Antibacterial Activities of Pyrenylated Coumarins from the Roots of Prangos hulusii
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Isolation of Compounds
3.4. 4′-Senecioiloxyosthol (1)
3.5. Antimicrobial Activity
3.6. Determination of Antibacterial Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pimenov, M.G.; Leonov, M.V. The Asian Umbelliferae biodiversity database (ASIUM) with particular feference to South-West Asian taxa. Turk. J. Bot. 2004, 28, 139–145. [Google Scholar]
- Güner, A.; Aslan, S.; Ekim, T.; Vural, M.; Babac, M.T. A Checklist of the Flora of Turkey (Vascular Plants); Nezahat Gokyigit Botanic Garden Publications, Flora Series I: İstanbul, Turky, 2012. [Google Scholar]
- Sharma, N.; Ashok, P.K.; Negi, A.; Lakshmayya, B. A review on ethnobotany, phytochemical and pharmacological dynamics of Prangos pabularia Lindl. J. Nat. Rem. 2013, 13, 68–75. [Google Scholar]
- Kafash-Farkhad, N.; Asadi-Samani, M.; Rafieian-Kopaei, M. A review on phytochemistry and pharmacological effects of Prangos ferulacea (L.) Lindl. Life Sci. J. 2013, 10, 360–367. [Google Scholar]
- Doković, D.D.; Bulatović, V.M.; Božić, B.D.; Kataranovski, M.V.; Zrakić, T.M.; Kovačević, N.N. 3,5-Nonadiyne isolated from the rhizome of Cachrys ferulacea inhibits endogenous nitric oxide release by rat peritoneal macrophages. Chem. Pharm. Bull. 2004, 52, 853–854. [Google Scholar]
- Ulubelen, A.; Topcu, G.; Tan, N.; Olcal, S.; Johansson, C.; Ucer, M.; Birman, H.; Tamer, S. Biological activities of a Turkish medicinal plant, Prangos platychlaena. J. Ethnopharmacol. 1995, 45, 193–197. [Google Scholar] [CrossRef]
- Shikishima, Y.; Takaishi, Y.; Honda, G.; Ito, M.; Takeda, Y.; Kodzhimatov, O.K.; Ashurmetov, O. Terpenoids and γ-pyrone derivatives from Prangos tschimganica. Phytochemistry 2001, 57, 135–141. [Google Scholar] [CrossRef]
