Analysis of variance (ANOVA) for the responses of
Chlorella sp. phenolic content and antioxidant activity was used to investigate the effects of each independent variable in the SW condition parameters to construct an empirical model that maximized the phenolic content and antioxidant activity from the microalgae. This model was also used to optimize each independent variable, namely extraction temperature, time, and microalgae concentration, during SW extraction.
Table 1 shows the experimental design employed, while
Table 2 summarizes the phenolic content and antioxidant activity data from all extracts examined.
The significance of each term at a specified level of confidence was determined by examining its respective
p-value and
F-value. In fact, the
p-value is the smallest level of significance that could be used to reject the null hypothesis, H
0. Therefore, the smaller the value is, the more significant its corresponding coefficient and the contribution towards the response variable. From the ANOVA in
Table 3, it was observed that some of the variables were highly significant to the regression model as indicated by the high
F-value. As can be seen in
Table 3, based on the
F-values, three linear factor terms (X
1, X
2, X
3), one quadratic term (X
12), and two interaction factors (X
1X
2, X
2X
3) for the phenolic response, and two linear factor terms (X
1, X
2), one quadratic term (X
12), and one interaction factor (X
1X
2) for antioxidant activity, had the largest effect on the investigated responses at a 95% confidence level, as indicated by the low
p-value (<0.05) and the high
F-value.
The
p-value of each of the other terms was greater than 0.05, which indicated that the effect of these terms on the response model was not statistically significant at the 95% confidence level. In other words, only the model terms with
