Chemical Composition, Antibacterial and Antifungal Activities of Crude Dittrichia viscosa (L.) Greuter Leaf Extracts
Abstract
:1. Introduction
2. Results and Discussion
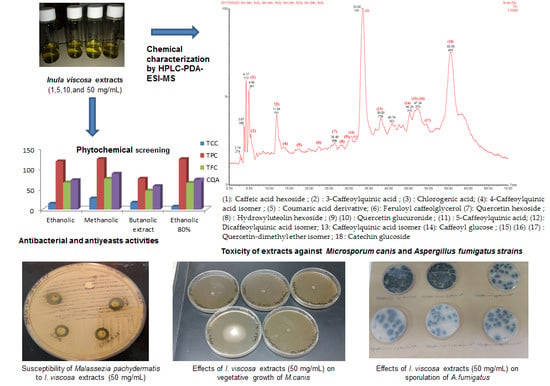
2.1. Phytochemical Screening
2.2. Phenolic Profile of D. viscosa Extracts
2.3. Antibacterial, Anti-Candida and Antifungal Activity of D. viscosa Extracts
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Plant Material
3.3. Preparation of Extracts
3.4. Phytochemical Screening
3.4.1. Condensed Tannins Content (CTC)
3.4.2. Total Phenol Content (TPC)
3.4.3. Total Flavonoid Content (TFC)
3.4.4. Caffeoylquinic Acid (CQC) Content
3.5. Characterization of PhenolicCcompounds by HPLC-PDA-ESI-MS
3.6. Antibacterial and Antifungal Activities
3.6.1. Bacterial Strains
3.6.2. Fungal Strains
Candida spp. Strains
Malassezia spp. Strains
Aspergillus fumigatusstrains
Microsporum canis strains
3.7. Determination of Antibacterial, Anti-Candida and Antifungal Activity of I. viscosa Extract
3.7.1. Toxicity Assay
3.7.2. Effect of D. viscosa Extract on Fungal Germination
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rossolini, G.M.; Arena, F.; Pecile, P.; Pollini, S. Update on the antibiotic resistance crisis. Curr. Opin. Pharmacol. 2014, 18, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Çelik, T.A.; Aslantürk, Ö.S. Evaluation of cytotoxicity and genotoxicity of Inula viscosa leaf extracts with Allium test. J. Biomed. Biotechnol. 2010, 2010, 189252. [Google Scholar]
- Parolin, P.; Scotta, M.I.; Bresch, I. Biology of Dittrichia viscosa, a Mediterranean ruderal plant. Фyton. 2013, 83, 251–262. [Google Scholar]
- Mahmoudi, H.; Hosni, K.; Zaouali, W.; Amri, I.; Zargouni, H.; Hamida, N.B.; Kaddour, R.; Hamrouni, L.; Nasri, M.B.; Ouerghi, Z. Comprehensive Phytochemical Analysis, Antioxidant and Antifungal Activities of Inula viscosa Aiton Leaves. J. Food Saf. 2015, 36, 77–88. [Google Scholar] [CrossRef]
- Omezzine, F.; Daami-Remadi, M.; Rinez, A.; Ladhari, A.; Haouala, R. In vitro assessment of Inula spp. organic extracts for their antifungal activity against some pathogenic and antagonistic fungi. Afr. J. Microbiol. Res. 2011, 5, 3527–3531. [Google Scholar]
- Assaf, A.M.; Amro, B.I.; Mashallah, S.; Haddadin, R.N. Antimicrobial and anti-inflammatory potential therapy for opportunistic microorganisms. J. Infect. Dev. Ctries 2016, 10, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Duek, L.; Kaufman, G.; Ulman, Y.; Berdicevsky, I. The pathogenesis of dermatophyte infections in human skin sections. J. Infect. 