- Shikishima, Y.; Takaishi, Y.; Honda, G.; Ito, M.; Takeda, Y.; Kodzhimatov, O.K.; Ashurmetov, O.; Lee, K.H. Chemical constituents of Prangos tschimganica; structure elucidation and absolute configuration of coumarin and furanocoumarin derivatives with anti-HIV activity. Chem. Pharm. Bull. 2001, 49, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Shikishima, Y.; Takaishi, Y.; Shibata, H.; Higuti, T.; Honda, G.; Ito, M.; Takeda, Y.; Kodzhimatov, O.K.; Ashurmetov, O.; et al. Coumarins and γ-pyrone derivatives from Prangos pabularia: Antibacterial activity and inhibition of cytokine release. Phytochemistry 2002, 59, 649–654. [Google Scholar] [CrossRef]
- Sajjadi, S.E.; Mehregan, I. Chemical composition of the essential oil of Prangos asperula Boiss. Subsp. haussknechtii (Boiss.) Herrnst. Etheyn fruits. DARU J. Fac. Pharm. Sci. 2003, 11, 79–81. [Google Scholar]
- Şenol, G.S.; Hasan, Y.; Özcan, S. Prangos hulusii sp. nov. (Apiaceae) from West Anatolia, Turkey. Nord. J. Bot. 2011, 29, 402–407. [Google Scholar]
- Tütüniş-Yazıcı, S.; Tan, N.; Meriçli, F.; Özsoy, N.; Tan, E. Biological activities of endemic Prangos hulusii. Planta Med. 2013, 79, PN113. [Google Scholar]
- Wei, Y.; Zhang, T.; Ito, Y. Preparative isolation of osthol and xanthotoxol from Common Cnidium Fruit (Chinese traditional herb) using stepwise elution by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1033, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.; Furukawa, H. Constituents of Murraya exotica L. structure elucidation of new coumarins. Chem. Pharm. Bull. 1987, 35, 4277–4285. [Google Scholar] [CrossRef]
- Nakatani, N.; Yamada, Y.; Fuwa, H. 7-Geranyloxycoumarin from juice oil of Hassaku (Citrus hassaku) and antimicrobial effects of related coumarins. Agric. Biol. Chem. 1987, 51, 419–423. [Google Scholar]
