The [Mo6Cl14]2− Cluster is Biologically Secure and Has Anti-Rotavirus Activity In Vitro
Abstract
:1. Introduction
2. Results and Discussion
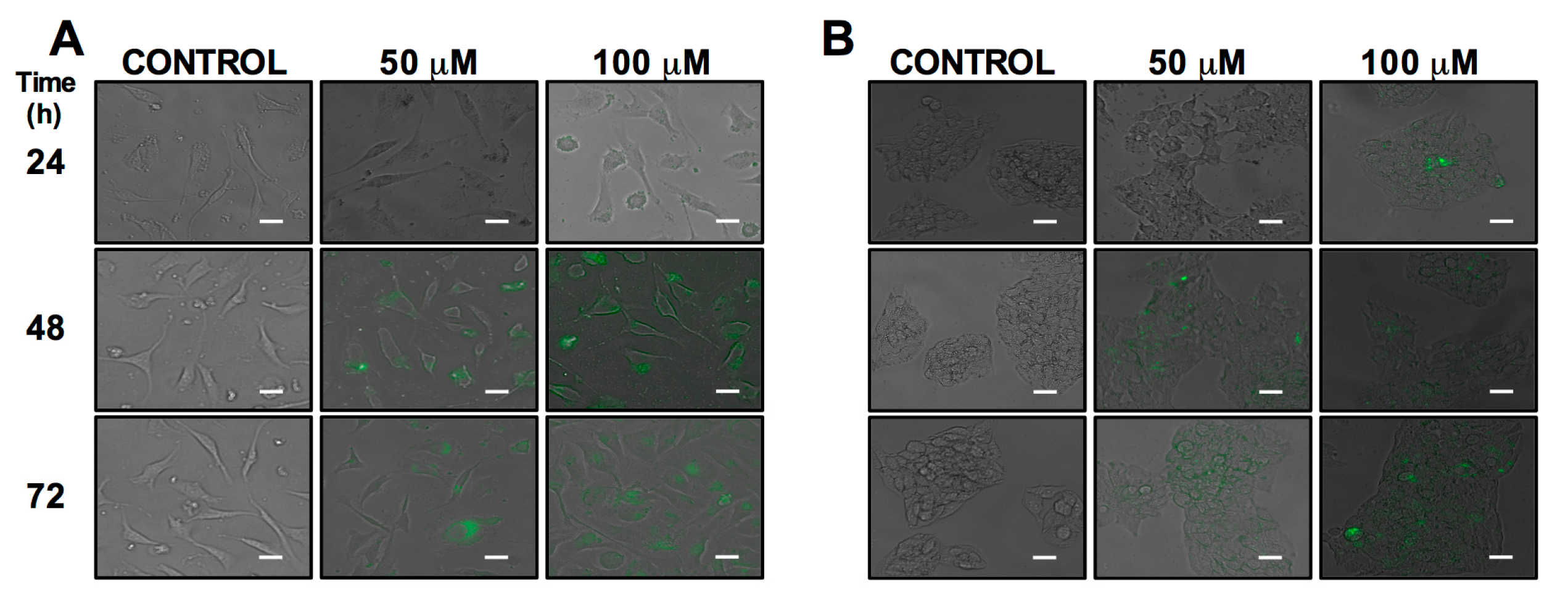
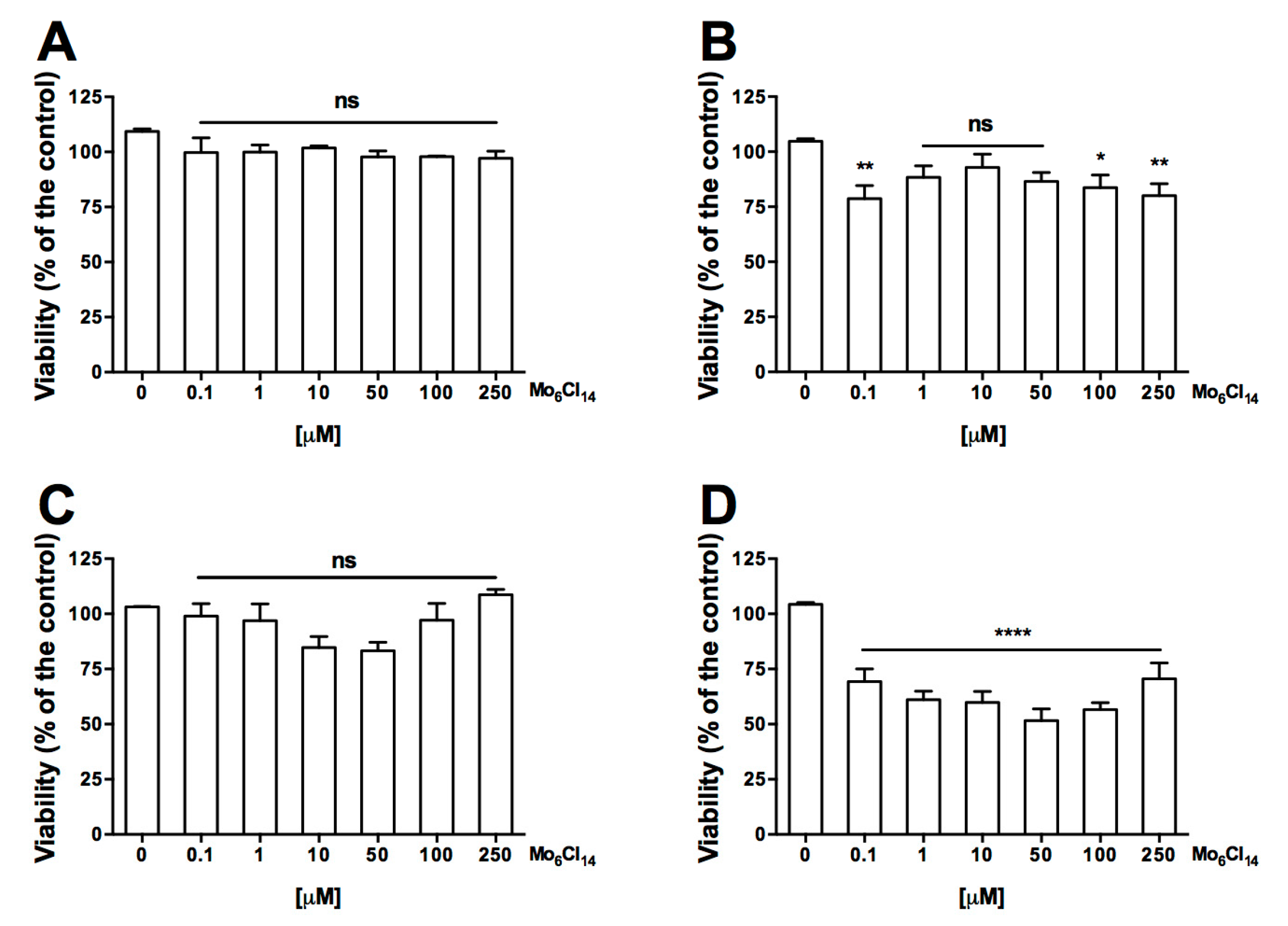
2.1. Cell Uptake and Viability
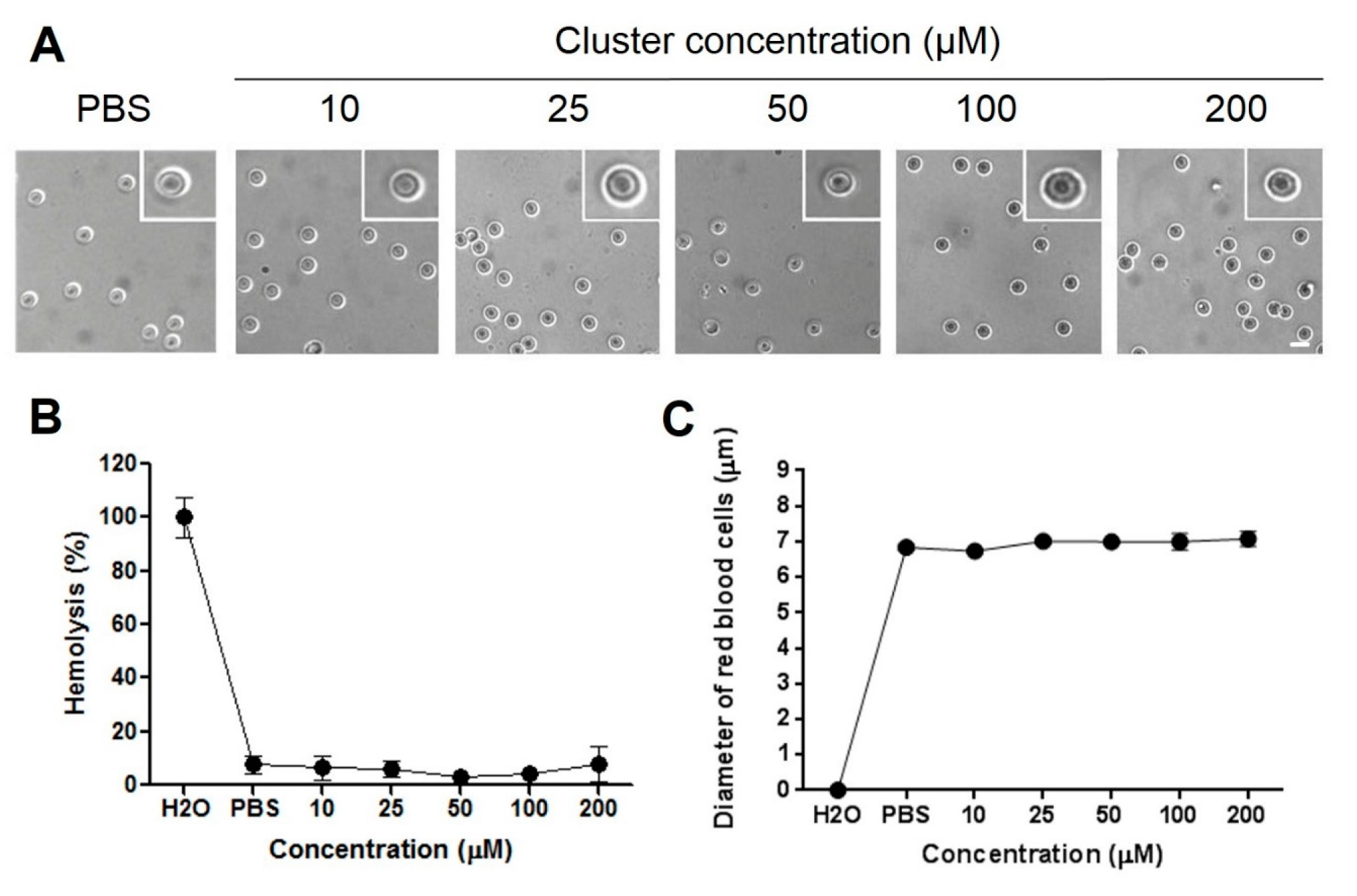
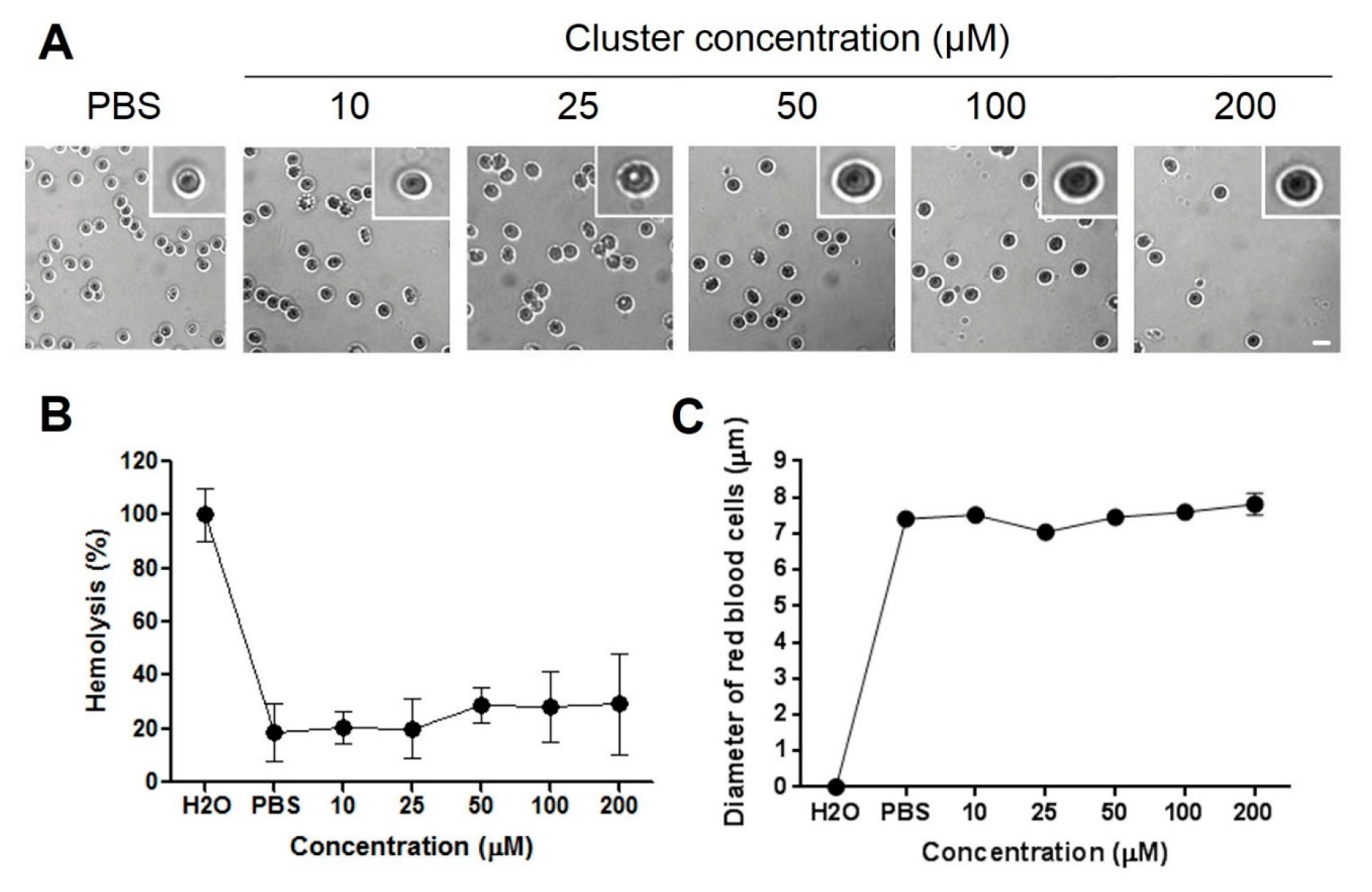
2.2. Hemolysis and Morphological Changes
2.3. Protein Binding
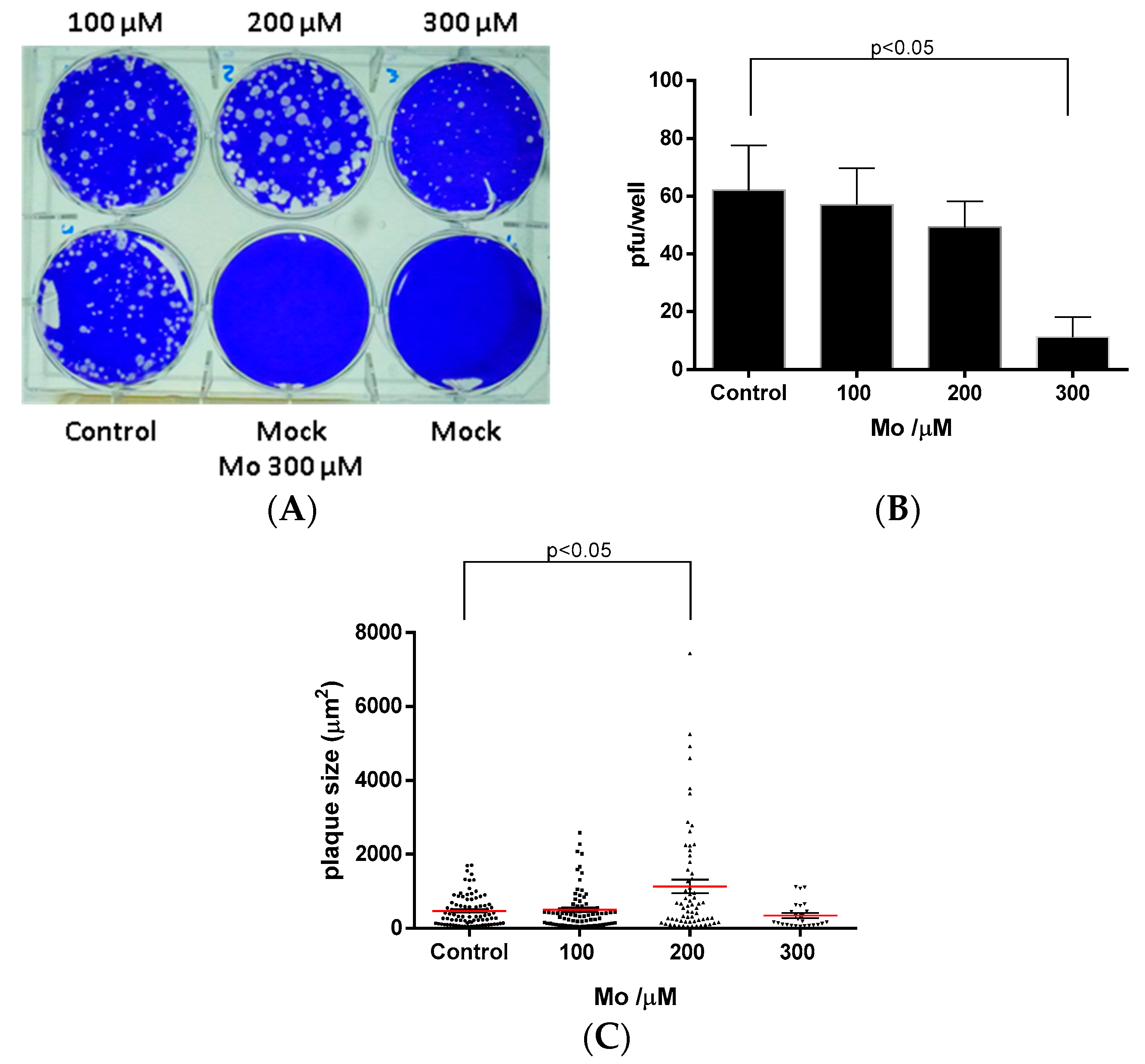
2.4. Antiviral Activity
3. Materials and Methods
3.1. Preparation of [Mo6Cl14]2− via Reduction of MoCl5 with Bismuth
3.2. Cell Culture
3.3. Cell Viability Assay
3.4. Epifluorescence Microscope in Live Cells
3.5. Human Blood Samples and Preparation of Red Blood Cells
3.6. Analysis of Red Blood Cells Morphology
3.7. Test of Hemolysis
3.8. Albumin Interaction Studies
3.9. Anti-Rotavirus Activity
3.10. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zarate, X.; Schott, E.; Alvarado-Soto, L.; Ramirez-Tagle, R. A family of octahedral molybdenum cluster complexes [Mo6Cl8(H2O)n(OH)6−n]n−2 with n = 0–6 as a pH-sensors: A theoretical study. Chem. Phys. Lett. 2013, 567, 39–42. [Google Scholar] [CrossRef]
- Ramirez-Tagle, R.; Arratia-Pérez, R. Electronic structure and molecular properties of the [Mo6X8L6]2−; X = Cl, Br, I; L = F, Cl, Br, I clusters. Chem. Phys. Lett. 2008, 460, 438–441. [Google Scholar] [CrossRef]
- Ramirez-Tagle, R.; Arratia-Pérez, R. Pyridine as axial ligand on the [Mo6Cl8]4+ core switches off luminescence. Chem. Phys. Lett. 2009, 475, 232–234. [Google Scholar] [CrossRef]
- Szczepura, L.F.; Edwards, J.A.; Cedeño, D.L. Luminescent Properties of Hexanuclear Molybdenum(II) Chloride Clusters Containing Thiolate Ligands. J. Clust. Sci. 2008, 20, 105–112. [Google Scholar] [CrossRef]
- Kirakci, K.; Kubát, P.; Dušek, M.; Fejfarová, K.; Šícha, V.; Mosinger, J.; Lang, K. A Highly Luminescent Hexanuclear Molybdenum Cluster—A Promising Candidate toward Photoactive Materials. Eur. J. Inorg. Chem. 2012, 2012, 3107–3111. [Google Scholar] [CrossRef]