2004, 48, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.B.; Shulman, S.A. Reports of zoonotic disease outbreaks associated with animal exhibits and availability of recommendations for preventing zoonotic disease transmission from animals to people in such settings. J. Am. Vet. Med. Assoc. 2004, 224, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Raad, I.I.; Graybill, J.R.; Bustamante, A.B.; Cornely, O.A.; Gaona-Flores, V.; Afif, C.; Graham, D.R.; Greenberg, R.N.; Hadley, S.; Langston, A.; et al. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin. Infect. Dis. 2006, 42, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Cafarchia, C.; Figueredo, L.A.; Otranto, D. Fungal diseases of horses. Vet. Microbiol. 2013, 167, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Cafarchia, C.; Figueredo, L.A.; Iatta, R.; Montagna, M.T.; Otranto, D. In vitro antifungal susceptibility of Malassezia pachydermatis from dogs with and without skin lesions. Vet. Microbiol. 2012, 155, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Cafarchia, C.; Figueredo, L.A.; Iatta, R.; Colao, V.; Montagna, M.T.; Otranto, D. In vitro evaluation of Malassezia pachydermatis susceptibility to azole compounds using E-test and CLSI microdilution methods. Med. Mycol. 2012, 50, 795–801. [Google Scholar] [PubMed]
- Seyedmousavi, S.; Guillot, J.; Arn, P.; De Hoog, G.S.; Mouton, J.W.; Melchers, W.J.G.; Verweij, P.E. Aspergillus and aspergilloses in wild and domestic animals: A global health concern with parallels to human disease. Med. Mycol. 2015, 53, 765–797. [Google Scholar] [CrossRef] [PubMed]
- Sefi, M.; Fetoui, H.; Makni, M.; Zeghal, N. Mitigating effects of antioxidant properties of Artemisia campestris leaf extract on hyperlipidemia, advanced glycation end products and oxidative stress in alloxan-induced diabetic rats. Food Chem. Toxicol. 2010, 48, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Trimech, I.; Weiss, E.K.; Chedea, V.S.; Marin, D.; Detsi, A.; Ioannou, E.; Roussis, V.; Kefalas, P. Evaluation of anti-oxidant and acetylcholinesterase activity and identification of polyphenolics of the invasive weed Dittrichia viscosa. Phytochem. Anal. 2014, 25, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.S.; Jayaprakasha, G. Antioxidant and Antibacterial Activities of Punica granatum Peel Extracts. J. Food Sci. 2003, 68, 1473–1477. [Google Scholar] [CrossRef]
- Geng, H.-M.; Zhang, D.-Q.; Zha, J.-P.; Qi, J.-L. Simultaneous HPLC Determination of Five Flavonoids in Flos Inulae. Chromatographia 2007, 66, 271–275. [Google Scholar] [CrossRef]