- Riviere, C.; Goossens, L.; Pommery, N.; Fourneau, C.; Delelis, A.; Henichart, J.P. Antiproliferative effects of isopentenylated coumarins isolated from Phellolophium madagascariense Baker. Nat. Prod. Res. 2006, 20, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhou, M.; Liu, Y.; Zhang, G.; Luo, Y. Chromones and coumarins from the dried fructus of Cnidium monnieri. Fitoterapia 2011, 82, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.D.; Kim, Y.J.; Ryu, H.W.; Kim, J.H.; Han, S.; Ra, J.; Seo, K.H.; Jang, K.C.; Leeb, J.H. Identification and characterisation of coumarins from the roots of Angelica dahurica and their inhibitory effects against cholinesterase. J. Func. Foods 2013, 5, 1421–1431. [Google Scholar] [CrossRef]
- Chunyan, C.; Bo, S.; Ping, L.; Jingmei, L.; Ito, Y. Isolation and purification of psoralen and bergapten from Ficus carica L leaves by high-speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Pierre, L.L.; Moses, M.N. Isolation and Characterisation of Stigmasterol and β-Sitosterol from Odontonema strictum (Acanthaceae). J. Innov. Pharm. Biol. Sci. 2015, 2, 88–96. [Google Scholar]
- Steck, W. Coumarins and chromones from Lomatium macrocarpum. Phytochemistry 1973, 12, 2283–2286. [Google Scholar] [CrossRef]
- Schinkovitz, A.; Gibbons, S.; Stavri, M.; Cocksedge, M.J.; Bucar, F. Ostruthin: An antimycobacterial coumarin from the roots of Peucedanum ostruthium. Planta Med. 2003, 69, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Stavri, M.; Gibbons, S. The antimycobacterial constituents of dill (Anethum graveolens). Phytother. Res. 2005, 19, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Walasek, M.; Grzegorczyk, A.; Malm, A.; Skalicka-Wozniak, K. Bioactivity-guided isolation of antimicrobial coumarins from Heracleum mantegazzianum Sommier & Levier (Apiaceae) fruits by high-performance counter-current chromatography. Food Chem. 2015, 186, 133–138. [Google Scholar] [PubMed]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the In Vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI (NCCLS)). M7-A7, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, Seventh Edition 1-56238-587-9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2006. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
