Comparative Analysis of Saponins from Different Phytolaccaceae Species and Their Antiproliferative Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Extraction Method
2.2. Qualitative and Quantitative Analysis of Saponins
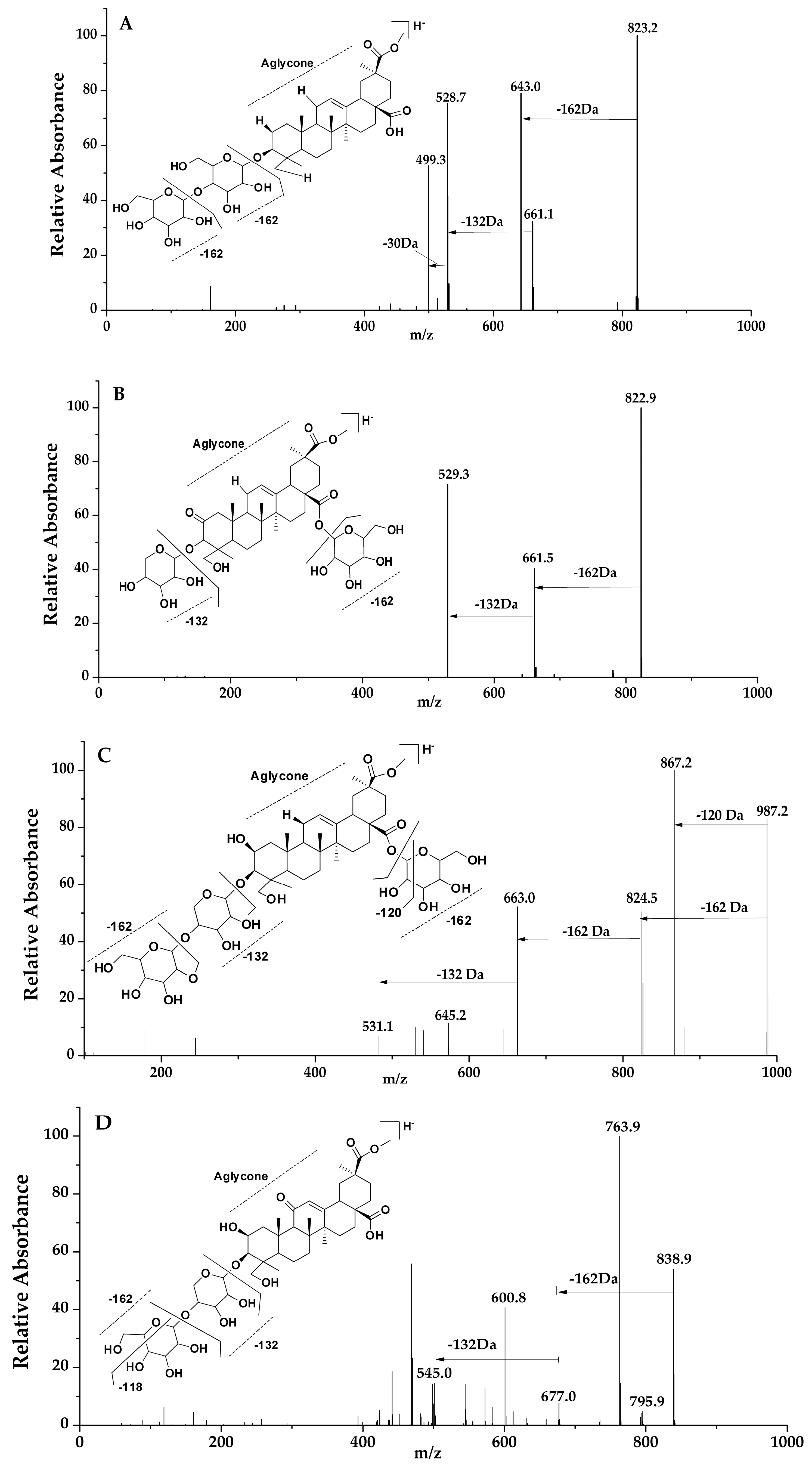
2.3. Structural Identifications of Saponins Using LC-ESI-MS/MS Analysis
2.3.1. Identification of the Peaks Based on Aglycone
Identification of Peaks with Phytolaccagenic Acid Aglycone
Identification of Peaks with 2-oxo-Phytolaccagenin Acid Aglycone
Identification of Peaks with Phytolaccagenin Aglycone
Identification of Peaks with 11α-Hydroxyphytolaccagenin Aglycone
Identification of Peaks with Serjanic Acid Aglycone
Identification of Peaks with 11-oxo-Phytolaccagenin Aglycone
Identification of Peaks with 11α-Methoxyphytolaccagenin Aglycone
2.4. Assessment of the Identified Triterpenoid Saponins in the Three Samples
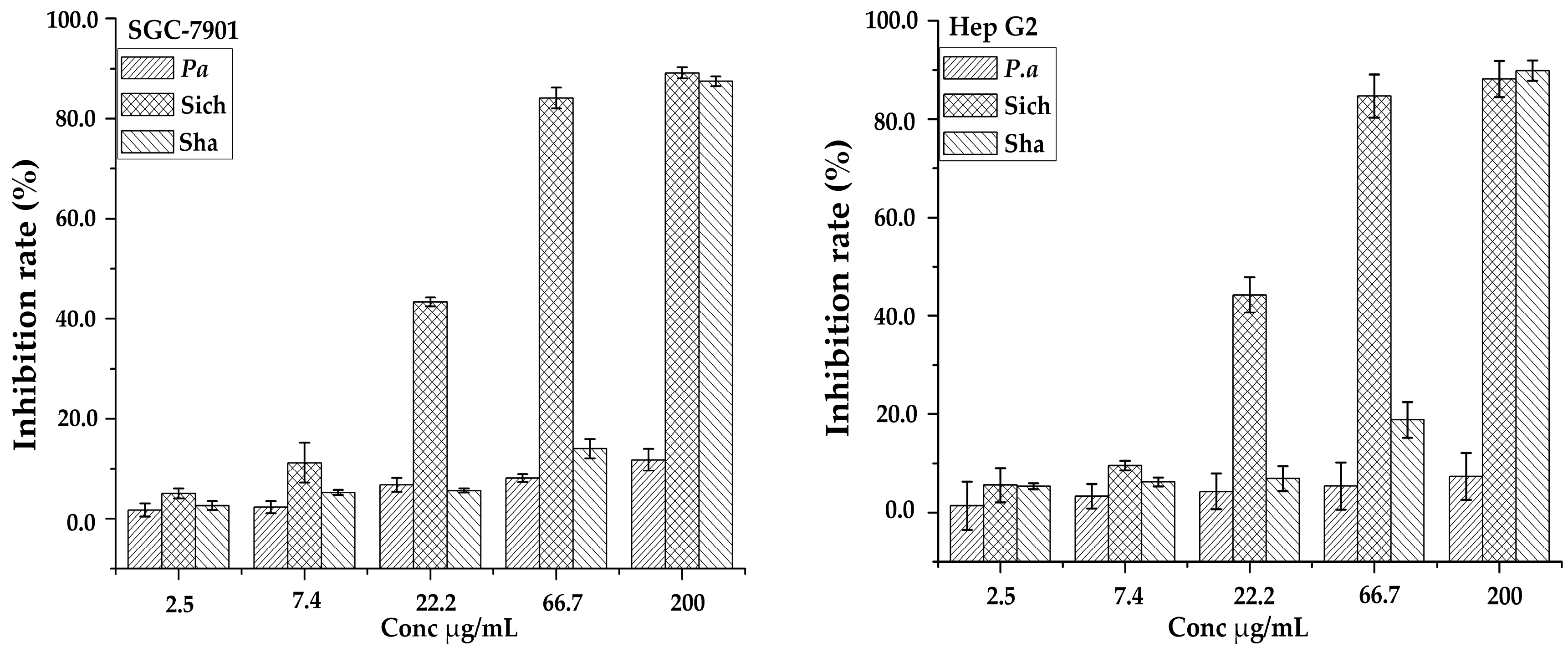
2.5. Antiproliferative Activity Assay
3. Experimental Section
3.1. Chemicals Reagents
3.2. Biological Material
3.3. Evaluation of Extraction Method
3.4. Colorimetric Method for Quantification Analysis
3.5. Extraction of Triterpenoid Saponins
3.6. Untargeted Metabolite Profiling by Liquid Chromatography-Mass Spectrometry
3.7. Antiproliferation Assays in Vitro
3.7.1. Cell Culture
3.7.2. Cytotoxic Analysis Using Sulforhodamine B (SRB) Growth Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lans, C. Comparison of plants used for skin and stomach problems in Trinidad and Tobago with Asian ethnomedicine. J. Ethnobiol. Ethnomed. 2007, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Kim, E.S.; Oh, K.; Kim, H.J.; Kim, Y.; Baek, K.H. Antibacterial effect of crude extract and metabolites of Phytolacca americana on pathogens responsible for periodontal inflammatory diseases and dental caries. BMC Complement. Altern. Med. 2014, 14, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Boo, H.-O.; Park, J.-H.; Woo, S.-H.; Park, H.-Y. Antimicrobial effect, antioxidant and tyrosinase inhibitory activity of the extract from different parts of Phytolacca americana L. Korean J. Crop. Sci. 2015, 60, 366–373. [Google Scholar] [CrossRef]