- Cordier, S.; Dorson, F.; Grasset, F.; Molard, Y.; Fabre, B.; Haneda, H.; Sasaki, T.; Mortier, M.; Ababou-Girard, S.; Perrin, C. Novel Nanomaterials Based on Inorganic Molybdenum Octahedral Clusters. J. Clust. Sci. 2008, 20, 9–21. [Google Scholar] [CrossRef]
- Kirakci, K.; Kubát, P.; Langmaier, J.; Polívka, T.; Fuciman, M.; Fejfarová, K.; Lang, K. A comparative study of the redox and excited state properties of (nBu4N)2[Mo6X14] and (nBu4N)2[Mo6X8(CF3COO)6] (X = Cl, Br, or I). Dalton Trans. 2013, 42, 7224–7232. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.N.; Baker, G.L.; Ruud, C.; Nocera, D.G. Fiber-optic oxygen sensor using molybdenum chloride cluster luminescence. Appl. Phys. Lett. 1999, 75, 2885–2887. [Google Scholar] [CrossRef]
- Inumaru, K.; Anzai, A.; Kikudome, T.; Harada, M.; Sakai, H.; Ide, Y.; Sano, T.; Yamanaka, S. Molybdenum Cluster Halide Compound Mo6Cl12(OH2)2 with Six-Handed Linkage Hydrogen Bonding. Bull. Chem. Soc. Jpn. 2011, 84, 379–385. [Google Scholar] [CrossRef]
- Ramirez-Tagle, R.; Arratia-Pérez, R. The luminescent [Mo6X8(NCS)6]2− (X = Cl, Br, I) clusters?: A computational study based on time-dependent density functional theory including spin-orbit and solvent-polarity effects. Chem. Phys. Lett. 2008, 455, 38–41. [Google Scholar] [CrossRef]
- Krasilnikova, A.A.; Shestopalov, M.A.; Brylev, K.A.; Kirilova, I.A.; Khripko, O.P.; Zubareva, K.E.; Khripko, Y.I.; Podorognaya, V.T.; Shestopalova, L.V.; Fedorov, V.E.; et al. Prospects of molybdenum and rhenium octahedral cluster complexes as X-ray contrast agents. J. Inorg. Biochem. 2015, 144, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Gasser, G. Metal Complexes and Medicine: A Successful Combination. Chimia (Aarau) 2015, 69, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Baggaley, E.; Weinstein, J.A.; Williams, J.A.G. Lighting the way to see inside the live cell with luminescent transition metal complexes. Coord. Chem. Rev. 2012, 256, 1762–1785. [Google Scholar] [CrossRef]
