Molecular Docking and Anticonvulsant Activity of Newly Synthesized Quinazoline Derivatives
Abstract
:1. Introduction
2. Results and Discussion
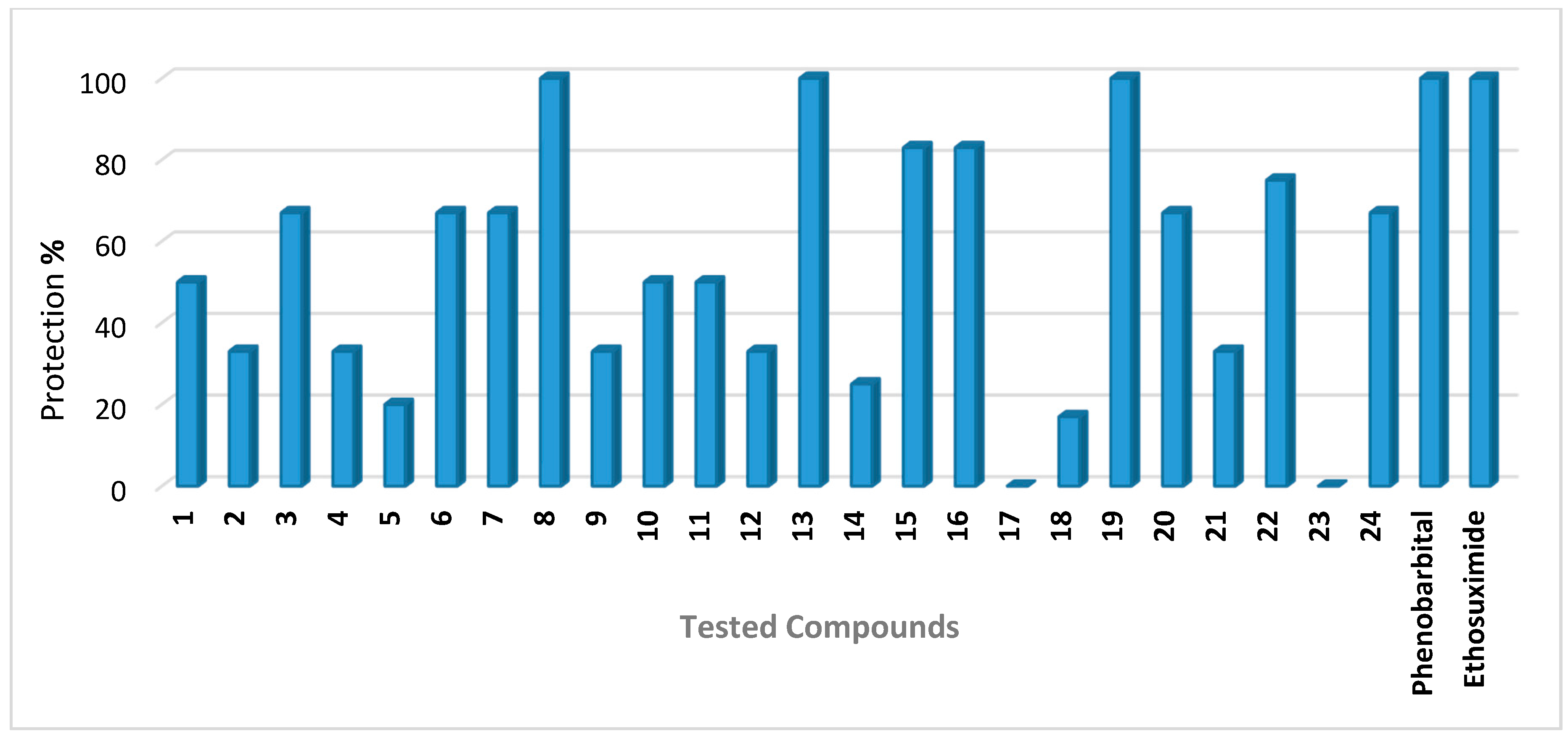
2.1. Anticonvulsant Activity
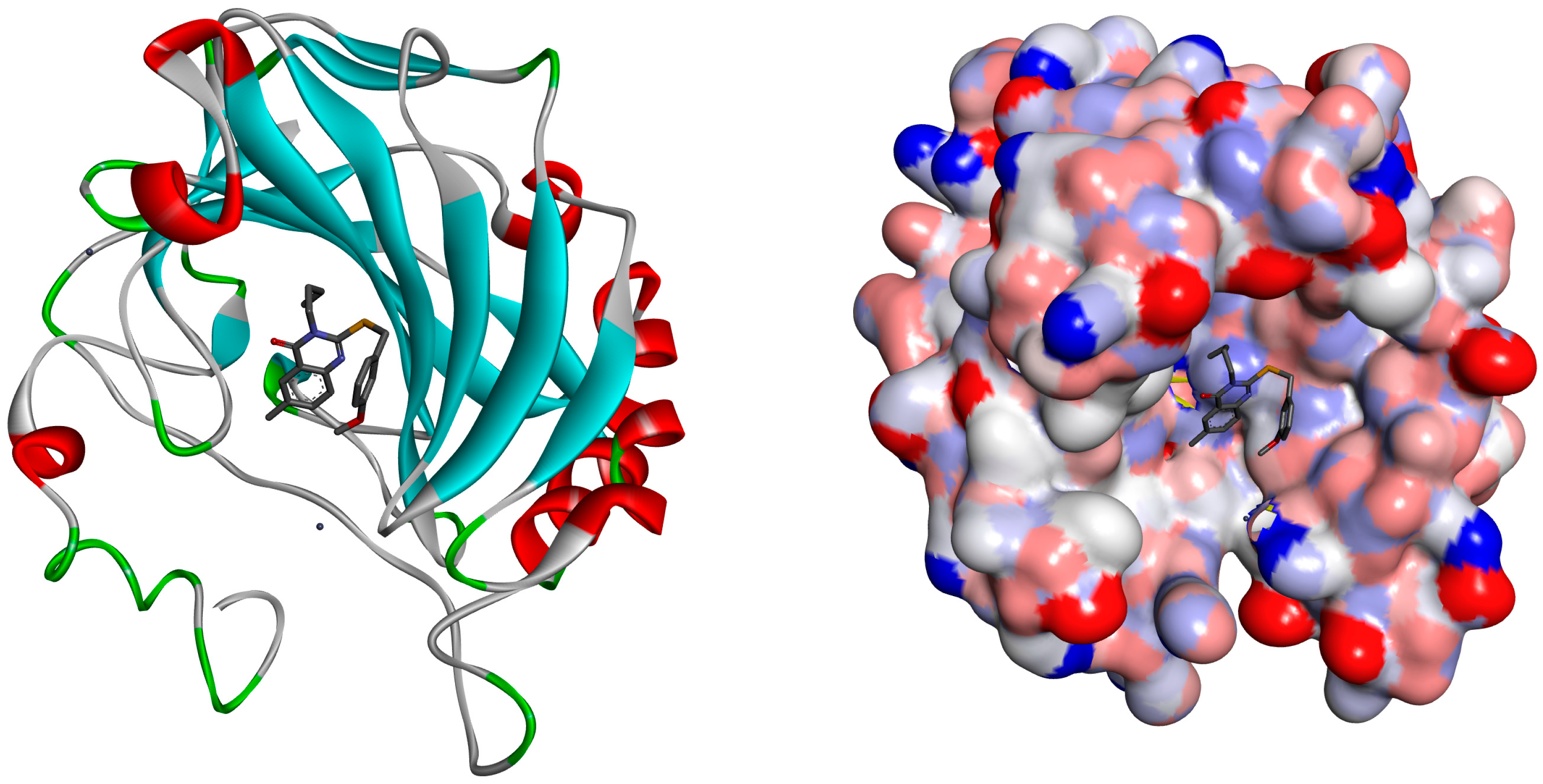
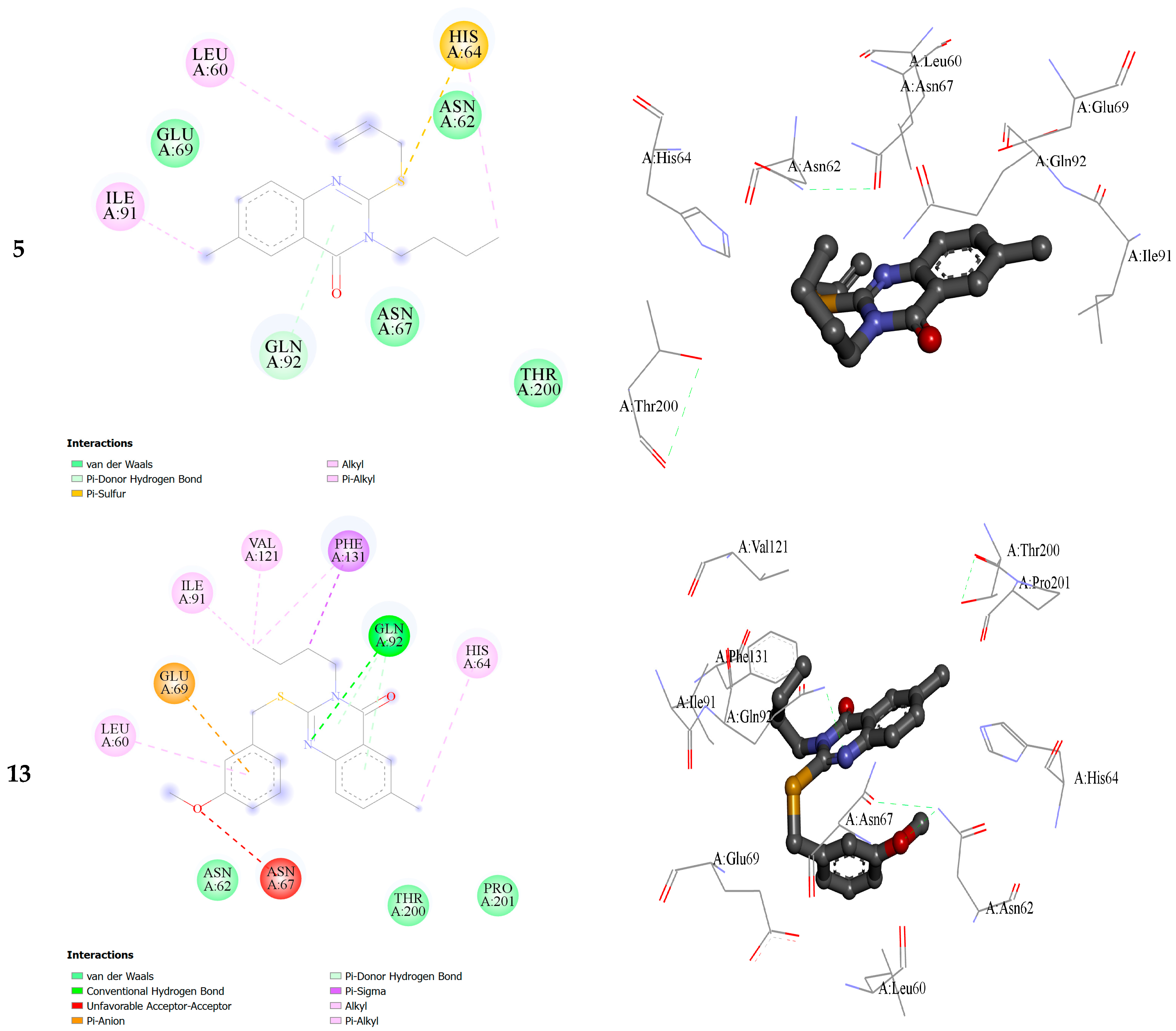
2.2. Molecular Docking
3. Materials and Methods
3.1. Animals
3.2. Drugs and Chemicals
3.3. Methods
3.3.1. Subcutaneous Pentylenetetrazol (scPTZ) Screen
3.3.2. Maximal Electroshock Seizure (MES) Screen
3.3.3. Neurotoxicity
3.4. Molecular Docking
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zayed, M.F.; Ihmaid, S.K.; Ahmed, H.E.A.; El-Adl, K.; Asiri, A.M.; Omar, A.M. Synthesis, modelling, and anticonvulsant studies of new quinazolines showing three highly active compounds with low toxicity and high affinity to the GABA-A receptor. Molecules 2017, 22, 188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-J.; Shen, Q.-K.; Jin, C.-M.; Quan, Z.-S. Synthesis and pharmacological evaluation of new 3,4-Dihydroisoquinolin derivatives dontaining heterocycle as potential anticonvulsant agents. Molecules 2016, 21, 1635. [Google Scholar] [CrossRef] [PubMed]
- Marjan, N.A.; Zamansoltani, F.; Torabinejad, B. Antiepileptic effects of quinine in the pentylenetetrazole model of seizure. Seizure 2009, 18, 129–132. [Google Scholar]
- Reddy, D.S. Pharmacotherapy of catamenial epilepsy. Ind. J. Pharmacol. 2005, 37, 288–293. [Google Scholar] [CrossRef]
- Giordano, C.; Marchiò, M.; Timofeeva, E.; Biagini, G. Neuroactive peptides as putative 366 mediators of antiepileptic ketogenic diets. Front. Neurol. 2014, 5, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Kneen, R.; Appleton, R.E. Alternative approaches to conventional antiepileptic drugs in the management of paediatric epilepsy. Arch. Dis. Child. 