A Metabolomics-Guided Exploration of the Phytochemical Constituents of Vernonia fastigiata with the Aid of Pressurized Hot Water Extraction and Liquid Chromatography-Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Various HCA Derivatives in V. fastigiata
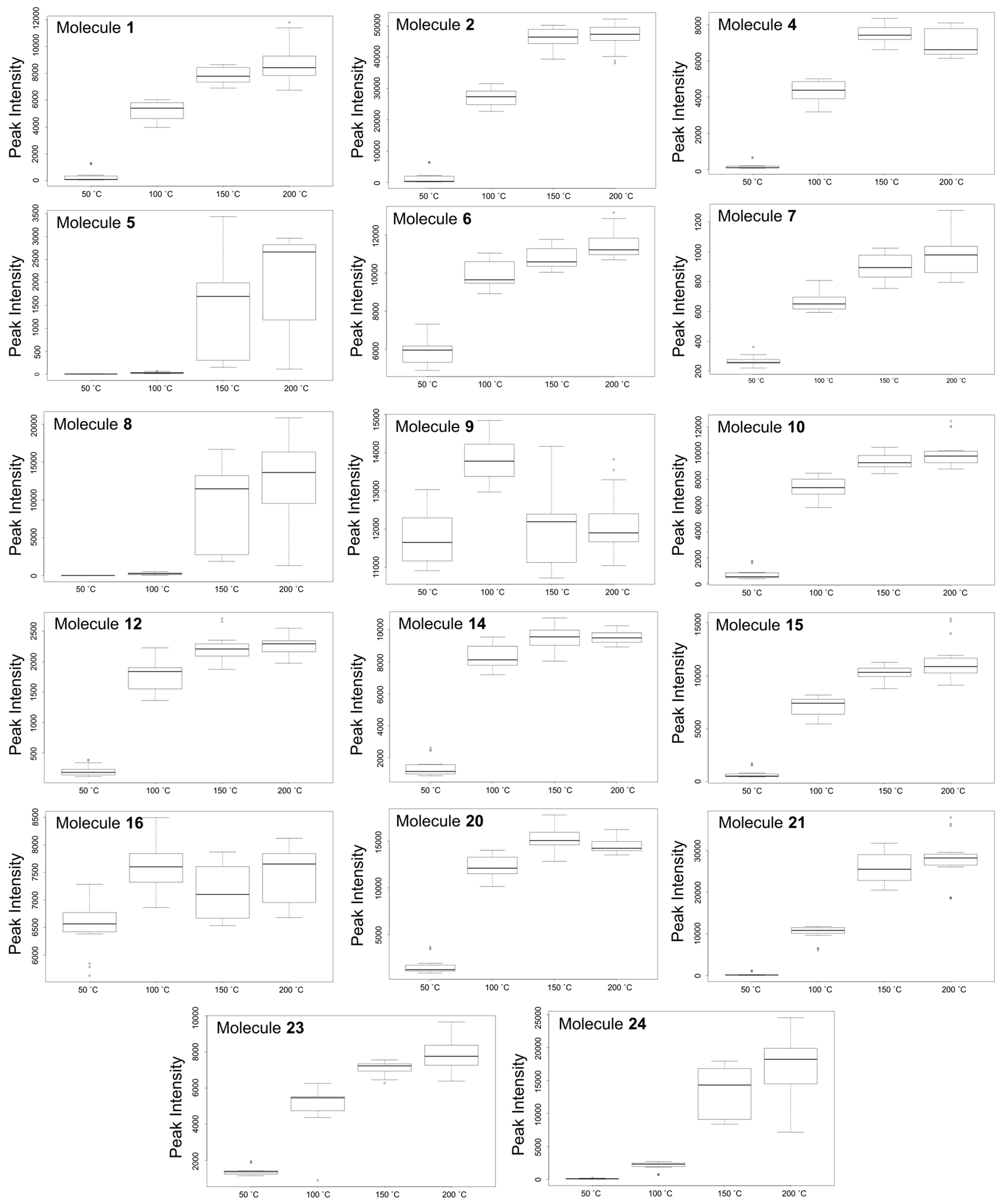
Biological Relevance of Identified HCA Derivatives
2.2. Identification of Various Glycosylated Quercetin Derivatives in V. fastigiata
Biological Relevance of Identified Quercetin Glycosylated Derivatives
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Extraction
3.2.2. Chromatography and Mass Spectrometry Analyses
3.2.3. Data Processing and Multivariate Data Analysis (MVDA)
3.2.4. Metabolite Annotation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martucci, M.E.P.; de Vos, R.C.; Carollo, C.A.; Gobbo-Neto, L. Metabolomics as a Potential Chemotaxonomical Tool: Application in the Genus Vernonia Schreb. PLoS ONE 2014, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Toyang, N.J.; Verpoorte, R.A. Review of the Medicinal Potentials of Plants of the Genus Vernonia (Asteraceae). J. Ethnopharmacol. 2013, 146, 681–723. [Google Scholar] [CrossRef] [PubMed]
- Erasto, P.; Grierson, D.; Afolayan, A. Bioactive sesquiterpene lactones from the leaves of Vernonia amygdalina. J. Ethnopharmacol. 2006, 106, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Bessong, P.O.; Obi, C.L.; Andréola, M.; Rojas, L.B.; Pouységu, L.; Igumbor, E.; Meyer, J.M.; Quideau, S.; Litvak, S. Evaluation of selected South African medicinal plants for inhibitory properties against Human Immunodeficiency Virus type 1 reverse transcriptase and integrase. J. Ethnopharmacol. 2005, 99, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Njan, A.A.; Adzu, B.; Agaba, A.G.; Byarugaba, D.; Díaz-Llera, S.; Bangsberg, D.R. The analgesic and antiplasmodial activities and toxicology of Vernonia amygdalina. J. Med. Food 2008, 11, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Igile, G.O.; Oleszek, W.; Jurzysta, M.; Burda, S.; Fafunso, M.; Fasanmade, A.A. Flavonoids from Vernonia amygdalina and their antioxidant activities. J. Agric. Food Chem. 1994, 42, 2445–2448. [Google Scholar] [CrossRef]