- Naeem, I.; Saddiqe, Z.; Patel, A.; Hellio, C. Analysis of flavonoid and antimicrobial activity of extracts of Hypericum perforatum. Asian J. Chem. 2010, 22, 3596–3600. [Google Scholar]
- Danino, O.; Gottlieb, H.E.; Grossman, S.; Bergman, M. Antioxidant activity of 1,3-dicaffeoylquinic acid isolated from Inula viscosa. Food Res. Int. 2009, 42, 1273–1280. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Ellepola, K.; Seneviratne, C.J.; Koga-Ito, C.Y. Potential use of phenolic acids as anti-Candida agents: A review. Front. Microbiol. 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Otang, W.M.; Grierson, D.S.; Ndip, R.N. The effect of the acetone extract of Arctotis arctotoides (Asteraceae) on the growth and ultrastructure of some opportunistic fungi associated with HIV/AIDS. Int. J. Mol. Sci. 2011, 12, 9226–9235. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Velegraki, A.; Cafarchia, C.; Gaitanis, G.; Iatta, R.; Boekhout, T. Malassezia. Infections in Humans and Animals: Pathophysiology, Detection, and Treatment. PLoS Pathog. 2015, 11, e1004523. [Google Scholar] [CrossRef] [PubMed]
- Berenji, F.; Rakhshandeh, H.; Ebrahimipour, H. In vitro study of the effects of henna extracts (Lawsonia inermis) on Malassezia species. Jundishapur J. Microbiol. 2010, 3, 125–128. [Google Scholar]
- Filip, R.; Davicino, R.; Anesini, C. Antifungal activity of the aqueous extract of Ilex paraguariensis against malassezia furfur. Phyther. Res. 2010, 24, 715–719. [Google Scholar]
- Von Nussbaum, F.; Brands, M.; Hinzen, B.; Weigand, S.; Häbich, D. Antibacterial natural products in medicinal chemistry–Exodus or revival? Angew. Chem. Int. Ed. 2006, 45, 5072–5129. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.S.; Lee, D.G. Antifungal action of chlorogenic acid against pathogenic fungi, mediated by membrane disruption. Pure Appl. Chem. 2010, 82, 219–226. [Google Scholar] [CrossRef]
- Magwedere, K.; Hemberger, M.Y.; Hoffman, L.C.; Dziva, F. Zoonoses: A potential obstacle to the growing wildlife industry of Namibia. Infect. Ecol. Epidemiol. 2012, 2, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multidrug Resistance: An Emerging Crisis. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 541340. [Google Scholar] [CrossRef] [PubMed]
- Chavan, U.; Shahidi, F.; Naczk, M. Extraction of condensed tannins from beach pea (Lathyrus maritimus L.) as affected by different solvents. Food Chem. 2001, 75, 509–512. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Zheng, C.J.; Yoo, J.-S.; Lee, T.-G.; Cho, H.-Y.; Kim, Y.-H.; Kim, W.-G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Lim, Y.Y.; Ling, S.K.; Tan, S.P.; Lim, K.K.; Khoo, M.G.H. Caffeoylquinic acids from leaves of Etlingera species (Zingiberaceae). LWT–Food Sci. Technol. 2009, 42, 1026–1030. [Google Scholar] [CrossRef]