2006, 91, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, M.M. Overview of drugs used for epilepsy and seizures etiology, diagnosis, and treatment. Pharm. Ther. 2010, 35, 392–415. [Google Scholar]
- D'Antuono, M.; Köhling, R.; Ricalzone, S.; Gotman, J.; Biagini, G.; Avoli, M. Antiepileptic drugs abolish ictal but not interictal epileptiform discharges in vitro. Epilepsia 2010, 51, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure-based drug discovery of carbonic anhydrase inhibitors. J. Enzym. Inhib. Med. Chem. 2012, 27, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Thiry, A.; Dogne, J.-M.; Supuran, C.T.; Masereel, B. Carbonic Anhydrase Inhibitors as Anticonvulsant Agents. Curr. Top. Med. Chem. 2007, 7, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Georgey, H.; Abdel-Gawad, N.; Abbas, S. Synthesis and anticonvulsant activity of some quinazolin-4-(3H)-one derivatives. Molecules 2008, 13, 2557–2569. [Google Scholar] [CrossRef] [PubMed]
- Boshta, N.M.; El-Essawy, F.A.; Ammar, R.M.; Ismail, A.; Wahba, N.E. Synthesis of some new quinazolin-4(3H)-one derivatives and evaluation of their anticonvulsant activity. Monatsh. Chem. Mon. 2016, 147, 2031–2042. [Google Scholar] [CrossRef]
- Jatav, V.; Mishra, P.; Kashaw, S.; Stables, J.P. CNS depressant and anticonvulsant activities of some novel 3-[5-substituted-1,3,4-thiadiazole-2-yl]-2-styrylquinazoline-4(3H)-ones. Eur. J. Med. Chem. 2008, 43, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Kashaw, S.K.; Kashaw, V.; Mishra, P.; Jain, N.K.; Stables, J.P. Synthesis, anticonvulsant and CNS depressant activity of some new bioactive 1-(4-substituted–phenyl)-3-(4-oxo-2-phenyl/ethyl-4H-quinazolin-3-yl)-urea. Eur. J. Med. Chem. 2009, 44, 4335–4343. [Google Scholar] [CrossRef] [PubMed]