- Robinson, H. Parapolydora (Asteraceae), a New Genus of Vernonieae from South Africa. Phytologia 2005, 87, 75–79. [Google Scholar]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, P.J.; Folb, P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Roos, G.; Prawat, H.; Walter, C.U.; Klaiber, I.; Vogler, B.; Guse, J.H.; Kraus, W. New sesquiterpene lactones with antibacterial activity from Vernonia fastigiata. Planta Med. 1998, 64, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Pressurized Hot Water Extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Gbashi, S.; Adebo, O.A.; Piater, L.; Madala, N.E.; Njobeh, P.B. Subcritical Water Extraction of Biological Materials. Sep. Purif. Rev. 2017, 46, 21–34. [Google Scholar] [CrossRef]
- Khoza, B.S.; Chimuka, L.; Mukwevho, E.; Steenkamp, P.A.; Madala, N.E. The Effect of Temperature on Pressurised Hot Water Extraction of Pharmacologically Important Metabolites as Analysed by UPLC-qTOF-MS and PCA. Evid. Based Complement. Altern. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Khoza, B.; Gbashi, S.; Steenkamp, P.; Njobeh, P.; Madala, N. Identification of hydroxylcinnamoyl tartaric acid esters in Bidens pilosa by UPLC-tandem mass spectrometry. S. Afr. J. Bot. 2016, 103, 95–100. [Google Scholar] [CrossRef]
- Gowda, H.; Ivanisevic, J.; Johnson, C.H.; Kurczy, M.E.; Benton, H.P.; Rinehart, D.; Nguyen, T.; Ray, J.; Kuehl, J.; Arevalo, B. Interactive XCMS Online: Simplifying advanced metabolomic data processing and subsequent statistical analyses. Anal. Chem. 2014, 86, 6931–6939. [Google Scholar] [CrossRef] [PubMed]
- XCMS Online. Available online: https://xcmsonline.scripps.edu (accessed on 16 May 2016).