- Ramírez-Tagle, R.; Arratia-Pérez, R. Electronic Structure and Molecular Properties of the Mixed Rhenium-Molybdenum [Re6−xMoxS8(CN)6]q−, (x = 0 to 6) Clusters. J. Clust. Sci. 2008, 20, 159–164. [Google Scholar] [CrossRef]
- Echeverría, C.; Becerra, A.; Nuñez-Villena, F.; Muñoz-Castro, A.; Stehberg, J.; Zheng, Z.; Arratia-Perez, R.; Simon, F.; Ramírez-Tagle, R. The paramagnetic and luminescent [Re6Se8I6]3− cluster. Its potential use as an antitumoral and biomarker agent. New J. Chem. 2012, 36, 927–932. [Google Scholar] [CrossRef]
- Rojas-Mancilla, E.; Oyarce, A.; Verdugo, V.; Zheng, Z.; Ramírez-Tagle, R. The Cluster [Re6Se8I6]3− Induces Low Hemolysis of Human Erythrocytes in Vitro: Protective Effect of Albumin. Int. J. Mol. Sci. 2015, 16, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-J.; Brylev, K.A.; Xu, J.-Z.; Mironov, Y.V.; Fedorov, V.E.; Sohn, Y.S.; Kim, S.-J.; Choy, J.-H. Cellular uptake and cytotoxicity of octahedral rhenium cluster complexes. J. Inorg. Biochem. 2008, 102, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Love, S.A.; Thompson, J.W.; Haynes, C.L. Development of screening assays for nanoparticle toxicity assessment in human blood: Preliminary studies with charged Au nanoparticles. Nanomedicine 2012, 7, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Sethu, S.; Vadukumpully, S.; Zhong, S.; Lim, C.T.; Hande, M.P.; Valiyaveettil, S. Investigations on the Structural Damage in Human Erythrocytes Exposed to Silver, Gold, and Platinum Nanoparticles. Adv. Funct. Mater. 2010, 20, 1233–1242. [Google Scholar] [CrossRef]
- Bern, M.; Sand, K.M.K.; Nilsen, J.; Sandlie, I.; Andersen, J.T. The role of albumin receptors in regulation of albumin homeostasis: Implications for drug delivery. J. Control Release 2015, 211, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, Q.-H.; Li, C.-R.; Wang, Z.; Ma, J.-J.; Zang, X.-H.; Qin, N.