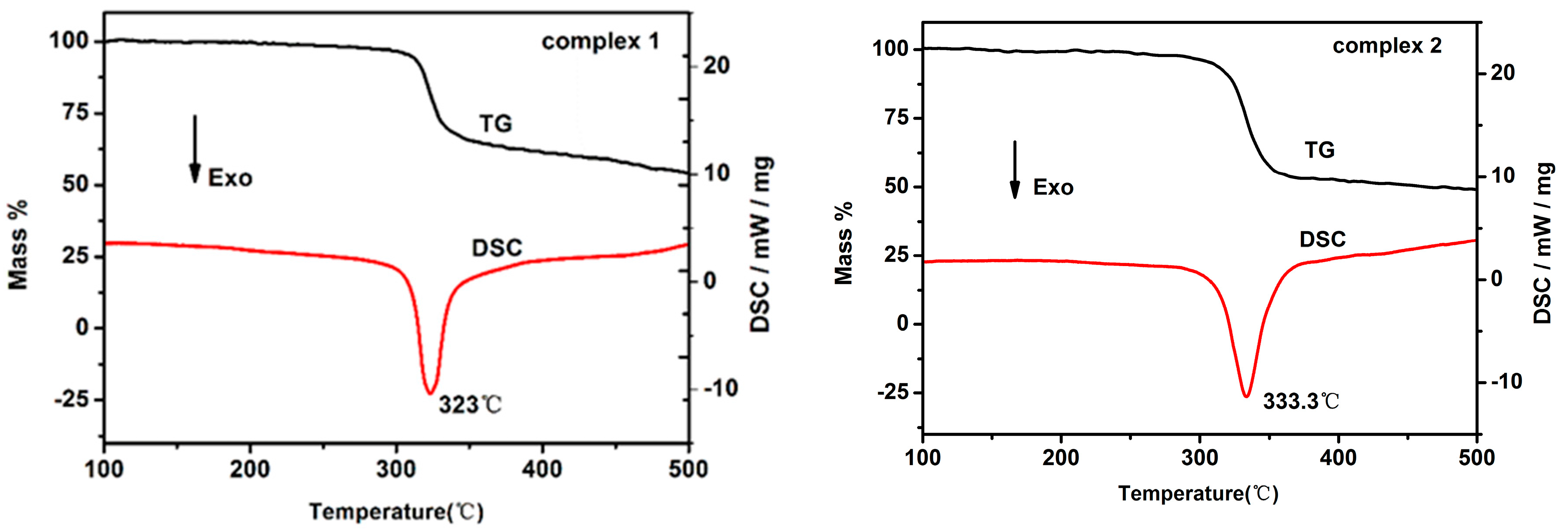
2.4. Stability and Detonation Properties
3. Experimental Materials and Methods
3.1. Chemical and Materials
3.2. Preparation of Ligands
3.3. Synthesis of the Energetic Metal Organic Framework
3.4. Measurement of Solid-State Ultraviolet Absorption
3.5. Measurement of Temperature Using Differential Scanning Calorimetry Measurement/Thermogravimetric
3.6. Measurement of Sensitivity
3.7. Measurement of Single-Crystal X-ray Diffraction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chavez, D.E.; Hiskey, M.A.; Gilardi, R.D. 3,3′-Azobis(6-amino-1,2,4,5-tetrazine): Aovel high-nitrogen energetic material. Angew. Chem. Int. Ed. 2000, 39, 1791–1793. [Google Scholar] [CrossRef]
- Dippold, A.A.; Klapotke, T.M. A study of dinitro-bis-1,2,4-triazole-1,1′-diol and derivatives: Design of high-performance insensitive energetic materials by the introduction of N-Oxides. J. Am. Chem. Soc. 2013, 135, 9931–9938. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Klapotke, T.M.; Stierstorfer, J. 1,5-Di(nitramino)tetrazole: High sensitivity and superior explosive performance. Angew. Chem. Int. Ed. 2015, 54, 10299–10302. [Google Scholar] [CrossRef] [PubMed]
- Gobel, M.; Karaghiosoff, K.; Klapotke, T.M.; Piercey, D.G.; Stierstorfer, J. Nitrotetrazolate-2N-oxides and the strategy of N-Oxide introduction. J. Am. Chem. Soc. 2010, 132, 17216–17226. [Google Scholar] [CrossRef] [PubMed]
- He, C.L.; Shreeve, J.M. Energetic materials with promising properties: Synthesis and characterization of 4,4-bis(5-nitro-1,2,3–2H-triazole) derivatives. Angew. Chem. Int. Ed. 2015, 54, 6260–6264. [Google Scholar] [CrossRef] [PubMed]
- Huynh, M.H. V.; Hiskey, M.A.; Hartline, E.L.; Montoya, D.P.; Gilardi, R. Polyazido high-nitrogen compounds: Hydrazo- and azo-1,3,5-triazine. Angew. Chem. Int. Ed. 2004, 43, 4924–4928. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; He, C.L.; Zhang, J.H.; Shreeve, J.M. Combination of 1,2,4-oxadiazole and 1,2,5-oxadiazole moieties for the generation of high-performance energetic materials. Angew. Chem. Int. Ed. 2015, 54, 9367–9371. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Parrish, D.A.; Shreeve, J.M. Energetic multifunctionalized nitraminopyrazoles and their ionic derivatives: Ternary hydrogen-bond induced high energy density materials. J. Am. Chem. Soc. 2015, 137, 4778–4786. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.; Fischer, D.; Klapotke, T.M.; Piercey, D.G.; Stierstorfer, J. Pushing the limits of energetic materials—The synthesis and characterization of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate. J. Mater. Chem. 2012, 22, 20418–20422. [Google Scholar] [CrossRef]
- Schmidt, R.D.; Lee, G.S.; Pagoria, P.R.; Mitchell, A.R.; Gilardi, R. Synthesis of 4-amino-3,5-dinitro-1H-pyrazole using vicarious nucleophilic substitution of hydrogen. J. Heterocycl. Chem. 2001, 38, 1227–1230. [Google Scholar] [CrossRef]