- Madala, N.E.; Piater, L.A.; Steenkamp, P.A.; Dubery, I.A. Multivariate statistical models of metabolomic data reveals different metabolite distribution patterns in isonitrosoacetophenone-elicited Nicotiana tabacum and Sorghum bicolor cells. SpringerPlus 2014, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tugizimana, F.; Piater, L.; Dubery, I. Plant Metabolomics: A New Frontier in Phytochemical Analysis. S. Afr. J. Sci. 2013, 109, 1–11. [Google Scholar] [CrossRef]
- Dictionary of Natural Products. Available online: dnp.chemnetbase.com (accessed on 14 March2016).
- KNApSAck. Available online: http://kanaya.naist.jp/KNApSAcK (accessed on 14 March2016).
- Ncube, E.N.; Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Dubery, I.A.; Madala, N.E. Analyses of chlorogenic acids and related cinnamic acid derivatives from Nicotiana tabacum tissues with the aid of UPLC-QTOF-MS/MS based on the in-source collision-induced dissociation method. Chem. Cent. J. 2014, 8, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Ramabulana, T.; Mavunda, R.; Steenkamp, P.; Piater, L.; Dubery, I.; Madala, N. Perturbation of pharmacologically relevant polyphenolic compounds in Moringa oleifera against photo-oxidative damages imposed by gamma radiation. J. Photochem. Photobiol. 2016, 156, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Kirkpatrick, J.; Kuhnert, N.; Roozendaal, H.; Salgado, P.R. LC-MSn Analysis of the cis isomers of chlorogenic acids. Food Chem. 2008, 106, 379–385. [Google Scholar] [CrossRef]
- Arlorio, M.; Locatelli, M.; Travaglia, F.; Coïsson, J.; Del Grosso, E.; Minassi, A.; Appendino, G.; Martelli, A. Roasting impact on the contents of clovamide (N-caffeoyl-l-DOPA) and the antioxidant activity of cocoa beans (Theobroma cacao L.). Food Chem. 2008, 106, 967–975. [Google Scholar] [CrossRef]
- Locatelli, M.; Travaglia, F.; Giovannelli, L.; Coïsson, J.D.; Bordiga, M.; Pattarino, F.; Arlorio, M. Clovamide and phenolics from cocoa beans (Theobroma cacao L.) inhibit lipid peroxidation in liposomal systems. Food Res. Int. 2013, 50, 129–134. [Google Scholar] [CrossRef]
- Schieber, A.; Keller, P.; Carle, R. Determination of phenolic acids and flavonoids of apple and pear by High-Performance Liquid Chromatography. J. Chromatogr. A 2001, 910, 265–273. [Google Scholar] [CrossRef]
- Chen, H.; Inbaraj, B.S.; Chen, B. Determination of phenolic acids and flavonoids in Taraxacum formosanum Kitam by liquid chromatography-tandem mass spectrometry coupled with a post-column derivatization technique. Int. J. Mol. Sci. 2012, 13, 260–285. [Google Scholar] [CrossRef] [PubMed]
- Fromm, M.; Loos, H.M.; Bayha, S.; Carle, R.; Kammerer, D.R. Recovery and characterisation of coloured phenolic preparations from apple seeds. Food Chem. 2013, 136, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Ablajan, K.; Abliz, Z.; Shang, X.; He, J.; Zhang, R.; Shi, J. Structural characterization of flavonol 3,7-di-O-glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry. J. Mass Spect. 