-X. Interaction of tetrandrine with human serum albumin: A fluorescence quenching study. Anal. Sci. 2007, 23, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Vicente, C.; de Haro, C.; Bautista, D. Novel bis-C,N-cyclometalated iridium(III) thiosemicarbazide antitumor complexes: Interactions with human serum albumin and DNA, and inhibition of cathepsin B. Inorg. Chem. 2013, 52, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Fiene, A.; Li, W.; Hanck, T.; Brylev, K.A.; Fedorov, V.E.; Lecka, J.; Haider, A.; Pietzsch, H.J.; Zimmermann, H.; et al. Polyoxometalates—Potent and selective ecto-nucleotidase inhibitors. Biochem. Pharmacol. 2015, 93, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Rhule, J.T.; Hill, C.L.; Judd, D.A.; Schinazi, R.F. Polyoxometalates in Medicine. Chem. Rev. 1998, 98, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Molitor, C.; Bijelic, A.; Rompel, A. In situ formation of the first proteinogenically functionalized [TeW6O24O2(Glu)]7− structure reveals unprecedented chemical and geometrical features of the Anderson-type cluster. Chem. Commun. 2016, 52, 12286–12289. [Google Scholar] [CrossRef] [PubMed]
- Das, J.K.; Bhutta, Z.A.; Lanata, C.F.; Fischer-Walker, C.L.; Olascoaga, A.C.; Torres, C.X.; Aryee, M.J.; Black, R.E.; Mi, K.; Ou, X.; et al. Comparative analysis of the immunogenicity of monovalent and multivalent rotavirus immunogens. PLoS ONE 2013, 15, 392–398. [Google Scholar] [CrossRef]
- Das, J.K.; Bhutta, Z.A. Global challenges in acute diarrhea. Curr. Opin. Gastroenterol. 2016, 32, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Knasmuller, S.; Schwab, C.; Uhl, M.; Mersch-Sundermann, V.; Williamson, G. Use of metabolically competent human hepatoma cells for the detection of mutagens and antimutagens. Mutat. Res. 1998, 402, 185–202. [Google Scholar] [CrossRef]