p-values less than 0.05 were determined to be significant to the model equation. The insignificant model terms were removed to improve the model. Model reduction involves this type of backward elimination procedure for all the insignificant terms, to ultimately produce a new and improved experimental model. The ANOVA analysis of the reduced model (new model) equation after eliminating the insignificant terms for both phenolic content and antioxidant activity is shown in
Table 4. In addition, the adequacy of the models was further justified through ANOVA. The R-squared value in the reduced model for phenolic content was 0.9679 and 0.9499 for antioxidant activity, indicating an excellent agreement between the experimental and predicted results. In addition, as shown in
Table 4, the model resulted in an
F-value of 51.61 for phenolic content and 41.11 for antioxidant activity, with an extremely low
p-value (<0.0001), implying that the model was highly significant and was adequate for the response variables that were tested. By performing multiple regression analysis on the experimental data, the model for the response variable could be expressed using the following quadratic polynomial equation in the form of coded values, after exclusion of the insignificant terms as shown in
Table 5.
Effect of Extraction Conditions on Phenolic Content and Antioxidant Activity
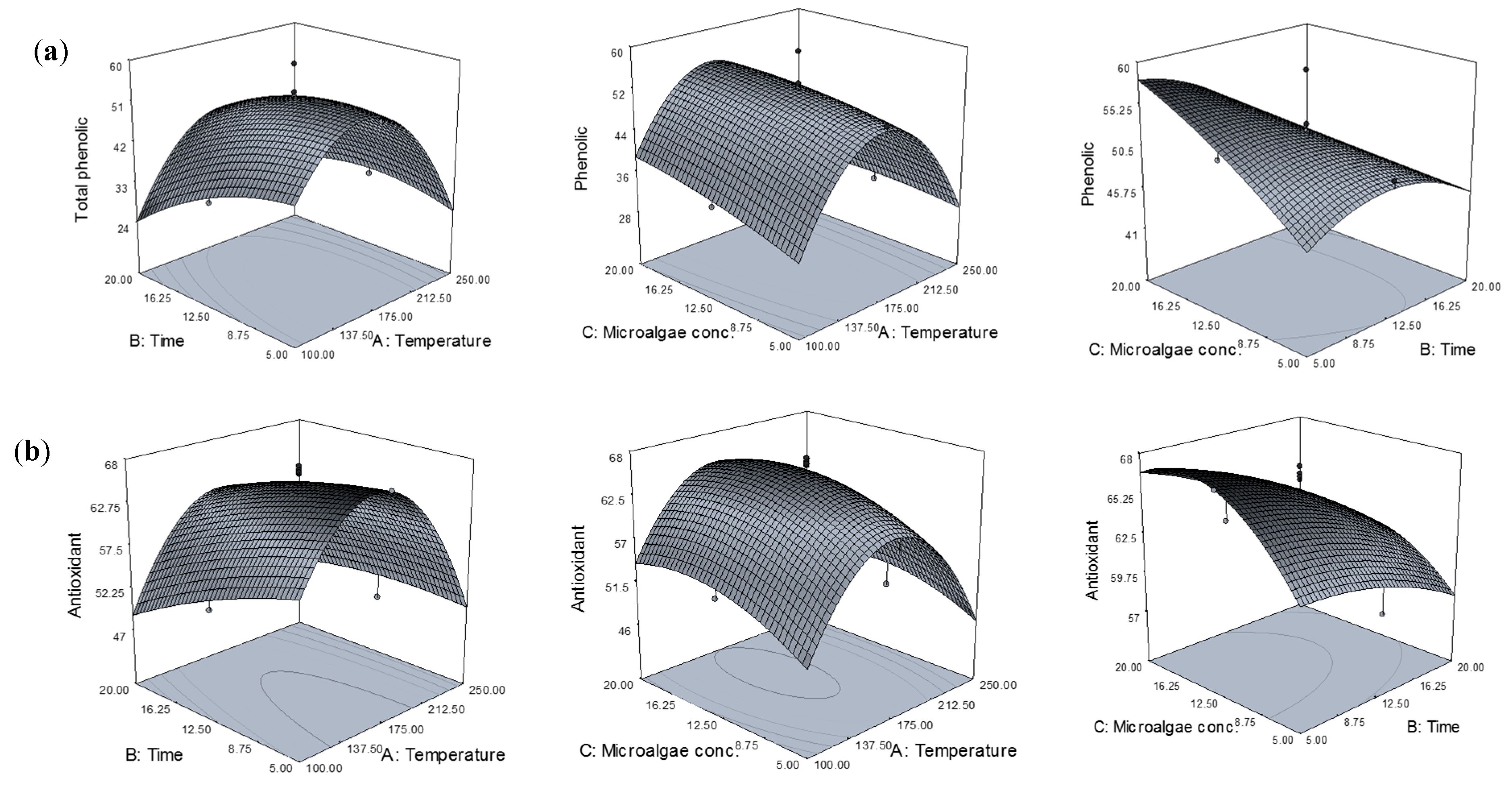
Three-dimensional representations of the response surfaces generated by the model are shown in
Figure 1. For the process with three variables, where two variables are illustrated in three-dimensional surface plots, the third variable is kept at the centre point. Varying the temperature during SW treatment allowed the solubility of different phenolic compounds to be modified. To obtain the highest phenolic content, it was important to determine appropriate operating conditions.