2006, 41, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Salinas, J.; Vergara, M.; Altamirano, C.; Gonzalez, Á.; Pérez-Correa, J. Characterization of Pressurized Hot Water Extracts of grape pomace: Chemical and biological antioxidant activity. Food Chem. 2015, 171, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Sanbongi, C.; Osakabe, N.; Natsume, M.; Takizawa, T.; Gomi, S.; Osawa, T. Antioxidative polyphenols isolated from Theobroma cacao. J. Agric. Food Chem. 1998, 46, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Makola, M.M.; Steenkamp, P.; Dubery, I.A.; Kabanda, M.M.; Madala, N.E. Preferential alkali metal adduct formation by cis geometrical isomers of dicaffeoylquinic acids allows for efficient discrimination from their trans isomers during ultra-high-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Dai, F.; Zhou, B.; Yang, L.; Liu, Z. Antioxidant activity of hydroxycinnamic acid derivatives in human low density lipoprotein: Mechanism and structure-activity relationship. Food Chem. 2007, 104, 132–139. [Google Scholar] [CrossRef]
- Li, Y.; Tang, W.; Chen, J.; Jia, R.; Ma, L.; Wang, S.; Wang, J.; Shen, X.; Chu, Z.; Zhu, C. Development of marker-free transgenic potato tubers enriched in caffeoylquinic acids and flavonols. J. Agric. Food Chem. 2016, 64, 2932–2940. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Cui, F.; Yin, F.; Zeng, X.; Sun, Y.; Li, Y. Caffeoylquinic acids competitively inhibit pancreatic lipase through binding to the catalytic triad. Int. J. Biol. Macromol. 2015, 80, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Villani, T.S.; Guo, Y.; Qi, Y.; Chin, K.; Pan, M.; Ho, C.; Simon, J.E.; Wu, Q. Phytochemistry, antioxidant capacity, total phenolic content and anti-inflammatory activity of Hibiscus sabdariffa leaves. Food Chem. 2016, 190, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Ooi, L.S.; Wang, H.; He, Z.; Ooi, V.E. Antiviral activities of purified compounds from Youngia japonica (L.) DC (Asteraceae, Compositae). J. Ethnopharmacol. 2006, 106, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Makola, M.M.; Dubery, I.A.; Koorsen, G.; Steenkamp, P.A.; Kabanda, M.M.; du Preez, L.L.; Madala, N.E. The effect of geometrical isomerism of 3,5-dicaffeoylquinic acid on its binding affinity to HIV-integrase enzyme: A molecular docking study. Evid. Based Complement. Altern. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhao, D.; Xiang, J.; Zhang, M.; Zhang, C.; Xu, X. Antitussive, expectorant, and anti-inflammatory activities of four caffeoylquinic acids isolated from Tussilago farfara. Pharm. Biol. 2015, 54, 1–8. [Google Scholar]
- Pereira-Caro, G.; Borges, G.; Nagai, C.; Jackson, M.C.; Yokota, T.; Crozier, A.; Ashihara, H. Profiles of phenolic compounds and purine alkaloids during the development of seeds of Theobroma cacao Cv. Trinitario. J. Agric. Food Chem. 2012, 61, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Gindro, R.; Travaglia, F.; Coïsson, J.; Rinaldi, M.; Arlorio, M. Study of the DPPH-scavenging activity: Development of a free software for the correct interpretation of data. Food Chem. 2009, 114, 889–897. [Google Scholar] [CrossRef]
- Fallarini, S.; Miglio, G.; Paoletti, T.; Minassi, A.; Amoruso, A.; Bardelli, C.; Brunelleschi, S.; Lombardi, G. Clovamide and rosmarinic acid induce neuroprotective effects in in vitro models of neuronal death. Brit. J. Pharmacol. 2009, 157, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, D.; Fuehrer, H.; Starzengrüber, P.; Swoboda, P.; Khan, W.A.; Reismann, J.A.; Mueller, M.S.; Chiba, P.; Noedl, H. Antiplasmodial activity of flavonol quercetin and its analogues in Plasmodium falciparum: Evidence from Clinical Isolates in Bangladesh and standardized parasite clones. Parasitol. Res. 2012, 110, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- METLIN. Available online: http://metlin.scripps.edu (accessed on 16 May 2016).