- Mersch-Sundermann, V.; Knasmüller, S.; Wu, X.J.; Darroudi, F.; Kassie, F. Use of a human-derived liver cell line for the detection of cytoprotective, antigenotoxic and cogenotoxic agents. Toxicology 2004, 198, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, S.M.; Chaplin, D.J.; Lee, F.; Stratford, M.R.L.; Locke, R.J.; Vojnovic, B.; Tozer, G.M. Effects of combretastatin A4 phosphate on endothelial cell morphology in vitro and relationship to tumour vascular targeting activity in vivo. Anticancer Res. 2001, 21, 93–102. [Google Scholar] [PubMed]
- Kou, L.; Sun, J.; Zhai, Y.; He, Z. The endocytosis and intracellular fate of nanomedicines: Implication for rational design. Asian J. Pharm. Sci. 2013, 8, 1–8. [Google Scholar] [CrossRef]
- Yeung, T.; Gilbert, G.E.; Shi, J.; Silvius, J.; Kapus, A.; Grinstein, S. Membrane Phosphatidylserine Regulates Surface Charge and Protein Localization. Science 2008, 319, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Perumal, O.P.; Inapagolla, R.; Kannan, S.; Kannan, R.M. The effect of surface functionality on cellular trafficking of dendrimers. Biomaterials 2008, 29, 3469–3476. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Qian, J.; Zhou, H.; Gan, Q.; Tang, W.; Lu, J.; Yuan, Y.; Liu, C. In vitro cytotoxicity and induction of apoptosis by silica nanoparticles in human HepG2 hepatoma cells. Int. J. Nanomed. 2011, 6, 1889–1901. [Google Scholar] [CrossRef]
- Liu, X; Sun, J. Silica nanoparticles induce apoptosis in human endothelial cells via reactive oxygen species. In Proceedings of the 3rd International Nanoelectronics Conference (INEC), HongKong, China, 3–8 January 2010; pp. 824–825. [Google Scholar]
- Feng, W.; Chen, L.; Qin, M.; Zhou, X.; Zhang, Q.; Miao, Y.; Qiu, K.; Zhang, Y.; He, C. Flower-like PEGylated MoS2 nanoflakes for near-infrared photothermal cancer therapy. Sci. Rep. 2015, 5, 17422. [Google Scholar] [CrossRef] [PubMed]
- Šebestová, L.; Havelek, R.; Řezáčová, M.; Honzíček, J.; Kročová, Z.; Vinklárek, J. Study of antitumor effect of selected vanadium and molybdenum organometallic complexes in human leukemic T-cells. Chem. Biol. Interact. 