Figure 1a shows the phenolic content as a function of extraction temperature, time, and microalgae concentration. The temperature had a large effect on the phenolic content extracted. Moreover, water at different temperatures during the SW extraction process had different dielectric constants, resulting in different polarities. Therefore, results might be related to water polarity and the solubility of phenolic compounds in
Chlorella sp. Increasing temperature led to a gradual increase in phenolic content above 100 °C, reaching a maximum at around 175 °C. Therefore, increasing the temperature improved total phenolic yield. Indeed, a higher temperature increased the solubility and diffusion coefficients of phenolic compounds, allowing a higher extraction rate [
18]. Furthermore, during the process, the viscosity and surface tension of the water were also reduced, whereas diffusivity is increased, allowing better penetration of the solvent into the matrix and enhancing the extraction process, in terms of both efficiency and speed. Therefore, mass transfer from the solid phase to the SW was improved. However, the amount of phenolic content began to decline above 175 °C due to some families of phenolic compounds becoming denatured beyond certain temperatures. In addition, previous studies have reported that, degradation of phenolic compounds was observed above 180 °C [
10]. Therefore, a temperature of 175 °C was considered adequate for the extraction of phenolic compounds from
Chlorella sp.In terms of extraction time, it has been reported that prolonged extraction times favor the extraction of the phenolic compounds. This might be due to the time requirement for the exposure of solute or compounds to the release medium when the water penetrates into the
Chlorella sp., dissolves the solute, and then diffuses out from the
Chlorella sp. However, in this study, extending the period of extraction time from 5 to 20 min led to a decrease in phenolic compound extraction. This might be due to the applied high extraction temperature causing decomposition of the phenolic compounds and structural destruction during extended extraction time. It has been reported that some families of phenolic compounds can be denatured beyond a certain temperature value [
19]. In addition, due to the applied extraction high temperature, longer extraction times increase the risk of phenolic reduction by increasing the loss of phenolic by oxidation [
20]. Therefore, 5 min was favored as the extraction time for phenolic compounds using SW extraction.
As shown in
Figure 1a, microalgae concentration also has a significant effect on the amount of phenolic content extracted. The amount of phenolic compounds extracted increased during extraction when increasing the microalgae concentration from 5 to 20 wt. %. This might be due to an increased rate of compound mass transfer resulting from the increased microalgae concentration during the extraction. This phenomenon was attributed to the mass transfer principle. Higher solid-to-solvent ratios gave higher concentration gradients, leading to the increased diffusion and extraction yield of phenolic compounds. Therefore, a microalgae concentration of 20 wt. % was sufficient for extracting the phenolic compounds from