- Jatav, V.; Mishra, P.; Kashaw, S.; Stables, J.P. Synthesis and CNS depressant activity of some novel 3-[5-substituted-1,3,4-thiadiazole-2-yl]-2-styrylquinazoline-4(3H)-ones. Eur. J. Med. Chem. 2008, 43, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, H.S.; Hegazy, G.H.; El-Taher, K.E.; El-Messery, S.M.; Al-Obaid, A.M.; El-Subbagh, H.I. Synthesis, anticonvulsant activity and molecular modeling study of some new hydrazinecarbothioamide, benzenesulfonohydrazide, and phenacylacetohy drazide analogues of 4(3H)-quinazolinone. Bioorg. Med. Chem. Lett. 2015, 25, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, A.S.; Abdel-Hamide, S.G.; Sayed-Ahmed, M.M.; Hassan, G.S.; El-Hadiyah, T.M.; Al-Shabanah, O.A.; Al-Deeb, O.A.; El-Subbagh, H.I. Novel 4(3H)-quinazolinone analogs: Synthesis and anticonvulsant activity. Med. Chem. Res. 2013, 22, 2815–2827. [Google Scholar] [CrossRef]
- Aly, M.M.; Mohamed, Y.A.; El-Bayouki, K.A.; Basyouni, W.M.; Abbas, S.Y. Synthesis of some new 4(3H)-quinazolinone-2-carboxaldehyde thiosemicarbazones and their metal complexes and a study on their anticonvulsant, analgesic, cytotoxic and antimicrobial activities—Part-1. Eur. J. Med. Chem. 2010, 45, 3365–3373. [Google Scholar] [CrossRef] [PubMed]
- Kashaw, S.K.; Gupta, V.; Kashaw, V.; Mishra, P.; Stables, J.P.; Jain, N.K. Anticonvulsant and sedative-hypnotic activity of some novel 3-[5-(4-substituted)411-phenyl-1,3,4-oxadiazol-2-yl]-2-styrylquinazolin-4(3H)-ones. Med. Chem. Res. 2010, 19, 250–261. [Google Scholar] [CrossRef]