- Nyau, V.; Prakash, S.; Rodrigues, J.; Farrant, J. HPLC-PDA-ESI-MS Identification of Polyphenolic Phytochemicals in Different Market Classes of Common Beans (Phaseolus vulgaris L.). Int. J. Biochem. Res. Rev. 2015, 8, 1–11. [Google Scholar] [CrossRef]
- Dhouioui, M.; Boulila, A.; Jemli, M.; Schiets, F.; Casabianca, H.; Zina, M.S. Fatty Acids Composition and Antibacterial Activity of Aristolochia longa L. and Bryonia dioïca Jacq. Growing Wild in Tunisia. J. Oleo Sci. 2016, 65, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Immediato, D.; Aguiar, L.; Iatta, R.; Camarda, A.; Lira, R.; De Luna, N.; Giangaspero, A.; Brandão-filho, S.P.; Otranto, D.; Cafarchia, C. Veterinary Parasitology Essential oils and Beauveria. bassiana against Dermanyssus gallinae (Acari: Dermanyssidae): Towards new natural acaricides. Vet. Parasitol. 2016, 229, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Tamai, M.A.; Alves, S.B.; Lopes, R.B.; Faion, M.; Padulla, L.F.L. Toxicidade De Produtos Fitossanitários para Beauveria Bassiana (Bals.) Vuill. Arq. Inst. Biol. (Sao Paulo) 2002, 69, 65–69. [Google Scholar]
Sample Availability: Samples of the phenolic and flavonoid compounds are available from the authors. |

| Polyphenols and Flavonoids Content | Ethanolic | Ethanolic 80% | Butanolic | Methanolic |
|---|---|---|---|---|
| CTC(mgCAE/g extract) | 14.29 ± 1.30 a | 7.05 ± 1.6 b | 16.86 ± 1.62 c | 27.15 ± 2.21 d |
| TPC (mgGAE/g extract) | 117.58 ± 1.29 a | 123.39 ± 1.22 b | 75.34 ± 1.30 c | 123.07 ± 1.69 b |
| TFC (mgQE/g extract) | 57.79 ± 1.76 a | 49.23 ± 1.039 b | 58.03 ± 1.85 a | 30.86 ± 50 c |
| CQC (mgCGAE/g extract) | 71.85 ± 0.35 a | 73.13 ± 1.06 a | 57.11 ± 0.98 b | 87.61 ± 1.06 c |
| Compound | RT (min) | λmax | [M − H]− | Fragment Ions | Proposed Structure | Occurrence |
|---|---|---|---|---|---|---|
| 1 | 4.856 | 292sh–322 | 377 | 341 (100), 179, 119 | Caffeic acid hexoside | ++ |
| 2 | 5.346 | 292sh–322 | 341 | 191 (100), 137, 128 | 3-Caffeoylquinic acid | ++ |
| 3 | 11.876 | 292sh–322 | 353 | 191 (100), 161 | Chlorogenic acid | +++ |
| 4 | 13.856 | 292sh–322 | 353 | 191, 161 (100) | 4-Caffeoylquinic acid isomer | + |
| 5 | 17.222 | 293–310 | 467 | 163 (100) | Coumaric acid derivative | + |
| 6 | 22.365 | - | 429 | 267 (100), 173, 161 | Feruloyl caffeolglycerol | + |
| 7 | 27.982 | 360, 262 | 463 | 301 (100), 331, 255 | Quercetin hexoside | + |
| 8 | 28.007 | 293sh–321 | 463 | 301(100) | Hydroxyluteolin hexoside | + |