- Dippold, A.A.; Klapotke, T.M.; Winter, N. Insensitive nitrogen-rich energetic compounds based on the 5,5′-dinitro-3,3′-bi-1,2,4-triazol-2-ide anion. Eur. J. Inorg. Chem. 2012, 21, 3474–3484. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; Brown, P.; Maiti, A.; Gee, R.H.; Peterson, G.R.; Weeks, B.L.; Hope-Weeks, L.J. Ionic polymers as a new structural motif for high-energy-density materials. J. Am. Chem. Soc. 2012, 134, 1422–1425. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.N.; Zhang, S.; Yang, Q.; Su, Z.Y.; Wei, Q.; Xie, G.; Chen, S.P. Silver(I)-based energetic coordination Polymers: Synthesis, structure and energy performance. New J. Chem. 2015, 39, 7849–7857. [Google Scholar] [CrossRef]
- Jiang, C.; Yu, Z.P.; Wang, S.J.; Jiao, C.; Li, J.M.; Wang, Z.Y.; Cui, Y. Rational design of metal-organic frameworks based on 5-(4-pyridyl)tetrazolate: From 2D grids to 3D porous networks. Eur. J. Inorg. Chem. 2004, 3662–3667. [Google Scholar] [CrossRef]
- Su, C.Y.; Goforth, A.M.; Smith, M.D.; Pellechia, P.J.; zur Loye, H.C. Exceptionally stable, hollow tubular metal-organic architectures: Synthesis, characterization, and solid-state transformation study. J. Am. Chem. Soc. 2004, 126, 3576–3586. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.H.; Parrish, D.A.; Shreeve, J.M. Nitrogen-rich 5-(1-methylhydrazinyl)tetrazole and its copper and silver complexes. Inorg. Chem. 2012, 51, 5305–5312. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.D.; Bi, Y.G.; Li, F.G.; Yang, L.; Zhou, Z.N.; Zhang, J.G.; Zhang, T.L. A novel stable high-nitrogen energetic compound: Copper(II) 1,2-diaminopropane azide. Z. Anorg. Allg. Chem. 2014, 640, 224–228. [Google Scholar] [CrossRef]
- Deblitz, R.; Hrib, C.G.; Blaurock, S.; Jones, P.G.; Plenikowski, G.; Edelmann, F.T. Explosive werner-type cobalt(III) complexes. Inorg. Chem. Front. 2014, 1, 621–640. [Google Scholar] [CrossRef]
- Friedrich, M.; Galvez-Ruiz, J.C.; Klapotke, T.M.; Mayer, P.; Weber, B.; Weigand, J.J. BTA copper complexes. Inorg. Chem. 2005, 44, 8044–8052. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Wang, Y.; Qi, C.; Zhao, X.X.; Zhang, J.C.; Zhang, S.W.; Pang, S.P. 3D energetic metal-organic frameworks: Synthesis and properties of high energy materials. Angew. Chem. Int. Ed. 2013, 52, 14031–14035. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.J.; Liu, X.Y.; Su, Z.Y.; Zhang, S.; Yang, Q.; Wei, Q.; Chen, S.P.; Xie, G.; Yang, X.W.; Gao, S.L. High-energy-density materials with remarkable thermostability and insensitivity: Syntheses, structures and physicochemical properties of Pb(II) compounds with 3-(tetrazol-5-yl) triazole. J. Mater. Chem. A 2014, 2, 11958–11965. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Liu, X.Y.; Duan, L.Q.; Yang, Q.; Wei, Q.; Xie, G.; Chen, S.P.; Yang, X.W.; Gao, S.L. In situ synthesized 3D heterometallic metal-organic framework (MOF) as a high-energy-density material shows high heat of detonation, good thermostability and insensitivity. Dalton Trans. 2015, 44, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Zhang, M.J.; Lin, P.; Malgras, V.; Tang, J.; Alshehri, S.M.; Yamauchi, Y.; Du, S.W.; Zhang, J. A highly energetic N-rich metal-organic framework as a new high-energy-density material. Chem. Eur. J. 2016, 22, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Zhao, X.Q.; Shi, W.; Liu, W.Y.; Chen, Z.L.; Cheng, P.; Liao, D.Z.; Yan, S.P. Anions-directed metal-mediated assemblies of coordination polymers based on the bis(4,4′-bis-1,2,4-triazole) ligand. Cryst. Growth Des. 2008, 8, 3652–3660. [Google Scholar] [CrossRef]