Sample Availability: Samples of the compounds are not available from the authors. |
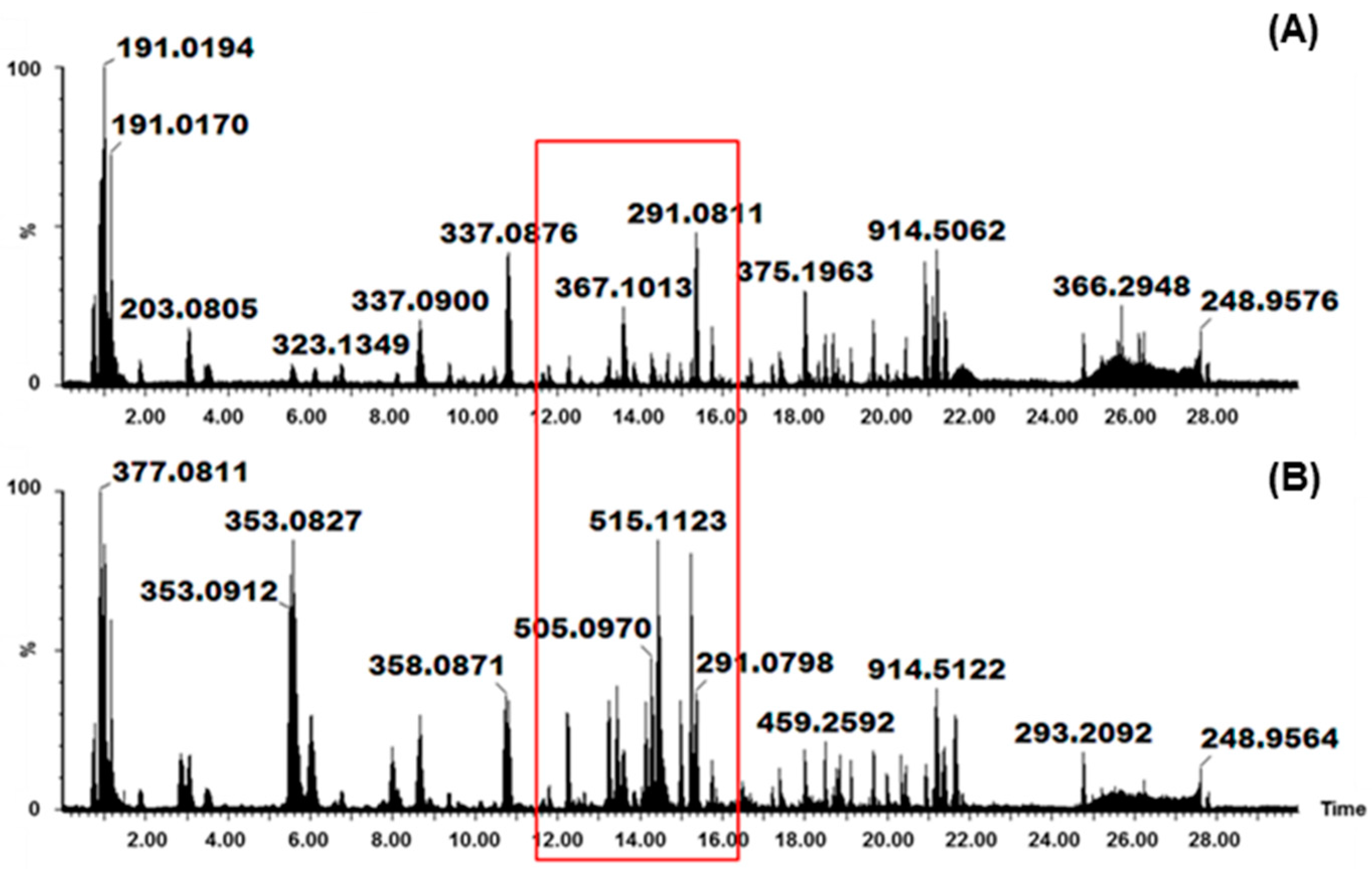