| 9 | 30.452 | 293sh–354 | 477 | 301 (100) | Quercetin glucuronide | + |
| 10 | 30.714 | 294sh–354 | 477 | 301 (100), 161 | Quercetin glucuronide | + |
| 11 | 32.888 | 292sh–322 | 353 | 191 (100), 179, 161 | 5-Caffeoylquinic acid | + |
| 12 | 33.463 | 292sh–322 | 353 | 191 (100), 179 (32) | Dicaffeoylquinic acid isomer | ++++ |
| 13 | 37.98 | 292–322 | 353 | 191 (78), 179 (100), 161 (80) | Caffeoylquinic acid isomer | +++ |
| 14 | 44.561 | 290, 320 | 339 | 135 (100) | Caffeoyl glucose | +++ |
| 15 | 47.234 | 253–349 | 329 | 314 (100), 299 (80), 285 (70), 271 (53), 243 (50) | Quercetin-dimethyl ether isomer | +++ |
| 16 | 47.395 | 253–349 | 329 | 314 (100), 299 (85) 271 (75), 241 (40) | Quercetin-dimethyl ether isomer | +++ |
| 17 | 49.315 | 253–349 | 329 | 314 (100), 299 (85), 285, 243 | Quercetin-dimethyl ether isomer | + |
| 18 | 55.566 | 278 | 493 | 289 (40), 165 (100), 139 (80) | Catechin glucoside | +++ |
| Bacterial spps. | Concentration (mg/mL) | Ethanol | Ethanol 80% | Butanol | Methanol |
|---|---|---|---|---|---|
| Eshershia coli | 50 | 12±1.41 a | 11.5 ± 0.70 a | 12.5 ± 0.70 a | 12 ± 0.70 a |
| 10 | 11 ± 1.41 a | 10 ± 0.0 a | 10.5 ± 0.0 a | 10 ± 0.0 a | |
| Sal Salmonella typhimurium | 50 | 10.5 ± 0.70 a | 9.5 ± 0.70 a | 10.5 ± 0.70 a | 10 ± 0.0 a |
| 10 | 9.5 ± 0.70 a | 0 ± 0.0 b | 9.5 ± 0.70 a | 9.5 ± 0.0 a | |
| Enterococcus feacium | 50 | 34 ± 1.41 a | 28.5 ± 0.0 b | 34.5 ± 0.70 a | 34.5 ± 0.7 a |
| 10 | 30 ± 0.0 a | 25 ± 0.0 b | 28 ± 0.0 c | 29 ± 0.0 d | |
| Streptococcus agalactiae | 50 | 28 ± 1.41 a | 28 ± 1.41 a | 29 ± 1.41 a | 29 ± 1.41 a |
| 10 | 18.5 ± 0.70 a | 17 ± 0.0 a | 21.5 ± 1.41 b | 18 ± 1.14 a | |
| Staphylococus aureus | 50 | 25 ± 0.0 a | 25 ± 0.0 a | 22.5 ± 0.70 b | 20 ± 0.0 c |
| 10 | 13.5 ± 0.70 a | 10 ± 0.0 b | 13 ± 1.41 a | 11 ± 0.0 c |
| Candida and Malassezia spp. | Concentration (mg/mL) | Ethanol | Ethanol 80% | Butanol | Methanol |
|---|---|---|---|---|---|
| Candida prapsilosis ATCC 22019 | 50 | 10.25 ± 0.58 a | 9.66 ± 1.52 a | 8.75 ± 1.73 a | 10.75 ± 0.95 a |
| 10 | 8.66 ± 1.73 a | 8.5 ± 1.73 a | 8.66 ± 1.73 a | 10 ± 0.95 a | |
| Candida krusei ATCC 6258 | 50 | 10 ± 1.41 a | 10.5 ± 0.57 a | 10 ± 1 a | 10 ± 0.0 a |
| 10 | 9.5 ± 0.7 a | 10 ± 0.0 a | 9 ± 0.82 a | 10 ± 0.0 a | |
| Candida albicans ATCC 10231 | 50 | 13.5 ± 0. 70 a | 13.5 ± 0.70 a | 14.5 ± 0.70 a | 14.5 ± 0.70 a |
| 10 | 12 ± 0.0 a | 11.5 ± 0.70 a | 13 ± 0.00 a | 12 ± 1.41 a | |
| Candida albicans CD 1358 | 50 | 10.5 ± 0.57 a | 11 ± 0.00 a | 10.25 ± 0.5 a | 10 ± 2.0 a |
| 10 | 10.25 ± 0.5 a | 10 ± 0.57 a | 9.5 ± 0.57 a | 9.5 ± 2.0 a | |