- Seth, S.; Matzger, A.J. Coordination polymerization of 5,5′-dinitro-2H,2H′-3,3′-bi-1,2,4-triazole leads to a dense explosive with high thermal stability. Inorg. Chem. 2017, 56, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Huynh, M.H.V.; Hiskey, M.A.; Chavez, D.E.; Gilardi, R.D. Preparation, characterization, and properties of 7-nitrotetrazolo [1,5-f]furazano[4,5-b]pyridine 1-oxide. J. Energ. Mater. 2005, 23, 99–106. [Google Scholar] [CrossRef]
- Talawar, M.B.; Sivabalan, R.; Mukundan, T.; Muthurajan, H.; Sikder, A.K.; Gandhe, B.R.; Rao, A.S. Environmentally compatible next generation green energetic materials (GEMs). J. Hazard. Mater. 2009, 161, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.T.; Chen, S.P.; Gao, S.L. Syntheses and characterization of lead(II) N,N-bis[1(2)H-tetrazol-5-yl]amine compounds and effects on thermal decomposition of ammonium perchlorate. Eur. J. Inorg. Chem. 2009, 23, 3475–3480. [Google Scholar] [CrossRef]
- Gao, H.X.; Shreeve, J.M. Azole-based energetic salts. Chem. Rev. 2011, 111, 7377–7436. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K.A.; Seth, S.; Matzger, A.J. Coordination polymers with high energy density: An emerging class of explosives. Cryst. Growth Des. 2015, 15, 5963–5972. [Google Scholar] [CrossRef]
- Bennion, J.C.; Vogt, L.; Tuckerman, M.E.; Matzger, A.J. Isostructural cocrystals of 1,3,5-trinitrobenzene assembled by halogen bonding. Cryst. Growth Des. 2016, 16, 4688–4693. [Google Scholar] [CrossRef]
- Bennion, J.C.; McBain, A.; Son, S.F.; Matzger, A.J. Design and synthesis of a series of nitrogen-rich energetic cocrystals of 5,5′-dinitro-2H,2H′-3,3′-bi-1,2,4-triazole (DNBT). Cryst. Growth Des. 2015, 15, 2545–2549. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Q.; Liu, X.Y.; Qu, X.N.; Wei, Q.; Xie, G.; Chen, S.P.; Gao, S.L. High-energy metal-organic frameworks (HE-MOFs): Synthesis, structure and energetic performance. Coord. Chem. Rev. 2016, 307, 292–312. [Google Scholar] [CrossRef]
- Cudzilo, S.; Nita, M. Synthesis and explosive properties of copper(II) chlorate(VII) coordination polymer with 4-amino-1,2,4-triazole bridging ligand. J. Hazard. Mater. 2010, 177, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.D.; Zhou, Z.N.; Li, F.G.; Yang, L.; Zhang, T.L.; Zhang, J.G. Preparation, crystal structures, thermal decompositions and explosive properties of two new high-nitrogen azide ethylenediamine energetic compounds. New J. Chem. 2013, 37, 646–653. [Google Scholar] [CrossRef]
- Liu, X.Y.; Gao, W.J.; Sun, P.P.; Su, Z.Y.; Chen, S.P.; Wei, Q.; Xie, G.; Gao, S.L. Environmentally friendly high-energy MOFs: Crystal structures, thermostability, insensitivity and remarkable detonation performances. Green Chem. 2015, 17, 831–836. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; Peterson, G.R.; Brown, P.; Maiti, A.; Gee, R.H.; Weeks, B.L.; Hope-Weeks, L.J. Metal-organic frameworks (MOFs) as safer, structurally reinforced energetics. Chem. Eur. J. 2013, 19, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, Y.; Dong, K.; Su, H.; Zhang, S.; Li, S.; Pang, S. Taming dinitramide anions within an energetic metal-organic framework: A new strategy for synthesis and tunable properties of high energy materials. Chem. Mater. 2016, 28, 1472–1480. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Su, H.; Li, S.; Zhang, S.; Pang, S. A simple method for the prediction of the detonation performances of metal-containing explosives. J. Phys. Chem. A 2014, 118, 4575–4581. [Google Scholar] [CrossRef] [PubMed]