- Tadeg, H.; Mohammed, E.; Asres, K.; Gebre-Mariam, T. Antimicrobial activities of some selected traditional Ethiopian medicinal plants used in the treatment of skin disorders. J. Ethnopharmacol. 2005, 100, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Taye, B.; Giday, M.; Animut, A.; Seid, J. Antibacterial activities of selected medicinal plants in traditional treatment of human wounds in Ethiopia. Asian Pac. J. Trop. Biomed. 2011, 1, 370–375. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Baek, K.-H.; Kim, E.-S.; Han, J.-E.; Kwak, M.-H. In vivo antifungal activities of the methanol extracts of invasive plant species against plant pathogenic fungi. J. Plant Pathol. 2012, 28, 317–321. [Google Scholar] [CrossRef]
- Tegegne, G.; Pretorius, J.C. In vitro and in vivo antifungal activity of crude extracts and powdered dry material from Ethiopian wild plants against economically important plant pathogens. Biol. Control 2007, 52, 877–888. [Google Scholar] [CrossRef]
- Escalante, A.M.; Santecchia, C.B.; López, S.N.; Gattuso, M.A. Isolation of antifungal saponins from Phytolacca tetramera, and Argentinean species in critic risk. J. Ethnopharmacol. 2002, 82, 29–34. [Google Scholar] [CrossRef]
- Di Liberto, M.; Svetaz, L.; Furlán, R.L.; Zacchino, S.A.; Delporte, C.; Novoa, M.A.; Asencio, M.; Cassels, B.K. Antifungal activity of saponin-rich extracts of Phytolacca dioica and of the sapogenin obtained through hydrolysis. Nat. Prod. Commun. 2010, 5, 1013–1018. [Google Scholar] [PubMed]
- Amuamuta, A.; Na-Bangchang, K. A review of ethnopharmacology of the commonly used antimalarial herbal agents for traditional medicine practice in Ethiopia. Afr. J. Pharm. Pharmacol. 2015, 9, 615–627. [Google Scholar]
- Adinew, G.M. Antimalarial activity of methanolic extract of Phytolacca dodecandra leaves against Plasmodium berghei infected Swiss albino mice. Int. J. Pharmacol. Clin. Sci. 2014, 3, 39–45. [Google Scholar]
- Guo, D.Z.; Chen, J.; Liu, Y.D.; Yao, H.; Han, F.A.; Pan, J. A high-performance molluscicidal ingredient against Oncomelania hupensis produced by a rhizospheric strain from Phytolacca acinosa Roxb. Pharmacogn. Mag. 2011, 28, 277–283. [Google Scholar]
- Treyvaud, V.; Marston, A.; Dyatmiko, W.; Hostettmann, K. Molluscicidal saponins from Phytolacca icosandra. Phytochemistry 2000, 55, 603–609. [Google Scholar] [CrossRef]
- Kiran, G.R.; Akondi, B.R. Antiobesity effect of Phytolacca berry in rats. Environ. Exp. Biol. 2014, 12, 95–99. [Google Scholar]