2015, 242, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alshamsan, A.; Majeed Khan, M.A.; Alrokayan, S.A. Antioxidative and cytoprotective response elicited by molybdenum nanoparticles in human cells. J. Colloid Interface Sci. 2015, 457, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A.; Guan, T.S.; Haque, R.A.; Khadeer Ahamed, M.B.; Abdul Majid, A.M.S. Mononuclear dioxomolybdenum(VI) thiosemicarbazonato complexes: Synthesis, characterization, structural illustration, in vitro DNA binding, cleavage, and antitumor properties. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 136 Pt C, 1335–1348. [Google Scholar] [CrossRef] [PubMed]
- Solovieva, A.O.; Vorotnikov, Y.A.; Trifonova, K.E.; Efremova, O.A.; Krasilnikova, A.A.; Brylev, K.A.; Vorontsova, E.V.; Avrorov, P.A.; Shestopalova, L.V.; Poveshchenko, A.F.; et al. Cellular internalisation, bioimaging and dark and photodynamic cytotoxicity of silica nanoparticles doped by {Mo6I8}4+ metal clusters. J. Mater. Chem. B 2016, 4, 4839–4846. [Google Scholar] [CrossRef]
- Hamoudeh, M.; Fessi, H.; Mehier, H.; Faraj, A.A.; Canet-Soulas, E. Dirhenium decacarbonyl-loaded PLLA nanoparticles: Influence of neutron irradiation and preliminary in vivo administration by the TMT technique. Int. J. Pharm. 2008, 348, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, E.M.; Szczupak, Ł.; Wong, S.; Skiba, J.; Guśpiel, A.; Solecka, J.; Vrček, V.; Kowalski, K.; Chen, Y. Antibacterial Properties of Metallocenyl-7-ADCA Derivatives and Structure in Complex with CTX-M β-Lactamase. Organometallics 2017, 36, 1673–1676. [Google Scholar] [CrossRef]
- Shestopalov, M.A.; Zubareva, K.E.; Khripko, O.P.; Khripko, Y.I.; Solovieva, A.O.; Kuratieva, N.V.; Mironov, Y.V.; Kitamura, N.; Fedorov, V.E.; Brylev, K.A. The first water-soluble hexarhenium cluster complexes with a heterocyclic ligand environment: Synthesis, luminescence, and biological properties. Inorg. Chem. 2014, 53, 9006–9013. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.W.-T.; Louie, M.-W.; Li, S.P.-Y.; Liu, H.-W.; Chan, B.T.-N.; Lam, T.C.-Y.; Lin, A.C.-C.; Cheng, S.-H.; Lo, K.K.