- The Committee on Data for Science and Technology (CODATA). Codata recommended key values for thermodynamics. J. Chem. Thermodyn. 1978, 10, 903–906. [Google Scholar]
- Qi, C.; Li, S.H.; Li, Y.C.; Wang, Y.A.; Chen, X.K.; Pang, S.P. A novel stable high-nitrogen energetic material: 4,4′-azobis(1,2,4-triazole). J. Mater. Chem. 2011, 21, 3221–3225. [Google Scholar] [CrossRef]
- Dippold, A.A.; Klapotke, T.M. Nitrogen-rich bis-1,2,4-triazoles-A comparative study of structural and energetic properties. Chem. Eur. J. 2012, 18, 16742–16753. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Zhang, J.C.; Du, Y.; Zhang, P.C.; Li, S.H.; Fang, T.; Pang, S.P. New roles for metal-organic frameworks: Fuels for environmentally friendly composites. RSC Adv. 2017, 7, 11142–11148. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (5,5′-dinitro-2H,2H′-3,3′-bi-1,2,4-triazole, 5,5′-dinitro-3,3′-bis-1,2,4-triazole-1-diol, 4,4′-azo-1,2,4-triazole) are available from the authors. |
[∆n = ∆ni (products, g) − ∆nj (reactants, g), ∆ni or ∆nj is the total molar amount of gases in the products or reactnts]
MOF(DNBT)
MOF(DNBTO)
| Compound | Tdec a | ρ b | N c | Ωco d | ISe | FS f | −Q g | P h | D i |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 323.2 | 1.93 | 52.44 | −26.97 | >40 | >360 | 4346.2 | 25.27 | 7.7 |
| 2 | 333.3 | 1.96 | 44.79 | −16.73 | 18 | 360 | 4476 | 27.62 | 7.86 |
| [Cu(ATZ)(ClO4)2]n [34] | >250 | 1.40 | 32.66 | −13.35 | 1 | 8.8 | - | - | 6.5 |
| [Cu(Pn)(N3)2]n [17] | 215.7 | 1.76 | 50.54 | −57.73 | 2.55 | - | - | - | - |
| [Cu2(En)2(N3)4]n [35] | 201.8 | 1.93 | 53.95 | −46.22 | 7.84 | - | - | - | - |
| [Cu(ATRZ)3(NO3)2]n [20] | 243 | 1.68 | 53.35 | −28.24 | 22.5 | 112 | 4388 | 35.68 | 9.16 |
| [Cu(Htztr)2(H2O)2]n [36] | 345 | 1.89 | 52.72 | −34.43 | >40 | >360 | - | 30.57 | 8.18 |
| RDX [12] | 210 | 1.8 | 37.84 | 0 | 7.5 | 120 | 1258.3 | 33.92 | 8.6 |
| TNT` [12] | 244 | 1.65 | 18.50 | −24.7 | 15 | 353 | 852 | 20.50 | 7.178 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Peng, P.; Hu, B.; Su, H.; Li, S.; Pang, S. High-Density Energetic Metal–Organic Frameworks Based on the 5,5′-Dinitro-2H,2′H-3,3′-bi-1,2,4-triazole. Molecules 2017, 22, 1068. https://doi.org/10.3390/molecules22071068
Dong Y, Peng P, Hu B, Su H, Li S, Pang S. High-Density Energetic Metal–Organic Frameworks Based on the 5,5′-Dinitro-2H,2′H-3,3′-bi-1,2,4-triazole. Molecules. 2017; 22(7):1068. https://doi.org/10.3390/molecules22071068
Chicago/Turabian StyleDong, Yalu, Panpan Peng, Baoping Hu, Hui Su, Shenghua Li, and Siping Pang. 2017. "High-Density Energetic Metal–Organic Frameworks Based on the 5,5′-Dinitro-2H,2′H-3,3′-bi-1,2,4-triazole" Molecules 22, no. 7: 1068. https://doi.org/10.3390/molecules22071068