- De Andrade, T.M.; de Melo, A.S.; Dias, R.G.; Varela, E.L.; de Oliveira, F.R.; Vieira, J.L.; de Andrade, M.A.; Baetas, A.C.; Monteiro, M.C.; Maia Cdo, S. Potential behavioral and pro-oxidant effects of Petiveria alliacea L. extract in adult rats. J. Ethnopharmacol. 2012, 143, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Das, S.; Samadder, A.; Bhadra, K.; Khuda-Bukhsh, A.R. Poly(lactide-co-glycolide) encapsulated extract of Phytolacca decandra demonstrates better intervention against induced lung adenocarcinoma in mice and on A549 cells. Eur. J. Pharm. Sci. 2012, 47, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.M.; Liu, J.X.; Wang, Z.M.; Wang, W.H. Phytolacacinoside A, a new triterpenoid saponin from Phytolacca acinosa Roxb. J. Asian Nat. Prod. Res. 2009, 11, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yi, Y.; Sun, P.; Zhang, D. Synthesis in vitro inhibitory activity towards COX-2 and haemolytic activity of derivatives of esculentoside A. Bioorg. Med. Chem. Lett. 2007, 17, 6430–6433. [Google Scholar] [CrossRef] [PubMed]
- Strauss, A.; Spengel, S.M.; Schaffner, W. Saponin from root culture of Phytolacca acinosa. Phytochemistry 1995, 38, 861–865. [Google Scholar] [CrossRef]
- Ncube, B.; Finnie, J.-F.; van Staden, J. Quality from the field: The impact of environmental factors as quality determinants in medicinal plants. S. Afr. J. Bot. 2012, 82, 11–20. [Google Scholar] [CrossRef]
- Szakiel, A.; Paczkowski, C.; Henry, M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem. Rev. 2011, 10, 471–491. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusià, J. Biogenic volatile organic compounds (BOVs) plant defense against climate warming. Trends Plant Sci. 2003, 8, 105–109. [Google Scholar] [CrossRef]
- Zeng, W.; Bai, Y.; Zhang, Q.; Zhao, Y. Chromatographic fingerprint analysis of semen Ziziphi spinosae by HPLC-DAD method. Anal. Lett. 2009, 42, 205–215. [Google Scholar] [CrossRef]
- An, M.; Fukazawa, S.; Usual, Y.; Nakamura, J.; Matura, M.; Zhu, F.; Nakayama, T. Antioxidant activity and constituents of propels collected in various areas of China. Food Chem. 2007, 101, 383–1392. [Google Scholar]
- Montoya, G.; Arango, G.J.; Ramirez-Pineda, J.R. Rapid differentiation of isobaric and positional isomers of structurally related glycosides from Phytolacca bogotensis. Rapid Commun. Mass Spectrom. 2009, 23, 3361–3371. [Google Scholar] [CrossRef] [PubMed]