- Kashaw, S.K.; Kashaw, V.; Mishra, P.; Jain, N.K.; Stables, J.P. Design, synthesis, and potential CNS activity of some new bioactive 1-(4-substituted-phenyl)-3-(4-oxo-2-415 methyl-4H-quinazolin-3-yl)-urea. Med. Chem. Res. 2011, 20, 738–745. [Google Scholar] [CrossRef]
- Pandey, S.; Srivastava, R.S. Recent advancement in the field of anticonvulsants: A review. Lett. Drug Des. Discov. 2010, 7, 694–706. [Google Scholar] [CrossRef]
- Zappalà, M.; Grasso, S.; Micale, N.; Zuccalà, G.; Menniti, F.S.; Ferreri, G.; de Sarro, G.; De Micheli, C. 1-Aryl-6,7-methylenedioxy-3H-quinazolin-4-ones as anticonvulsant agents. Bioorg. Med. Chem. Lett. 2003, 13, 4427–4430. [Google Scholar] [CrossRef] [PubMed]
- Abuelizz, H.A.; Marzouk, M.; Ghabbour, H.; Al-Salahi, R. Synthesis and anticancer activity of new quinazoline derivatives. Saudi Pharm. J. 2017, in press. [Google Scholar] [CrossRef]
- Al-Omar, M.A.; Abdel-Hamide, S.G.; Al-Khamees, H.A.; El-Subbagh, H.I. Synthesis and biological screening of some new substituted-3H-quinazolin-4-one analogs as antimicrobial agents. Saudi Pharm. J. 2004, 12, 63–71. [Google Scholar]
- Alafeefy, A.M.; Ceruso, M.; Al-Tamimi, A.M.S.; del Prete, S.; Capasso, C.; Supuran, C.T. Quinazoline-sulfonamides with potent inhibitory activity against the α-carbonic anhydrase from Vibrio Cholera. Bioorg. Med. Chem. 2014, 22, 5133–5140. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, K.; Rapacz, A.; Łuszczki, J.J.; Latacz, G.; Obniska, J.; Kieć-Kononowicz, K.; Filipek, B. Design, synthesis and biological evaluation of new hybrid anticonvulsants derived from N-benzyl-2-(2,5-dioxopy rrolidin-1-yl) propanamide and 2-(2,5-dioxopyrrolidin-1-yl) butan amide derivatives. Bioorg. Med. Chem. 2015, 23, 2548–2561. [Google Scholar] [CrossRef] [PubMed]