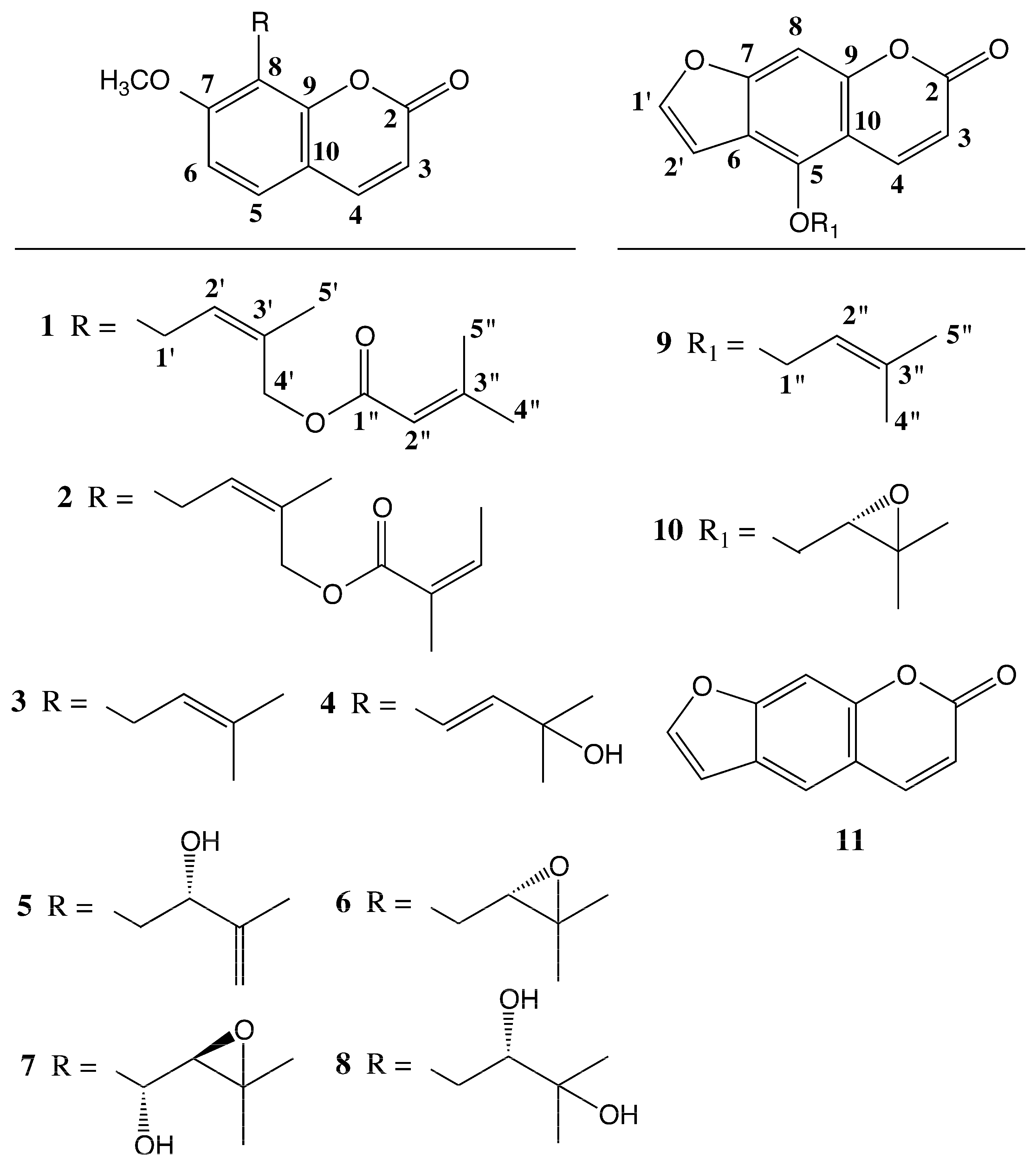
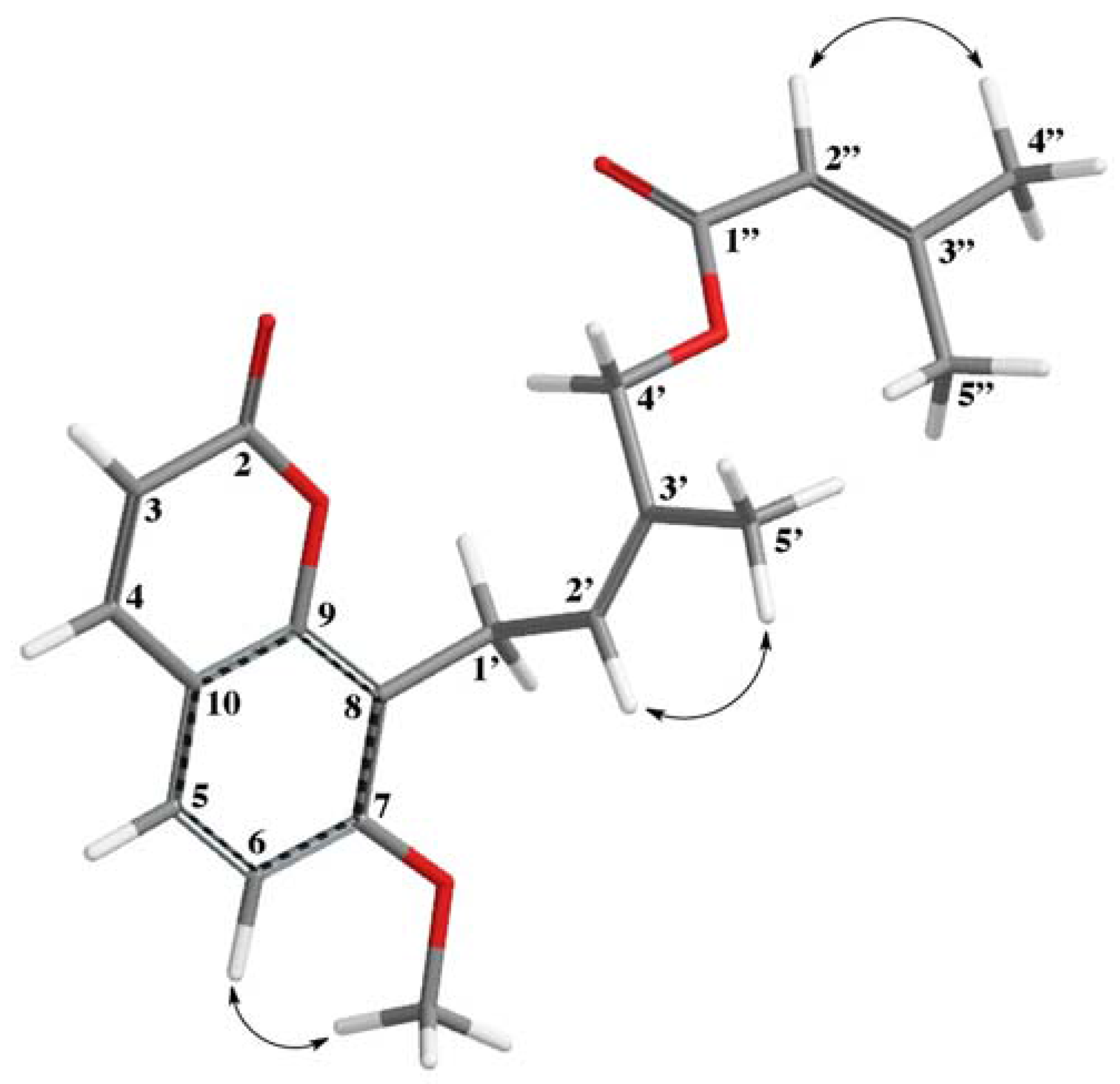
| Positions | ΔH (J in Hz) | ΔC, Type |
|---|---|---|
| 2 | 161.14, C | |
| 3 | 6.23 d (9.8), 1H | 116.08, CH |
| 4 | 7.60 d (9.8), 1H | 143.65, CH |
| 5 | 7.30 d (8.4), 1H | 126.46, CH |
| 6 | 6.82 d (8.4), 1H | 107.3, CH |
| 7 | 160.09, C | |
| 8 | 116.66, C | |
| 9 | 152.86, C | |
| 10 | 112.92, C | |
| -OCH3 | 3.89 s, 3H | 56.01, CH3 |
| 1′ | 3.62 d (7.9), 2H | 21.45, CH2 |
| 2′ | 5.50 br t (7.9), 1H | 113.04, CH |
| 3′ | 131.24, C | |
| 4′ | 4.87 s, 2H | 62.40, CH2 |
| 5′ | 1.72 br s, 3H | 21.61, CH3 |
| 1″ | 166.8, C | |
| 2″ | 5.72 quint (1.3), 1H | 126.51, CH |
| 3″ | 156.41, C | |
| 4″ | 1.90 br d (1.3), 3H | 27.39, CH3 |
| 5″ | 2.17 br d (1.3), 3H | 20.2, CH3 |
| Test Strains | Prenylated Coumarins (μg/mL) ** | Extracts (μg/mL) *** | References **** | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 * | 3 | 4 | 5 | 6 | 8 | 9 | 10 | DCM | PE | MeOH | TTR | OXA | CEF | CPR | |
| Minimum Inhibitory Concentration (MIC) μg/mL | |||||||||||||||
| S. epidermidis ATCC 12228 | 625 | >125 | >125 | 63 | >125 | 125 | >125 | 125 | 625 | 313 | 625 | 64 | 0.5 | 4 | 0.5 |
| S. aureus ATCC 25923 | 625 | 125 | >250 | >250 | >250 | 250 | >250 | 250 | 1250 | 625 | 1250 | 0.5 | 0.5 | 2 | 1 |
| E. faecalis ATCC 29212 | 313 | >250 | >250 | >250 | >250 | 250 | >250 | 250 | 313 | 313 | 313 | 16 | 8 | 2 | 1 |
| K. pneumoniae ATCC 4352 | 313 | 125 | 63 | 63 | 125 | 250 | 125 | 250 | 625 | 625 | 313 | 8 | 4 | 2 | 0.5 |
| B. subtilis ATCC 9372 | 5 | 125 | 63 | 63 | >250 | 250 | >250 | 125 | 1250 | 1250 | 1250 | 0.3 | 0.1 | 0.5 | 0.5 |
| E. coli ATCC 10799 | 313 | >125 | >125 | >125 | >125 | 250 | >125 | 250 | 156 | 313 | 313 | 2 | 32 | 1 | 0.3 |