-W. Emissive behavior, cytotoxic activity, cellular uptake, and PEGylation properties of new luminescent rhenium(I) polypyridine poly(ethylene glycol) complexes. Inorg. Chem. 2012, 51, 13289–13302. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, N.K.; Gallud, A.; Ytterberg, A.J.; Chernobrovkin, A.; Aranzaes, J.R.; Astruc, D.; Antipov, A.; Fedutik, Y.; Fadeel, B.; Zubarev, R.A. Cytotoxic and Proinflammatory Effects of Metal-Based Nanoparticles on THP-1 Monocytes Characterized by Combined Proteomics Approaches. J. Proteome Res. 2017, 16, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhang, M.; Xia, B.; Xiong, J.; Zong, Y.; Hu, G.; Zhang, C. Effects of Molybdenum or/and Cadmium on mRNA Expression Levels of Inflammatory Cytokines and HSPs in Duck Spleens. Biol. Trace Elem. Res. 2016, 170, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.; Li, K.; Riegler, V.; Barrack, R.L. Patient-Reported Metal Allergy: A Risk Factor for Poor Outcomes After Total Joint Arthroplasty? J. Arthroplasty 2016, 31, 1910–1915. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, H.M.; Xue, Y.; Robinson, C.D.; Canalizo-Hernandez, M.A.; Marvin, R.G.; Kelly, R.A.; Mondragon, A.; Penner-Hahn, J.E.; O’Halloran, T.V. Tetrathiomolybdate Inhibits Copper Trafficking Proteins Through Metal Cluster Formation. Science 2010, 327, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Vyskočil, A.; Viau, C. Assessment of molybdenum toxicity in humans. J. Appl. Toxicol. 1999, 19, 185–192. [Google Scholar] [CrossRef]
- Aubert, T.; Burel, A.; Esnault, M.-A.; Cordier, S.; Grasset, F.; Cabello-Hurtado, F. Root uptake and phytotoxicity of nanosized molybdenum octahedral clusters. J. Hazard. Mater. 2012, 219–220, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Keller, A. Stability and Aggregation of Metal Oxide Nanoparticles in Natural Aqueous Matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-E.; Kwak, J.