- Curia, G.; Longo, D.; Biagini, G.; Jones, R.S.; Avoli, M. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods 2008, 172, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Krall, R.L.; Penry, J.K.; White, B.G.; Kupferberg, H.J.; Swinyard, E.A. Antiepileptic drug development: II. Anticonvulsant drug screening. Epilepsia 1978, 19, 409–428. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure 2011, 20, 359–368. [Google Scholar] [CrossRef] [PubMed]
- White, H.S. Preclinical development of antiepileptic drugs: Past, present, and future directions. Epilepsia 2003, 44, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Dunham, N.W.; Miya, T.S. A note on a simple apparatus for detecting neurological deficit in rats and mice. J. Am. Pharm. Assoc. Am. Pharm. Assoc. 1957, 46, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Gitto, R.; Ferro, S.; Agnello, S.; de Luca, L.; de Sarro, G.; Russo, E.; Vullo, D.; Supuran, C.T.; Chimirri, A. Synthesis and evaluation of pharmacological profile of 1-aryl-6,7-dimethoxy-3,4-dihydroisoquinoline-2(1H)-sulfonamides. Bioorg. Med. Chem. 2009, 17, 3659–3664. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Scozzafava, A.; Supuran, C.T. Which carbonic anhydrases are targeted by the antiepileptic sulfonamides and sulfamates? Chem. Biol. Drug Des. 2009, 74, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Aggarwal, M.; Maresca, A.; Scozzafava, A.; McKenna, R.; Supuran, C.T. Dithiocarbamates: A new class of carbonic anhydrase inhibitors. Crystallographic and kinetic investigations. Chem. Commun. 2012, 48, 1868–1870. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Karataş, M.O.; Uslu, H.; Sarı, S.; Alagöz, M.A.; Karakurt, A.; Alıcı, B.; Bilen, C.; Yavuz, E.; Gencer, N.; Arslan, O. Coumarin or benzoxazinone based novel carbonic anhydrase inhibitors: Synthesis, molecular docking and anticonvulsant studies. J. Enzym. Inhib. Med. Chem. 2016, 31, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Behairy, M.F.; Aboul-Enein, M.N.; El-Azzouny, A.A.; Saleh, O.A.; Maklad, Y.A.; Aboutabl, M.E.; Maghraby, A.S. Design, synthesis, and biological profile of novel N-(5-aryl-1,3,4-thiadiazol-2-yl) hydrazinecarboxamides. Eur. J. Chem. 2014, 5, 488–496. [Google Scholar] [CrossRef]
- Alam, O.; Mullick, P.; Verma, S.P.; Gilani, S.J.; Khan, S.A.; Siddiqui, N.; Ashsan, W. Synthesis, anticonvulsant and toxicity screening of newer pyrimidine semicarbazone derivatives. Eur. J. Med. Chem. 2010, 45, 2467–2472. [Google Scholar] [CrossRef] [PubMed]
- Luszczki, J.J.; Czuczwar, M.; Gawlik, P.; Sawiniec-Pozniak, G.; Czuczwar, K.; Czuczwar, S.J. 7-Nitroindazole potentiates the anticonvulsant action of some second-generation antiepileptic drugs in the mouse maximal electroshock-induced seizure model. J. Neural. Transm. 2006, 113, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, K.; Carradori, S.; Monti, S.M.; Buonanno, M.; Secci, D.; Vullo, D.; Supuran, C.T.; de Simone, G. Out of the active site binding pocket for carbonic anhydrase inhibitors. Chem. Commun. 2015, 51, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Temperini, C.; Vu, H.; Pham, N.B.; Poulsen, S.-A.; Scozzafava, A.; Quinn, R.J.; Supuran, C.T. Non-Zinc Mediated Inhibition of Carbonic Anhydrases: Coumarins Are a New Class of Suicide Inhibitors. J. Am. Chem. Soc. 2009, 131, 3057–3062. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–24 are available from the authors. |
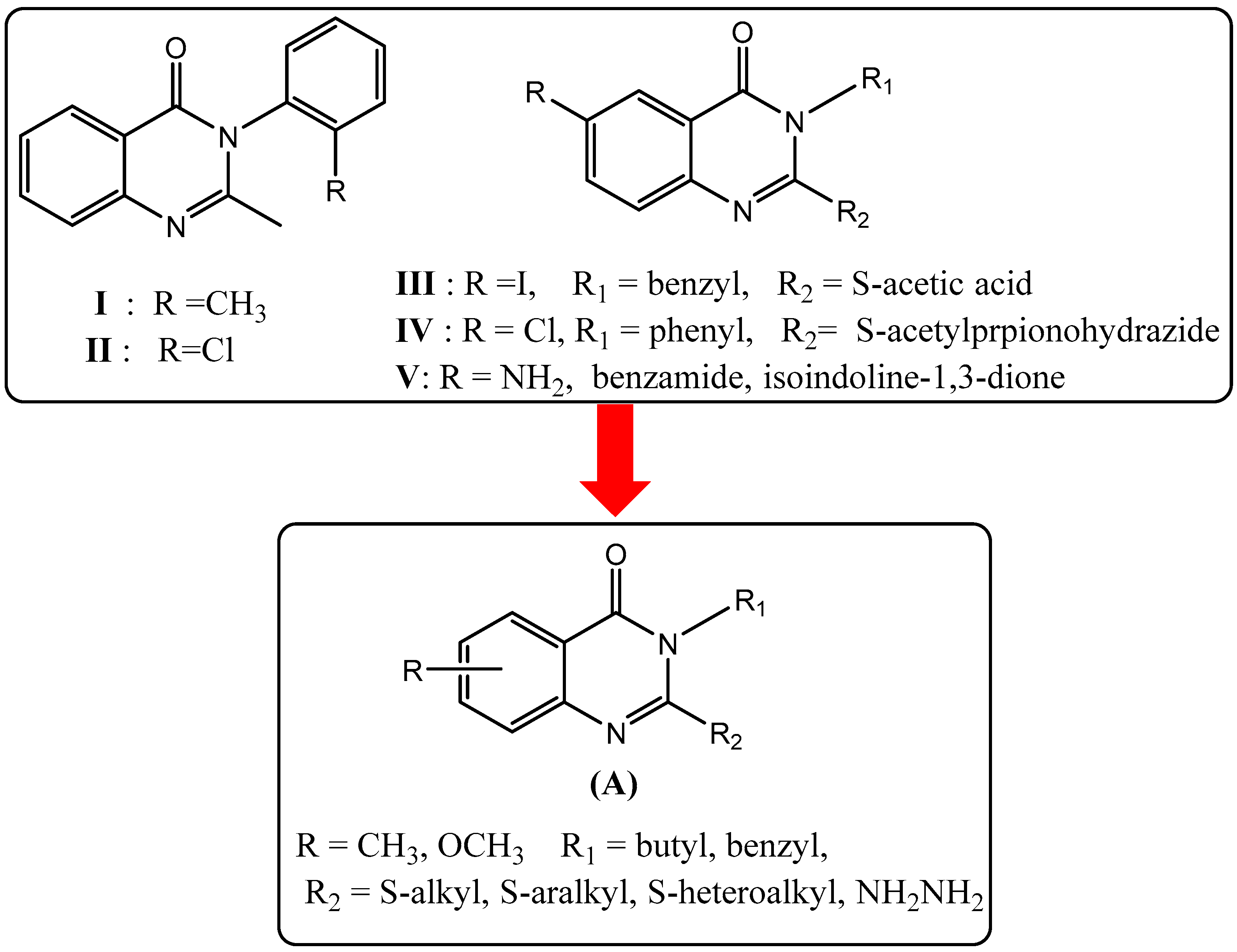
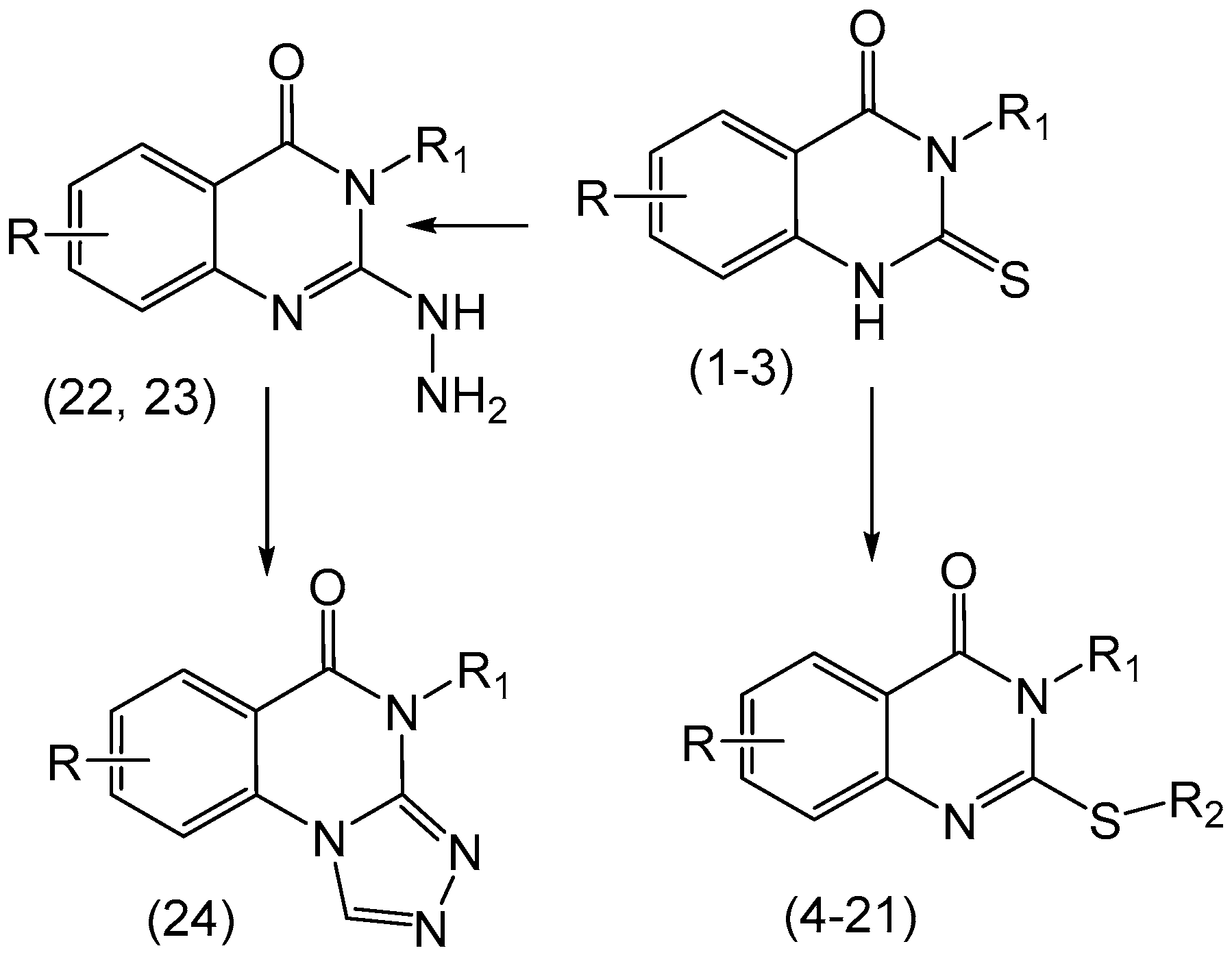