- Montoya, G.; Arango, G.J.; Unger, M.; Holzgrabe, U. O-glycoside sequence of pentacyclic triterpene saponins from Phytolacca bogotensis using HPLC-ESI/multi-stage tandem mass spectrometry. Phytochem. Anal. 2009, 20, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.M.; Porzel, A.; Wessjohann, A.L. Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC–MS, LC–MS and 1D NMR techniques. Phytochemistry 2012, 76, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Chen, S.; Liang, Y.-Z.; Wang, X.; Tian, R.; Upton, R. Chromatographic fingerprint analysis—A rational approach for quality assessment of traditional Chinese herbal medicine. J. Chromatogr. A 2006, 1112, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.S. An overview of extraction techniques for medicinal and aromatic plants. In Extraction Technology for Medicinal and Aromatic Plants; Handa, S.S., Khanuja, S.P.S., Longo, G., Rakesh, D.D., Eds.; International Centre for Science and High Technology: AREA Science Park, Trieste, Italy, 2008; Chapter 1; pp. 21–54. [Google Scholar]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles of herbs. Ultrason. Sonochem. 2001, 8, 303–311. [Google Scholar] [CrossRef]
- Yi, C.; Xie, M.Y.; Gong, X.F. Microwave-Assisted extraction used for the isolation of total triterpenoid saponins from Ganoderma atrum. J. Food Eng. 2007, 81, 162–170. [Google Scholar]
- Marston, A.; Wolfender, J.L.; Hostettmann, K. Analysis and isolation of saponin from plant material. In Saponins in Food, Feedstuffs and Medicinal Plants; Loesser, W., Matson, A., Eds.; Springer: Dordrecht, Netherlands, 2000; Chapter 1; pp. 281–286. ISBN 978-90-481-5341-1. [Google Scholar]
- Perret, C.; Wolfender, J.L.; Hostettmann, K. LC/ESI-MS analysis of triterpene glycosides: Rapid estimation of the saponin content of dried berries of Phytolacca dodecandra. Phytochem. Anal. 1999, 10, 272–278. [Google Scholar] [CrossRef]
- Madl, T.; Sterk, H.; Mittelbach, M.; Rechberger, G.N. Tandem mass spectrometric analysis of a complex triterpene saponin mixture of Chenopodium quinoa. J. Am. Soc. Mass Spectrom. 2006, 17, 795–806. [Google Scholar] [PubMed]
- Yi, Y.H. A triterpenoid and its saponin from Phytolacca esculenta. Phytochemistry 1991, 30, 4179–4181. [Google Scholar] [PubMed]