| Candida albicans CD 1378 | 50 | 10.25 ± 0.5 a | 11.0 ± 0 b | 10 ± 0.0 a | 10 ± 0.81 a |
| 10 | 10 ± 0.5 a | 10.33 ± 0.0 a | 10 ± 0.5 a | 10 ± 0.0 a | |
| Candida albicans CD 1407 | 50 | 10.66 ± 0 a | 10.33 ± 1.89 a | 10.75 ± 0.5 a | 11 ± 0.81 a |
| 10 | 10 ± 0.5 a | 8.25 ± 1.89 a | 10.33 ± 0.57 a | 9.66 ± 0.57 a | |
| Candida albicans C13F3A | 50 | 9.5 ± 1.91 a | 10.5 ± 0.57 a | 10.75 ± 0.5 a | 11 ± 0.81 a |
| 10 | 7 ± 1.15 a | 10 ± 0.57 a | 6.66 ± 0.57 a | 9.66 ± 0.57 a | |
| Malassezia pachydermatis CBS1879 | 50 | 10 ± 0.0 a | 10 ± 0.0 a | 10.33 ± 0.57 a | 11 ± 00 a |
| 10 | 9.33 ± 1.15 a | 9.33 ± 0.57 a | 9.66 ± 1.52 a | 9.33 ± 0.57 a | |
| Malassezia pachydermatis CD 112 | 50 | 10.33 ± 0.57 a | 10.66 ± 0.57 a | 9.33 ± 1.15 a | 10.66 ± 0.57 a |
| 10 | 7.66 ± 0.57 a | 7.66 ± 0.57 a | 7 ± 0.0 a | 10.33 ± 0.57 b | |
| Malassezia pachydermatis CD 90 | 50 | 10.33 ± 1.55 a | 10.33 ± 0.57 a | 9.66 ± 0.57 a | 9.66 ± 0.57 a |
| 10 | 0 ± 0.0 a | 7.66 ± 0.57 b | 7.33 ± 0.57 b | 9.33 ± 0.57 c | |
| Malassezia furfur CBS1978 | 50 | 10.66 ± 1.54 a | 10.33 ± 1.52 a | 8.33 ± 0.57 a | 9.66 ± 1.15 a |
| 10 | 0 ± 0.0 a | 7 ± 0.0 b | 0 ± 0.0 a | 8 ± 1.0 b | |
| Malassezia furfur CD 1006 | 50 | 9.33 ± 0.57 a | 9.66 ± 1.52 a | 8.33 ± 1.52 a | 9 ± 1.73 a |
| 10 | 0 ± 0.0 a | 0 ± 0.0 a | 0 ± 0.0 a | 8 ± 1.0 b | |
| Malassezia furfur CD 1029 | 50 | 8 ± 1.0 a | 9 ± 0.0 a | 0 ± 0.0 b | 9 ± 1.0 a |
| 10 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 |
| [C] mg/mL | Ethanol | Ethanol 80% | Butanol | Methanol | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | Germination (Log10 CFU/mL) | Growth (Ø cm) | Sporulation (Log10 CFU/mL) | T Value | Germination (Log10 CFU/mL) | Growth (Ø cm) | Sporulation (Log10 CFU/mL) | T Value | Germination (Log10 CFU/mL) | Growth (Ø cm) | Sporulation (Log10 CFU/mL) | T Value | Germination (Log10 CFU/mL) | Growth (Ø cm) | Sporulation (Log10 CFU/mL) | T Value | |
| CD 1279 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 5 | 0 | 2.4 ± 0.3 | 3.54 ± 0.1 | 14.71 | 0 | 2.55 ± 0.1 | 4.06 ± 0.1 | 23.08 | 0 | 2.5 ± 0.1 | 3.47 ± 0.1 | 14.54 | 0 | 1.8 ± 0.1 | 2.97 ± 0.1 | 9.31 | |
| 1 | 3.72 ± 0.1 | 3.65 ± 0.4 | 4.79 ± 0.1 | 77.78 | 3.87 ± 0.1 | 3.85 ± 0.1 | 4.77 ± 0.1 | 76.91 | 3.56 ± 0.2 | 3.5 ± 0.1 | 4.58 ± 0.1 | 53.55 | 3.49 ± 0.1 | 2.55 ± 0.1 | 4.43 ± 0.1 | 38.45 | |
| Control | 4.30 ± 0.1 | 5.45 ± 0.1 | 4.91 ± 0.1 | 100 | 4.30 ± 0.2 | 5.45 ± 0.1 | 4.91 ± 0.1 | 100 | 4.30 ± 0.2 | 5.45 ± 0.1 | 4.91 ± 0.1 | 100 | 4.3 ± 0.2 | 5.45 ± 0.1 | 4.91 ± 0.1 | 100 | |