Chlorella sp. by SW.
Most of the valuable characteristics of phenolic compounds are associated with their antioxidant activities [
21]. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay has been used extensively in antioxidant assays because it is fast, reliable, and reproducible, and can be used to test the general antioxidant activity of various natural substances, including algae extracts, in vitro [
22]. Therefore, the DPPH radical scavenging assay has been used to monitor the capacity of extracted compounds to scavenge free radicals in hydrophilic systems. The effects of extraction temperature, time, and microalgae concentration on DPPH radical scavenging activity are shown in
Figure 1b.
The effect of extraction temperature on the antioxidant activity of extracts was similar that on the phenolic content extracted. The antioxidant activity increased as the temperature increased from 100 to 175 °C. Certain antioxidant compounds might be mobilized at high temperatures, while possibly promoting concurrent decomposition of antioxidants already mobilized at lower temperatures. It has been stated that the rate of extraction of thermally stable antioxidants at elevated temperatures is higher than the rate of decomposition of less soluble antioxidants [
23]. This was implied by the relatively high percent of inhibition of the extracts obtained at higher temperatures. Increasing the temperature above 175 °C during SW treatment reduced the antioxidant activity. These results showed that mobilization of the antioxidants from the substrate (algae) might occur up to a certain level, followed by their possible loss due to decomposition at higher temperatures. The antioxidant activity of the extracts was high after extraction for 5 min, but declined as the extraction time increased. As mentioned earlier, this decline was due to the longer exposure of active compounds to high temperatures that caused decomposition and structural destruction during longer extraction times. Longer extraction times increase the risk of phenolic oxidation unless reducing agents are added to the solvent system [
24]. Therefore, it cause the reduction in the percent of inhibition of the extracts. Microalgae concentration during SW extraction had a similar effect on the antioxidant capacity, but with a smaller impact. The results obtained in the present study also correlated with the amount of phenolic content extracted from
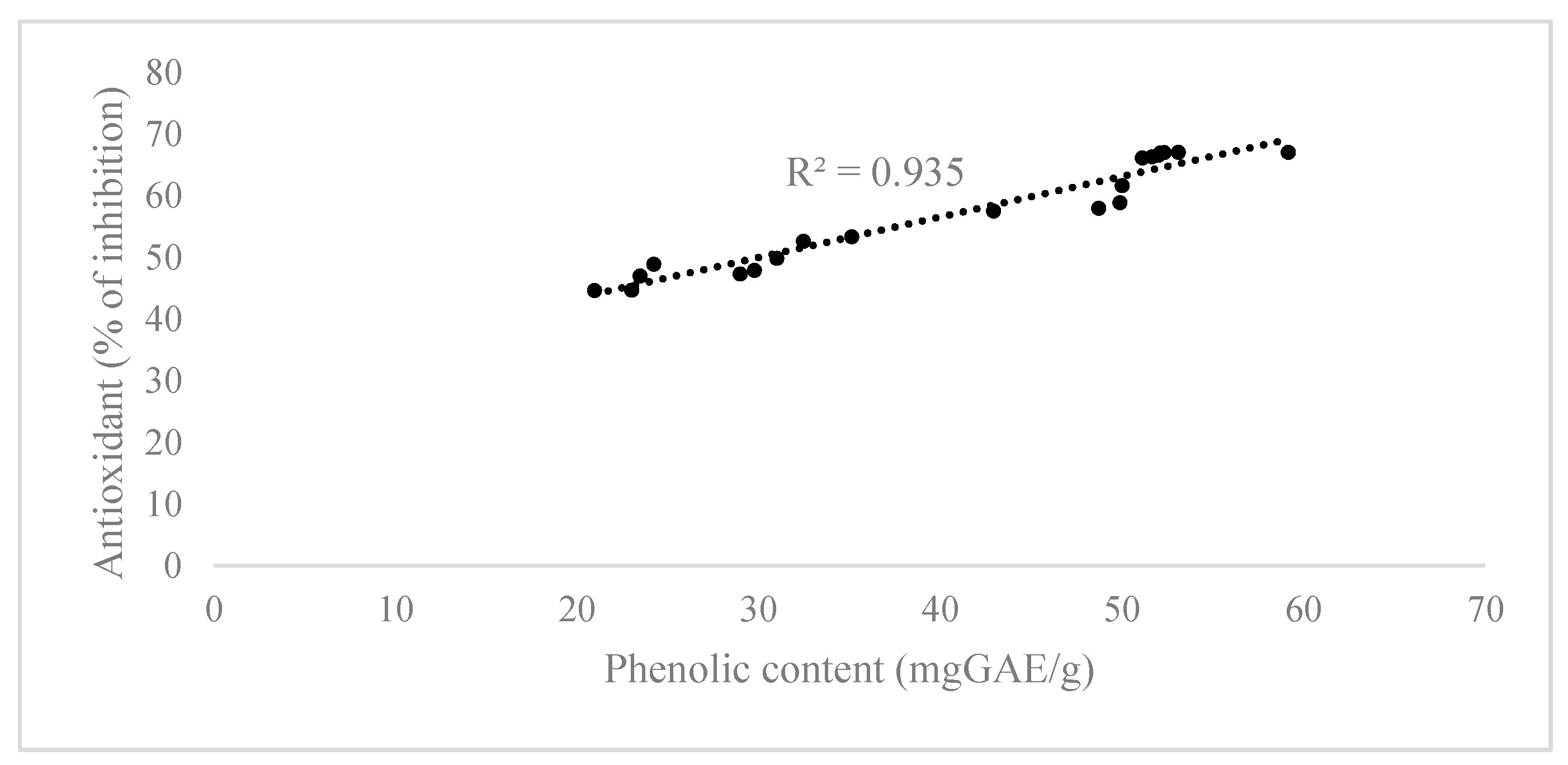
Chlorella sp., which could be responsible for the antioxidant activity observed. Based on these results, it could be concluded that the obtained
Chlorella sp. extract with the highest phenolic content also showed the highest antioxidant activity.