| Compounds | R | R1 | R2 |
|---|---|---|---|
| 1 | methyl | benzyl | - |
| 2 | methoxy | benzyl | - |
| 3 | methyl | butyl | - |
| 4 | methyl | butyl | ethyl |
| 5 | methyl | butyl | allyl |
| 6 | methyl | butyl | 2-Me-benzyl |
| 7 | methyl | butyl | 3-Me-benzyl |
| 8 | methyl | butyl | 4-Cl-benzyl |
| 9 | methyl | butyl | 4-NO2-benzyl |
| 10 | methyl | butyl | 2-CN-benzyl |
| 11 | methyl | butyl | 3-CN-benzyl |
| 12 | methyl | butyl | 4-CN-benzyl |
| 13 | methyl | butyl | 3-methoxy-benzyl |
| 14 | methyl | butyl | (1H-Benzoimidazol-2-yl)methyl |
| 15 | methyl | butyl | morphilinoethyl |
| 16 | methyl | benzyl | 4-CN-benzyl |
| 17 | methyl | benzyl | 2-methyl-benzyl |
| 18 | methyl | benzyl | 4-NO2-benzyl |
| 19 | methyl | benzyl | 7-NO2-benzoxadiazole |
| 20 | methoxy | benzyl | 3-(Phthalimido-2-yl)propyl |
| 21 | methoxy | benzyl | morphilinoethyl |
| 22 | methyl | butyl | hydrazine |
| 23 | methyl | benzyl | hydrazine |
| 24 | methyl | butyl | - |
| Compounds | Dose a | % Protection | Neurotoxicity b | ||
|---|---|---|---|---|---|
| (mg/kg) | (mmol/kg) | scPTZ | MES | ||
| 1 | 100 | 0.301 | 50 | Nd | 0/6 |
| 2 | 100 | 0.376 | 33 | Nd | 0/6 |
| 3 | 100 | 0.234 | 67 | Nd | 0/6 |
| 4 | 100 | 0.239 | 33 | Nd | 0/6 |
| 5 | 100 | 0.253 | 20 | Nd | 0/6 |
| 6 | 100 | 0.267 | 67 | Nd | 0/6 |
| 7 | 100 | 0.212 | 67 | Nd | 0/6 |
| 8 | 100 | 0.248 | 100 | 33 | 0/6 |
| 9 | 100 | 0.241 | 33 | Nd | 0/6 |
| 10 | 100 | 0.204 | 50 | Nd | 0/6 |
| 11 | 100 | 0.255 | 50 | Nd | 0/6 |
| 12 | 100 | 0.355 | 33 | Nd | 0/6 |
| 13 | 100 | 0.239 | 100 | 33 | 0/6 |
| 14 | 100 | 0.373 | 25 | Nd | 0/6 |
| 15 | 100 | 0.245 | 83 | Nd | 0/6 |
| 16 | 100 | 0.252 | 83 | Nd | 0/6 |
| 17 | 100 | 0.329 | 0 | Nd | 0/6 |