| CD 1243 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 5 | 0 | 2.1 ± 0.14 | 3.13 ± 0.1 | 10.62 | 0 | 2.25 ± 0.1 | 3.41 ± 0.04 | 13.85 | 0 | 1.85 ± 0.1 | 3.06 ± 0.1 | 9.52 | 0 | 1.85 ± 0.1 | 2.86 ± 0.1 | 8.61 | |
| 1 | 3.73 ± 0.1 | 2.55 ± 0.1 | 4.22 ± 0.2 | 48.97 | 3.81 ± 0.1 | 2.85 ± 0.1 | 4.22 ± 0.02 | 45.41 | 3.24 ± 0.1 | 2.85 ± 0.1 | 3.79 ± 0.1 | 25.71 | 3.24 ± 0.1 | 2.35 ± 0.1 | 3.43 ± 0.1 | 15.11 | |
| C | 4.18 ± 0.1 | 5.55 ± 0.1 | 4.51±0.1 | 100 | 4.18 ± 0.1 | 5.55 ± 0.1 | 4.51±0.1 | 100 | 4.18 ± 0.1 | 5.55 ± 0.1 | 4.51 ± 0.1 | 100 | 4.18 ± 0.1 | 5.55 ± 0.1 | 4.51 ± 0.1 | 100 | |
| CD 1447 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 5 | 0 | 2.5 ± 0.1 | 3.36 ± 0.2 | 16.58 | 0 | 2.85 ± 0.1 | 3.55 ± 0.2 | 21.12 | 0 | 2.45 ± 0.1 | 3.81 ± 0.1 | 14.77 | 0 | 1.65 ± 0.1 | 2.81 ± 0.1 | 9.16 | |
| 1 | 3.85 ± 0.1 | 3.45 ± 0.1 | 4.43 ± 0.1 | 66.31 | 3.88 ± 0.3 | 3.8 ± 0.1 | 4.26 ± 0.1 | 17.19 | 2.52 ± 0.1 | 3 ± 0.1 | 4.29 ± 0.1 | 50.23 | 2.29 ± 0. 3 | 2.65 ± 0.1 | 4.26 ± 0.1 | 46.31 | |
| C | 4.46 ± 0.1 | 4.15 ± 1 | 4.61 ± 0.1 | 100 | 4.46 ± 0.1 | 4.15 ± 0.1 | 4.61 ± 0.2 | 100 | 4.46 ± 0.1 | 4.15 ± 0.1 | 4.61 ± 0.1 | 100 | 4.46 ± 0.1 | 4.15 ± 0.1 | 4.61 ± 0.1 | 100 | |
| [C] mg/mL | Ethanol | Ethanol 80% | Butanol | Methanol | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | Germination (Log10 CFU/mL) | Growth (Ø cm) | Sporulation (Log10 CFU/mL) | T Value | Germination (Log10 CFU/mL) | Growth (Ø cm) | Sporulation (Log10 CFU/mL) | T Value | Germination (Log10 CFU/mL) | Growth (Ø cm) | Sporulation (Log10 CFU/mL) | T Value | Germination (Log10 CFU/mL) | Growth (Ø cm) | Sporulation (Log10 CFU/mL) | T Value | |
| CD 1435 | 50 | 0 | 1.25 ±0.1 | 5.09 ± 0.2 | 2.88 | 0 | 1.55 ± 0.1 | 6.25 ± 0.2 | 4.71 | 0 | 1.35 ± 0.1 | 5.12 ± 0.2 | 3.11 | 0 | 1.15 ± 0.1 | 4.08 ± 0.3 | 2.59 |
| 10 | 3.66 ± 0.1 | 2.35 ± 0.1 | 6.45 ± 0.2 | 7.13 | 3.51 ± 0.1 | 2.85 ± 0.1 | 6.81 ± 0.1 | 13.62 | 3.47 ± 0.3 | 3.35 ± 0.2 | 6.18 ± 0.1 | 8.56 | 3.46 ± 0.3 | 1.95 ± 0.1 | 5.08 ± 0.6 | 4.75 | |
| 5 | 4.06 ± 0.1 | 5.9 ± 0.1 | 6.72± 0.2 | 19.33 | 4.20 ± 0.1 | 6.25 ± 0.1 | 7.23 ± 0.0 | 29.86 | 4.03 ± 0.3 | 5.7 ± 0.14 | 6.51 ± 0.1 | 16.44 | 4.14 3 ± 0.1 | 5.35 ± 0.1 | 6.18±0.3 | 14.45 | |
| 1 | 5.21 ± 0.2 | 7.85 ± 0.1 | 7.75 ± 0.1 | 73.53 | 5.03 ± 0.1 | 8.15 ± 0.1 | 7.79 ± 0.1 | 78.41 | 4.97 ± 0.1 | 8.15 ± 0.1 | 7.74 ± 0.1 | 73.71 | 4. 7 ± 0.1 | 8.25 ± 0.1 | 7.66±0.1 | 64.9 | |
| C | 5.24 ± 0.1 | 8.9 ± 1.0 | 7.91 ± 0.1 | 100 | 5.24 ± 0.1 | 8.9 ± 1.0 | 7.91 ± 0.6 | 100 | 5.24 ± 0.1 | 8.95 ± 1.0 | 7.91 ± 0.1 | 100 | 5.24 ± 0.1 | 8.95 ± 1.1 | 7.91±0.1 | 100 | |
| CD 1441 | 50 | 0 | 1.2 ± 0.0 | 5.35 ± 0.2 | 2.86 | 0 | 1.65 ± 0.1 | 5.23 ± 0.2 | 3.85 | 0 | 1.2 ± 0 | 5.13 ± 0.3 | 2.81 | 0 | 1.1 ± 0 | 4. 67 ± 0.1 | 2.54 |