| P. aeruginosa ATCC 27853 | 156 | >125 | >125 | >125 | >125 | 250 | >125 | 125 | 313 | 313 | 625 | 32 | >64 | 16 | 0.5 |
| S. choleraesuis ATCC 14028 | 313 | >125 | >125 | >125 | >125 | 125 | >125 | 125 | 625 | 625 | 1250 | 4 | >64 | 0.3 | 0.3 |
| P. mirabilis ATCC 7002 | 313 | >125 | >125 | >125 | >125 | 250 | >125 | 250 | 313 | 625 | 625 | 64 | 32 | 1 | 1 |
| Methicillin-sensitive Staphylococcus aureus (MSSA) | 625 | 125 | >250 | >250 | >250 | 250 | >250 | 250 | 1250 | 1250 | 2500 | 64 | 64 | 2 | 0.1 |
| Methicillin-resistant Staphylococcus aureus (MRSA) | 625 | 16 | >250 | >250 | >250 | 250 | 16 | 125 | 1250 | 1250 | 2500 | 8 | 8 | 16 | 0.5 |
| Methicillin Resistant Coagulase-Negative Staphylococci (MRCNS) | 313 | >250 | 125 | 125 | 125 | 125 | >250 | 125 | 1250 | 1250 | 1250 | 8 | 8 | 16 | 4 |
| K. pneumoniae | 313 | >125 | >125 | >125 | >125 | 250 | >125 | 250 | 2500 | 2500 | 2500 | 2 | >64 | >64 | 2 |
| A. baumannii | n.t. | >125 | >125 | >125 | >125 | 125 | >125 | 125 | 2500 | 1250 | 2500 | n.t. | n.t. | n.t. | >16 |
| E. coli | 156 | >125 | >125 | >125 | >125 | 250 | >125 | 250 | 2500 | 2500 | 2500 | >64 | >64 | >64 | 0.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, N.; Yazıcı-Tütüniş, S.; Bilgin, M.; Tan, E.; Miski, M. Antibacterial Activities of Pyrenylated Coumarins from the Roots of Prangos hulusii. Molecules 2017, 22, 1098. https://doi.org/10.3390/molecules22071098
Tan N, Yazıcı-Tütüniş S, Bilgin M, Tan E, Miski M. Antibacterial Activities of Pyrenylated Coumarins from the Roots of Prangos hulusii. Molecules. 2017; 22(7):1098. https://doi.org/10.3390/molecules22071098
Chicago/Turabian StyleTan, Nur, Seçil Yazıcı-Tütüniş, Merve Bilgin, Emir Tan, and Mahmut Miski. 2017. "Antibacterial Activities of Pyrenylated Coumarins from the Roots of Prangos hulusii" Molecules 22, no. 7: 1098. https://doi.org/10.3390/molecules22071098