-W.; Park, J.W. Nanotechnology for early cancer detection. Sensors 2010, 10, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Kubát, P.; Kučeráková, M.; Šícha, V.; Gbelcová, H.; Lovecká, P.; Grznárová, P.; Ruml, T.; Lang, K. Water-soluble octahedral molybdenum cluster compounds Na2[Mo6I8(N3)6] and Na2[Mo6I8(NCS)6]: Syntheses, luminescence, and in vitro studies. Inorg. Chim. Acta 2016, 441, 42–49. [Google Scholar] [CrossRef]
- Hay, D.N.T.; Adams, J.A.; Carpenter, J.; DeVries, S.L.; Domyancich, J.; Dumser, B.; Goldsmith, S.; Kruse, M.A.; Leone, A.; Moussavi-Harami, F.; et al. Facile reduction of early transition metal halides with nonconventional, mild reductants. 6. A new, lower-temperature, solid-state synthesis of the cluster hexamolybdenum dodecachloride Mo6Cl12 from MoCl5, via chloromolybdic acid, (H3O)2[Mo6(μ3-Cl)8Cl6]·6H2O. Inorg. Chim. Acta 2004, 357, 644–648. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
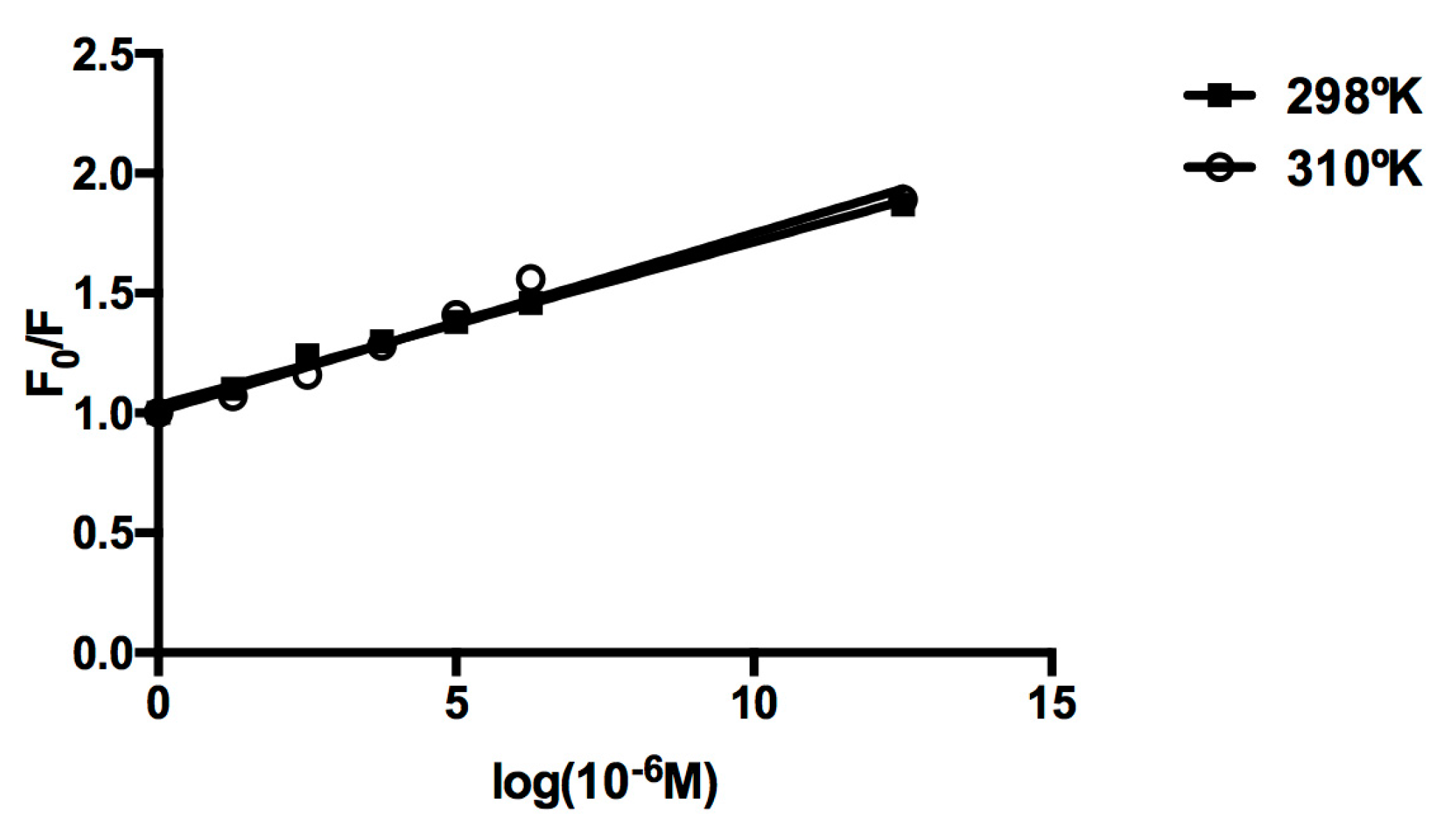
| T (°K) | Ksv (L mol−1) | R |
|---|---|---|
| 298 | 0.12 | 0.89 |
| 310 | 0.14 | 0.93 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Mancilla, E.; Oyarce, A.; Verdugo, V.; Morales-Verdejo, C.; Echeverria, C.; Velásquez, F.; Chnaiderman, J.; Valiente-Echeverría, F.; Ramirez-Tagle, R. The [Mo6Cl14]2− Cluster is Biologically Secure and Has Anti-Rotavirus Activity In Vitro. Molecules 2017, 22, 1108. https://doi.org/10.3390/molecules22071108
Rojas-Mancilla E, Oyarce A, Verdugo V, Morales-Verdejo C, Echeverria C, Velásquez F, Chnaiderman J, Valiente-Echeverría F, Ramirez-Tagle R. The [Mo6Cl14]2− Cluster is Biologically Secure and Has Anti-Rotavirus Activity In Vitro. Molecules. 2017; 22(7):1108. https://doi.org/10.3390/molecules22071108
Chicago/Turabian StyleRojas-Mancilla, Edgardo, Alexis Oyarce, Viviana Verdugo, Cesar Morales-Verdejo, Cesar Echeverria, Felipe Velásquez, Jonas Chnaiderman, Fernando Valiente-Echeverría, and Rodrigo Ramirez-Tagle. 2017. "The [Mo6Cl14]2− Cluster is Biologically Secure and Has Anti-Rotavirus Activity In Vitro" Molecules 22, no. 7: 1108. https://doi.org/10.3390/molecules22071108