- Wang, L.; Bai, L.; Nagasawa, T.; Hasegawa, T.J. Bioactive triterpene saponins from the roots of Phytolacca americana. J. Nat. Prod. 2008, 71, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Suga, Y.; Maruyama, Y.; Kawanishi, S.; Shoji, J. Studies on the constituents of Phytolaccaceae plants; on the structures of Phytolaccasaponin B, E and G from the roots of Phytolacca americana L. J. Pharm. Soc. Jpn. 1978, 26, 520–525. [Google Scholar]
- Yi, Y.H. Esculentoside L and K: Two new saponins from Phytolacca esculenta. Planta Med. 1990, 56, 301–303. [Google Scholar] [PubMed]
- Woldemichael, M.G.; Wink, M. Identification and biological activities of triterpenoid saponins from Chenopodium quinoa. J. Agric. Food Chem. 2001, 49, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Hostettmann, K.; Wolfender, J.L. The search for biologically active secondary metabolites. J. Pestic. Sci. 1997, 51, 471–482. [Google Scholar] [CrossRef]
- Xiang, Z.B.; Tang, C.H.; Chen, G.; Shi, Y.S. Studies on colorimetric determination of oleanolic acid in Chinese quince. Nat. Prod. Res. 2001, 13, 23–26. [Google Scholar]
- Han, L.; Pan, G.; Wang, Y.; Song, X.; Gao, X.; Ma, B.; Kang, L. Rapid profiling and identification of triterpenoid saponins in crude extracts from Albizia julibrissin Durazz. by ultra high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Pharm. Biomed. Anal. 2011, 55, 996–1009. [Google Scholar] [PubMed]
- Santhanam, R.K.; Ahmad, S.; Abas, F.; Safinar, I.I.; Rukayadi, Y.; Tayyab, M.A.; Shaari, K. Bioactive constituents of Zanthoxylum rhetsa bark and its cytotoxic potential against B16-F10 melanoma cancer and normal human dermal fibroblast (HDF) cell lines. Molecules 2016, 21, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Mu, R.H.; Li, L.; Liang, F.; Yao, L.; Su, L. Intracellular doxorubicin delivery of a core cross-linked, redox-responsive polymeric micelles. Int. J. Pharm. 2016, 498, 195–204. [Google Scholar]
- Rodriguez-Chavez, J.L.; Coballase-Urrutia, E.; Sicilia-Argumedo, G.; Ramirez-Apan, T.; Delgado, G. Toxicological evaluation of the natural products and some semisynthetic derivatives of Heterotheca inuloides Cass (Asteraceae). J. Ethnopharmacol. 2015, 175, 256–265. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
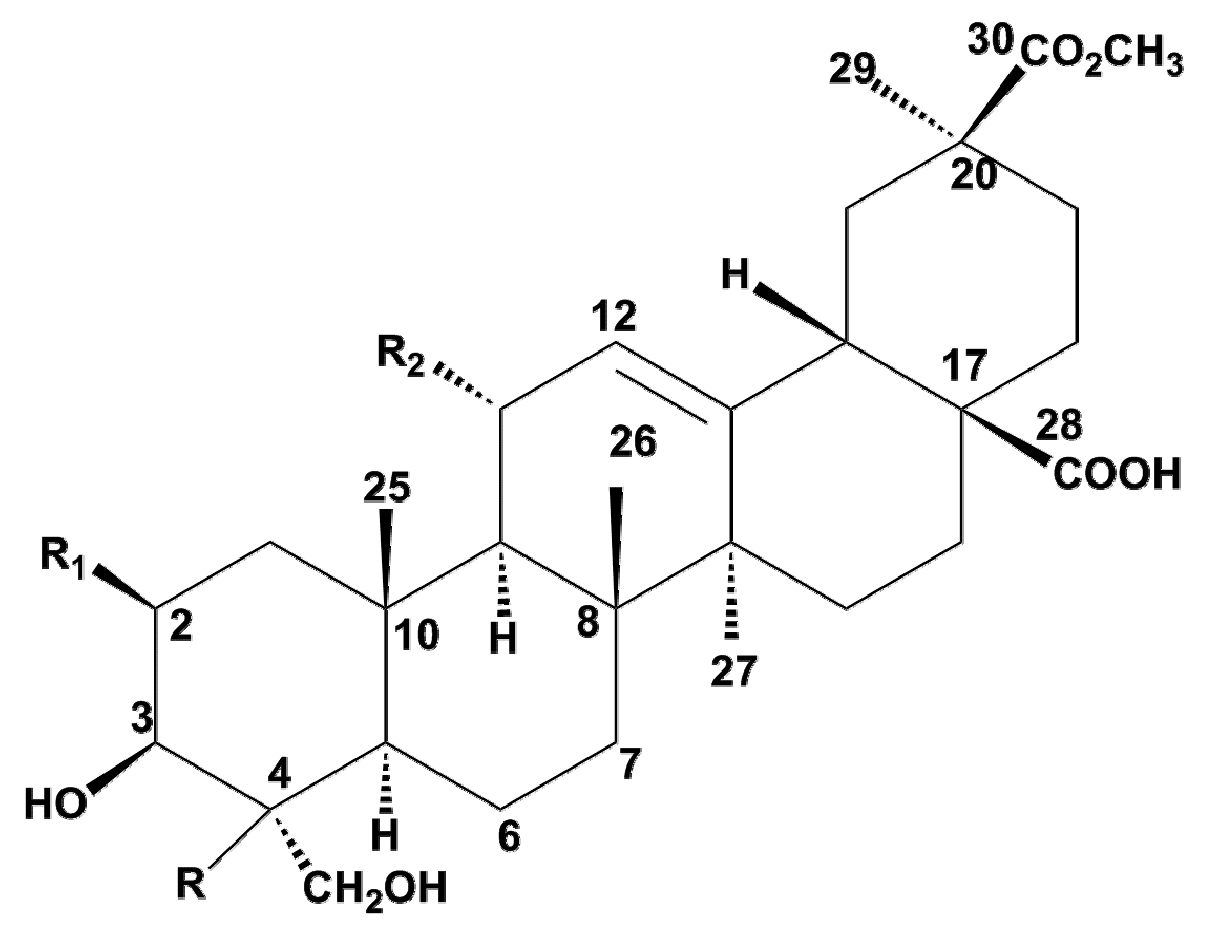
| Aglycone | MW | R | R1 | R2 |
| Serjanic acid (1) | 500 | H | H | H |
| Phytolaccagenic acid (2) | 516 | OH | H | H |
| 2-oxo-Phytolacagenin acid (3) | 530 | OH | =O | H |