| 18 | 100 | 0.309 | 17 | Nd | 0/6 |
| 19 | 100 | 0.338 | 100 | 17 | 0/6 |
| 20 | 100 | 0.331 | 67 | Nd | 0/6 |
| 21 | 100 | 0.251 | 33 | Nd | 0/6 |
| 22 | 100 | 0.352 | 75 | Nd | 0/6 |
| 23 | 100 | 0.291 | 0 | Nd | 0/6 |
| 24 | 100 | 0.325 | 67 | Nd | 0/6 |
| Phenobarbital | 30 | 0.130 | 100 | Nd | Nd |
| Ethosuximide | 150 | 1.060 | 100 | Nd | Nd |
| Diphenyl Hydantoin | 45 | 0.160 | - | 100 | Nd |
| Within the Active Binding Site of hCA II Isomer. Quinazoline Derivatives | Free Binding Energy (kcal/mol) | H-Bonds (HBs) | Closest Interaction of Quinazoline and Active Site Residues |
|---|---|---|---|
| 3 | −3.55 | One HB: (GLN92-Q3) | ASN67, HIS64, GLN92, ILE 91 |
| 5 | −3.19 | One HB: (GLN92-Q5) | HIS64, ASN62, ASN67, GLU69, GLN92 |
| 13 | −4.01 | Two HBs ASN62-Q13 GLN92-Q13 | ILE91, PHE131, GLU69, ASN62, LEU60, ASN67, GLN92, THR200, HIS64, PRO201 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuelizz, H.A.; Dib, R.E.; Marzouk, M.; Anouar, E.-H.; A. Maklad, Y.; N. Attia, H.; Al-Salahi, R. Molecular Docking and Anticonvulsant Activity of Newly Synthesized Quinazoline Derivatives. Molecules 2017, 22, 1094. https://doi.org/10.3390/molecules22071094
Abuelizz HA, Dib RE, Marzouk M, Anouar E-H, A. Maklad Y, N. Attia H, Al-Salahi R. Molecular Docking and Anticonvulsant Activity of Newly Synthesized Quinazoline Derivatives. Molecules. 2017; 22(7):1094. https://doi.org/10.3390/molecules22071094
Chicago/Turabian StyleAbuelizz, Hatem A., Rabab El Dib, Mohamed Marzouk, El-Hassane Anouar, Yousreya A. Maklad, Hanan N. Attia, and Rashad Al-Salahi. 2017. "Molecular Docking and Anticonvulsant Activity of Newly Synthesized Quinazoline Derivatives" Molecules 22, no. 7: 1094. https://doi.org/10.3390/molecules22071094