| 10 | 3.55 ± 0.1 | 3.4 ± 0.3 | 6.59 ± 0.1 | 9.93 | 3.68 ± 0.1 | 3.4 ± 0.0 | 6.56 ± 0.1 | 9.8 | 3.19 ± 0.1 | 3.3 ± 0.5 | 6.17 ± 0. 2 | 8.33 | 2.67 ± 0.1 | 2.95 ± 0.1 | 5.64 ± 0.1 | 8.25 | |
| 5 | 4.07 ± 0.2 | 5.25 ± 0.1 | 6.96 ± 0.1 | 17.2 | 4.34 ± 0.1 | 5.4 ± 0.3 | 7.04 ± 0.10 | 18.78 | 3.95 ± 0.1 | 4.55 ± 0.2 | 6.84 ± 0.1 | 14.34 | 3.8 ± 0.1 | 4.4 ± 0.2 | 6.72 ± 0.1 | 13.1 | |
| 1 | 4.99 ± 0.1 | 7.55 ± 0.2 | 7.73 ± 0.5 | 47.5 | 5.06 ± 0.1 | 7.95 ± 0.1 | 7.9 ± 0.1 | 63.81 | 4.83 ± 0.1 | 7.4 ± 0.1 | 7.57 ± 0.1 | 38.74 | 4.66 ± 0.1 | 7.8 ± 0.1 | 7.47 ± 0.1 | 34.82 | |
| C | 5.35 ± 0.1 | 8.8 ± 0.1 | 8.15 ± 0.1 | 100 | 5.53 ± 0.1 | 8.8 ± 0.1 | 8.15 ± 0.1 | 100 | 5.35 ± 0.1 | 8.84 ± 0.1 | 8.15 ± 0.1 | 100 | 5.35 ± 0.1 | 8.8 ± 0.1 | 8.15 ± 0.1 | 100 | |
| CD 1438 | 50 | 0 | 1.25 ± 0.1 | 5.06 ± 0.1 | 2.84 | 0 | 1.6 ± 0 | 5.19 ± 1.1 | 3.83 | 0 | 0 | 0 | 0 | 0 | 1.2 ± 0.0 | 0.00 | 0 |
| 10 | 4.3 ± 0.1 | 3.05 ± 0.2 | 5.76 ± 0.5 | 7.2 | 4.58 ± 0.1 | 3.8 ± 0.1 | 6.5 ± 0.2 | 9.72 | 2.67 ± 0.1 | 2.95 ± 0.1 | 5.85 ± 0.1 | 6.94 | 4.3 ± 0.1 | 2.55 ± 0.2 | 5.61 ± 0.1 | 5.97 | |
| 5 | 5.08 ± 0.1 | 5.55 ± 0.2 | 6.69 ± 0.1 | 14.37 | 5.19 ± 0.1 | 6.6 ± 0.1 | 7.78 ± 0.1 | 23.54 | 3.96 ± 0.1 | 3.95 ± 0.1 | 6. 84 ± 0.1 | 12.23 | 4.68 ± 0.1 | 3.6 ± 0.1 | 6.18 ± 0.2 | 8.84 | |
| 1 | 5.45 ± 0.1 | 7.65 ± 0.2 | 7.89 ± 0.1 | 49.38 | 5.76 ± 0.1 | 8.0 ± 0.3 | 7.78 ± 0.01 | 41.49 | 4.12 ± 0.1 | 7.62± 0.1 | 7.78 ± 0.1 | 40.84 | 5.3 ± 0 | 7.55 ± 0.2 | 7.69 ± 0.1 | 40.73 | |
| C | 5.92 ± 0.1 | 8.95 ± 1.0 | 8.31 ± 0.2 | 100 | 5.92 ± 0.1 | 8.95 ± 1.0 | 8.31 ± 0.2 | 100 | 5.92 ± 0.1 | 8.95 ± 1.0 | 8.31 ± 0.2 | 100 | 5.92 ± 0.1 | 8.95 ± 1.0 | 8.31 ± 0.2 | 100 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhimi, W.; Ben Salem, I.; Immediato, D.; Saidi, M.; Boulila, A.; Cafarchia, C. Chemical Composition, Antibacterial and Antifungal Activities of Crude Dittrichia viscosa (L.) Greuter Leaf Extracts. Molecules 2017, 22, 942. https://doi.org/10.3390/molecules22070942
Rhimi W, Ben Salem I, Immediato D, Saidi M, Boulila A, Cafarchia C. Chemical Composition, Antibacterial and Antifungal Activities of Crude Dittrichia viscosa (L.) Greuter Leaf Extracts. Molecules. 2017; 22(7):942. https://doi.org/10.3390/molecules22070942
Chicago/Turabian StyleRhimi, Wafa, Issam Ben Salem, Davide Immediato, Mouldi Saidi, Abdennacer Boulila, and Claudia Cafarchia. 2017. "Chemical Composition, Antibacterial and Antifungal Activities of Crude Dittrichia viscosa (L.) Greuter Leaf Extracts" Molecules 22, no. 7: 942. https://doi.org/10.3390/molecules22070942