| Phytolaccagenin (4) | 532 | OH | OH | H |
| 11-oxo-Phytolacagenin (5) | 546 | OH | OH | =O |
| 11α-Methoxyphytolaccagenin (6) | 562 | OH | OH | OCH3 |
| 11α-Hydroxyphytolaccagenin (7) | 548 | OH | OH | OH |
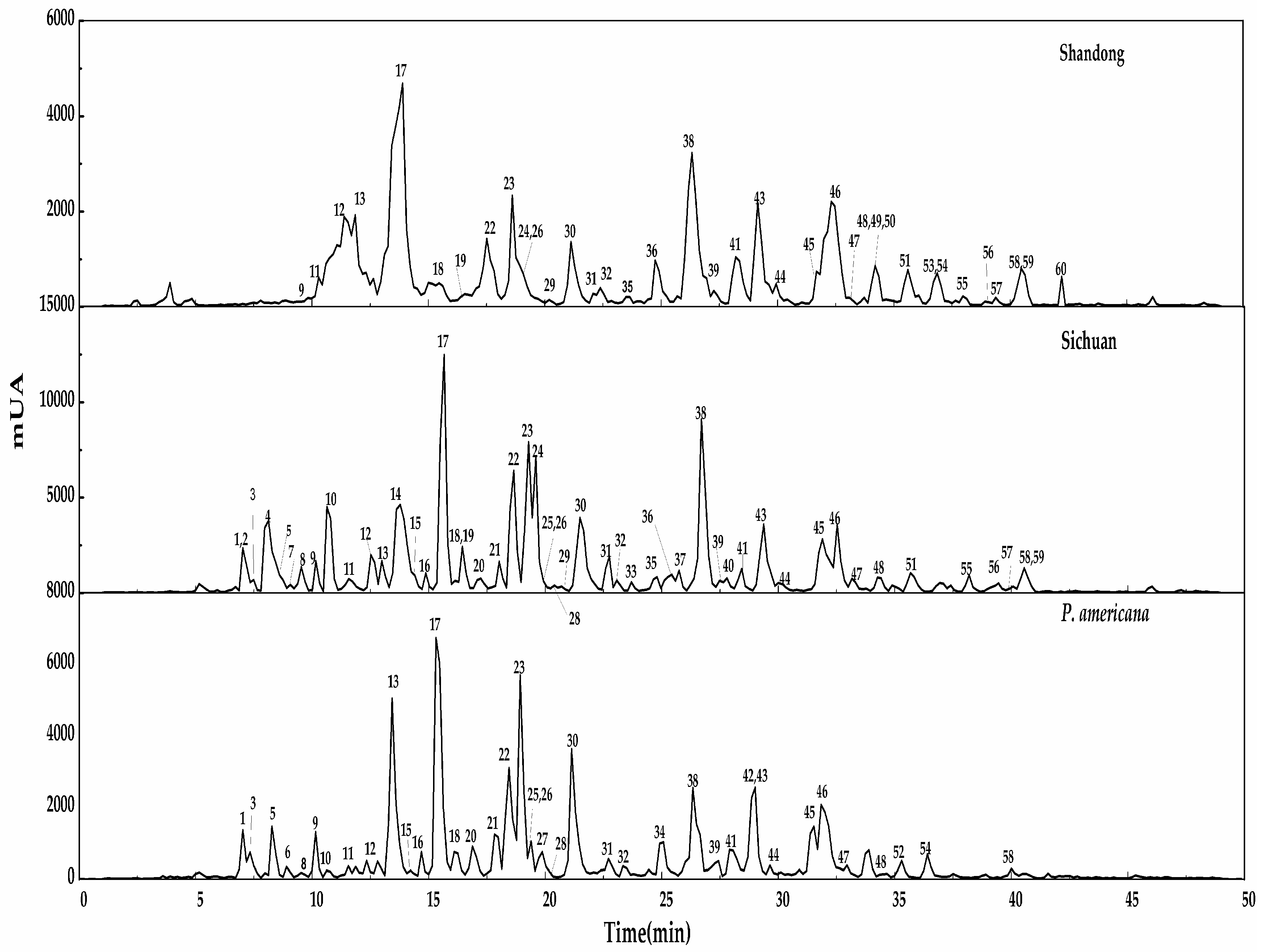

| Peak | RT (min) | m/z | Fragmentation [M − H]− | P. a | Sha | Sich | Tentative Structural Elucidation | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 7.02 | 841 | 841, 779, 679, 617, 547, 529 | √ | * | √ | 3-[Xyl]-28-Glc-11α-hydroxyphytolaccagenin | [25] |
| 2 | 7.34 | 1003 | 1003, 841, 679,661, 547, 529 | * | * | √ | 3-[Xyl-(1→4)-Glc]-28-Glc-11α-hydroxyphytolaccagenin | [25] |
| 3 | 7.36 | 883 | 883, 547, 499 | √ | * | √ | Unknown | |
| 4 | 8.12 | 973 | 973, 811, 795, 779, 649, 631, 517 | * | * | √ | 3-[Glc-(1→3)-Ara]-28-Glc-phytolaccagenic acid | [34] |
| 5 | 8.30 | 839 | 839, 795, 763, 677, 633, 601, 545,515 | √ | * | √ | 3-[Xyl-(1→4)-Glc)]-28-Glc-11-oxophytolaccagenin | [35,36] |
| 6 | 9.00 | 633 | 633, 531 | √ | * | * | Unknown | |
| 7 | 9.06 | 1149 | 1149, 987, 825, 663, 645, 531 | * | * | √ | 3-[Xyl-(1→4)-Glc-(1→2)-Glc]-28-Glc-phytolaccagenin | [25,26] |
| 8 | 9.53 | 855 | 855, 779, 693,679, 663, 617, 531 | √ | * | √ | 3-[Xyl]-28-Glc-11α-methoxyphytolaccagenin | [36] |
| 9 | 10.16 | 1031 | 1031, 693, 531 | √ | √ | √ | Unknown | |
| 10 | 10.71 | 811 | 811, 691, 649, 631, 515, 499 | √ | * | √ | 3-[Ara]-28-Glc-phytolaccagenic acid | [34] |
| 11 | 11.55 | 823 | 823, 661, 643, 531, 529 | √ | √ | √ | 3-[Xyl-(1→4)-Glc]-2-oxophytolaccagenic acid | [25,36] |
| 12 | 12.42 | 987 | 987, 825, 663, 645, 531 | √ | √ | √ | 3-[Xyl-(1→4)-Glc]-28-Glc-phytolaccagenin | [26] |
| 13 | 12.90 | 825 | 825, 663, 645, 627, 531 | √ | √ | √ | 3-[Xyl]-28-Glc-phytolaccagenin | [26,37] |
| 14 | 13.77 | 825 | 825, 663, 645, 531 | * | * | √ | Isomer 13 | |
| 15 | 14.12 | 1133 | 1133, 971, 809, 663, 647, 515 | √ | * | √ | 3-[Xyl-(1→4)-Glc-(1→2)-Glc]-28-Glc-phytolaccagenic acid | [25,36] |
| 16 | 14.74 | 867 | 867, 529 | √ | * | √ | Unknown | |
| 17 | 14.85 | 869 | 869, 531 | √ | √ | √ | Unknown | |
| 18 | 16.35 | 809 | 809, 647, 629, 515 | √ | √ | √ | 3-[Xyl]-28-Glc-phytolaccagenic acid | [38] |
| 19 | 16.57 | 973 | 973, 811, 795, 779, 649, 631, 515, | * | √ | √ | Isomer 4 | |
| 20 | 16.98 | 855 | 855, 779, 693,679, 663, 617, 531 | √ | * | √ | Isomer 8 | |
| 21 | 18.00 | 867 | 867, 529 | √ | * | √ | Isomer 16 | |
| 22 | 18.20 | 963 | 963, 933, 591, 569, 545, 529 | √ | √ | √ | unknown | |
| 23 | 18.93 | 811 | 811, 795, 693, 649, 631, 515 | √ | √ | √ | Isomer 10 | |
| 24 | 19.08 | 885 | 885, 663, 617, 531 | * | √ | √ | Unknown | |
| 25 | 19.61 | 955 | 955, 809, 647,629, 515 | √ | * | √ | 3-[Xyl-(1→4)-Glc-(1→2)-Rha]-phytolaccagenic acid | [25] |
| 26 | 19.93 | 1117 | 1117, 955, 809, 647, 629,515 | √ | √ | √ | 3-[LRha-(1→2)-Glc-(1→2)-Rha]-28-Glc-phytolaccagenic acid | [25,26] |
| 27 | 19.97 | 841 | 841, 679, 547 | √ | * | * | Isomer 1 | |
| 28 | 20.39 | 795 | 795, 633, 531, 501 | √ | * | √ | 3-[Ara]-28-Glc-serjanic | [33] |
| 29 | 20.44 | 663 | 663, 531 | * | √ | √ | 3-[Xyl]-phytolaccagenin | [25] |
| 30 | 21.25 | 839 | 839, 677, 545 | √ | √ | √ | Isomer 5 | |
| 31 | 22.62 | 823 | 823, 661, 643, 531, 529 | √ | √ | √ | Isomer 11 | |
| 32 | 22.79 | 809 | 809, 647, 529,515 | √ | √ | √ | Isomer 18 | |
| 33 | 23.43 | 649 | 649, 517 | * | * | √ | Unknown | |
| 34 | 24.25 | 653 | 653, 571, 529, 515 | √ | * | * | Unknown | |
| 35 | 24.86 | 855 | 855, 779, 693, 663, 531 | * | √ | √ | 3-[Xyl-(1→4)-Glc]-11α-methoxyphytolaccagenin | [25] |
| 36 | 25.02 | 853 | 853, 775,630,515 | * | √ | √ | Unknown | |
| 37 | 26.21 | 1355 | 1355, 1163, 677, 661, 531,515 | * | * | √ | Unknown | |
| 38 | 26.45 | 945 | 945, 915, 707, 591, 561, 545 | √ | √ | √ | Unknown | |
| 39 | 27.02 | 915 | 915, 779, 677, 633, 603, 529, 515 | √ | √ | √ | Unknown | |
| 40 | 27.80 | 855 | 855, 779, 693, 663, 531 | * | * | √ | Isomer 35 | |
| 41 | 28.23 | 823 | 823, 661, 529 | √ | √ | √ | 3-[Xyl]-28-2-oxophytolaccagenic acid | [25] |
| 42 | 28.99 | 779 | 779, 617, 545 | √ | * | * | Unknown | |
| 43 | 29.14 | 823 | 823, 779, 661, 631, 617, 529 | √ | √ | √ | 3-[Xyl-(1→4)-Glc]-2-oxophytolaccagenic acid | [25] |
| 44 | 29.89 | 971 | 971, 809, 647, 515 | √ | √ | √ | 3-[Glc-(1→3)-LAra]-28-Glc-phytolaccagenic acid | [39] |
| 45 | 31.69 | 839 | 839, 677, 545, 515 | √ | √ | √ | 3-[Glc-(1→4)-Glc]-phytolaccagenic acid | [25] |
| 46 | 32.33 | 825 | 825, 663, 645, 531, 499 | √ | √ | √ | 3-[Xyl-(1→4)-Glc]-phytolaccagenin | [25] |
| 47 | 33.06 | 1045 | 1045, 915, 799, 663, 547 | √ | √ | √ | Unknown | |
| 48 | 33.68 | 841 | 841, 679, 547, 515 | √ | √ | √ | Isomer 1 | |
| 49 | 33.70 | 901 | 901, 841, 547 | * | √ | * | Unknown | |
| 50 | 33.72 | 1171 | 1171, 841, 679, 547 | * | √ | * | Unknown | |
| 51 | 35.56 | 955 | 955, 809, 791, 647, 629, 515 | * | √ | √ | Isomer 25 | |
| 52 | 35.62 | 835 | 835, 515 | √ | * | * | Unknown | |
| 53 | 36.63 | 737 | 737, 677, 665, 545 | * | √ | * | Unknown | |
| 54 | 36.86 | 809 | 809, 647, 629, 515 | √ | √ | * | 3-[Xyl-(1→4)-Glc]-phytolaccagenic acid | [25] |
| 55 | 38.10 | 1045 | 1045, 915, 799, 709, 663, 547 | * | √ | √ | Isomer 47 | |
| 56 | 39.05 | 925 | 925, 779, 617, 545 | * | √ | √ | 3-[Glc-(1→4)-Rha]-11-oxo-phytolaccagenin | [40] |
| 57 | 39.36 | 721 | 721, 661, 529 | * | √ | √ | Unknown | |
| 58 | 40.69 | 723 | 723, 663, 531 | √ | √ | √ | Unknown | |
| 59 | 41.01 | 1329 | 1329, 663, 562, 531 | * | √ | √ | Unknown | |
| 60 | 44.68 | 649 | 649, 631, 517 | * | √ | * | Isomer 33 |
| % Relative Peak Area of Common Peaks | ||||
|---|---|---|---|---|
| Peak | m/z | P. a | Sha | Sich |
| 9 | 1031 | 1.97 | 0.43 | 1.00 |
| 11 | 823 | 0.42 | 0.32 | 1.26 |
| 12 | 987 | 0.58 | 7.20 | 1.98 |
| 13 | 825 | 8.12 | 7.20 | 1.26 |
| 17 | 869 | 11.48 | 18.84 | 14.37 |
| 18 | 809 | 1.59 | 2.55 | 1.46 |
| 22 | 963 | 5.21 | 4.39 | 8.52 |
| 23 | 811 | 7.20 | 3.24 | 6.86 |
| 26 | 1117 | 0.59 | 0.13 | 0.70 |
| 30 | 839 | 6.23 | 3.02 | 6.21 |
| 31 | 823 | 1.25 | 0.34 | 1.92 |
| 32 | 809 | 0.64 | 0.70 | 0.62 |
| 38 | 945 | 4.14 | 9.23 | 9.01 |
| 39 | 915 | 1.21 | 0.75 | 0.47 |
| 41 | 823 | 2.13 | 2.94 | 1.29 |
| 43 | 823 | 2.53 | 2.44 | 4.52 |
| 44 | 971 | 0.38 | 0.77 | 0.74 |
| 45 | 839 | 2.73 | 3.11 | 5.49 |
| 46 | 825 | 5.89 | 7.28 | 3.69 |
| 47 | 1045 | 1.56 | 0.37 | 1.02 |
| 48 | 841 | 0.79 | 5.58 | 1.24 |
| 58 | 723 | 0.47 | 1.01 | 1.96 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleri, F.D.; Chen, G.; Li, X.; Guo, M. Comparative Analysis of Saponins from Different Phytolaccaceae Species and Their Antiproliferative Activities. Molecules 2017, 22, 1077. https://doi.org/10.3390/molecules22071077
Saleri FD, Chen G, Li X, Guo M. Comparative Analysis of Saponins from Different Phytolaccaceae Species and Their Antiproliferative Activities. Molecules. 2017; 22(7):1077. https://doi.org/10.3390/molecules22071077
Chicago/Turabian StyleSaleri, Flora Didii, Guilin Chen, Xun Li, and Mingquan Guo. 2017. "Comparative Analysis of Saponins from Different Phytolaccaceae Species and Their Antiproliferative Activities" Molecules 22, no. 7: 1077. https://doi.org/10.3390/molecules22071077