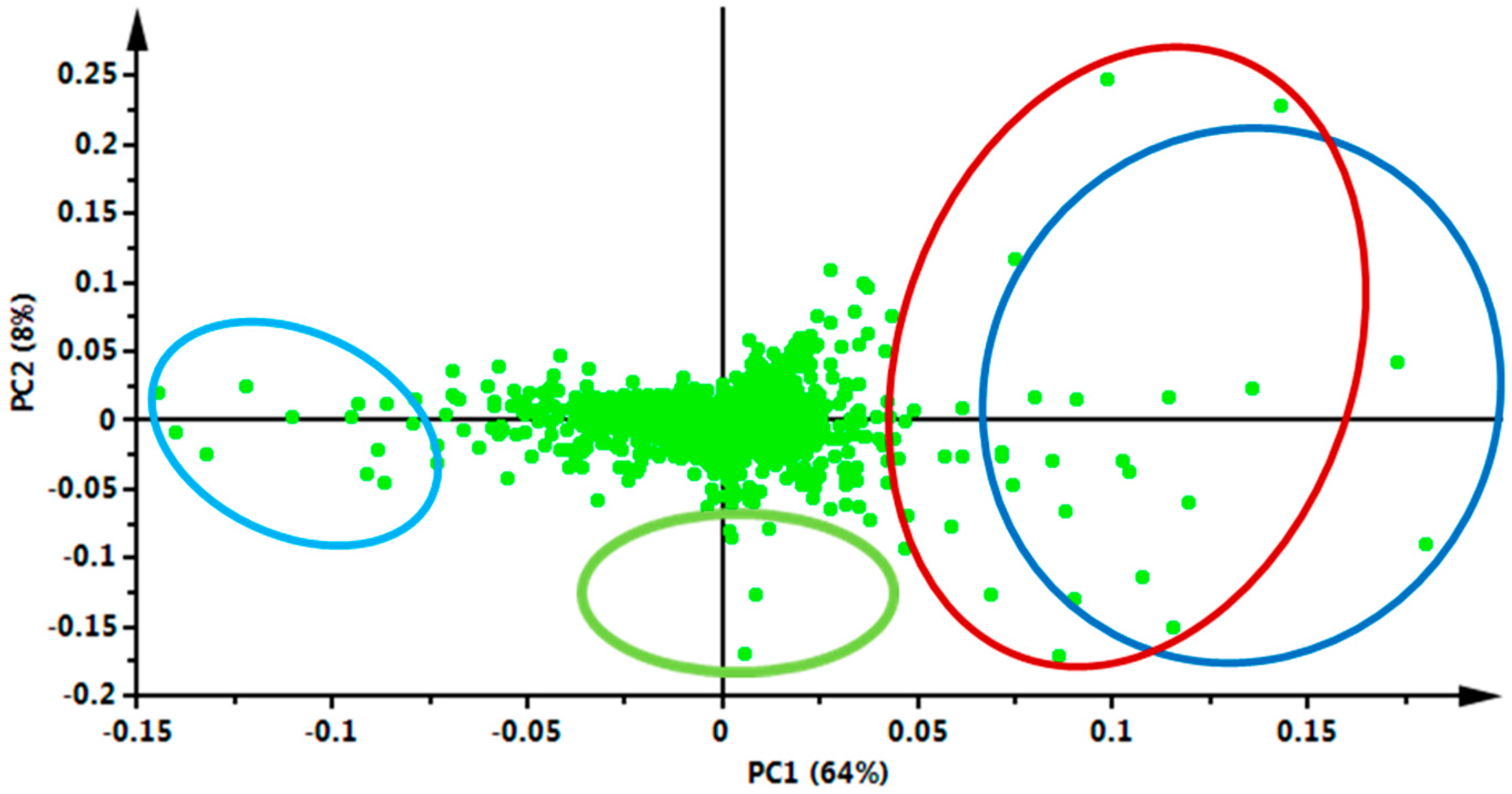
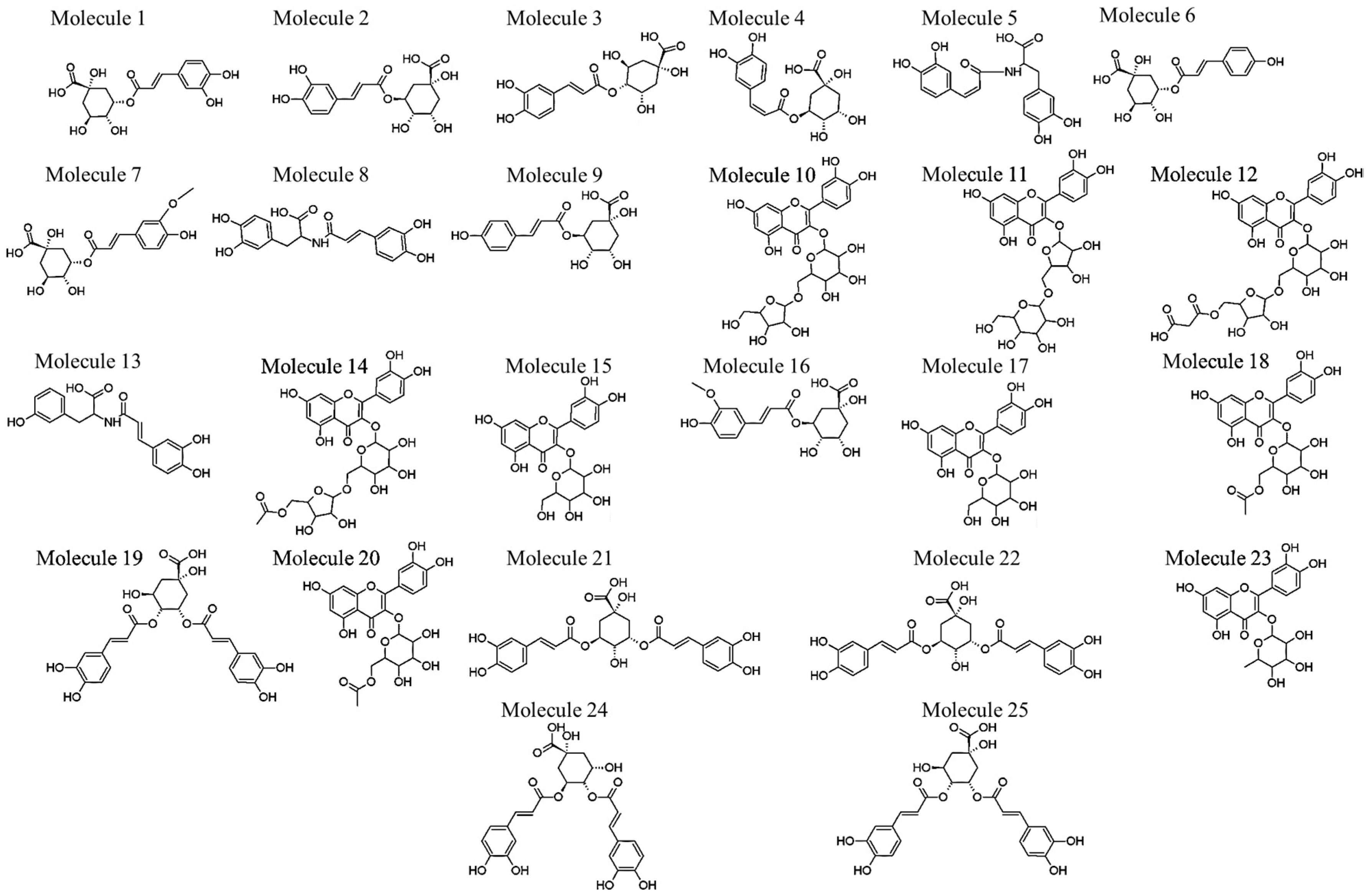
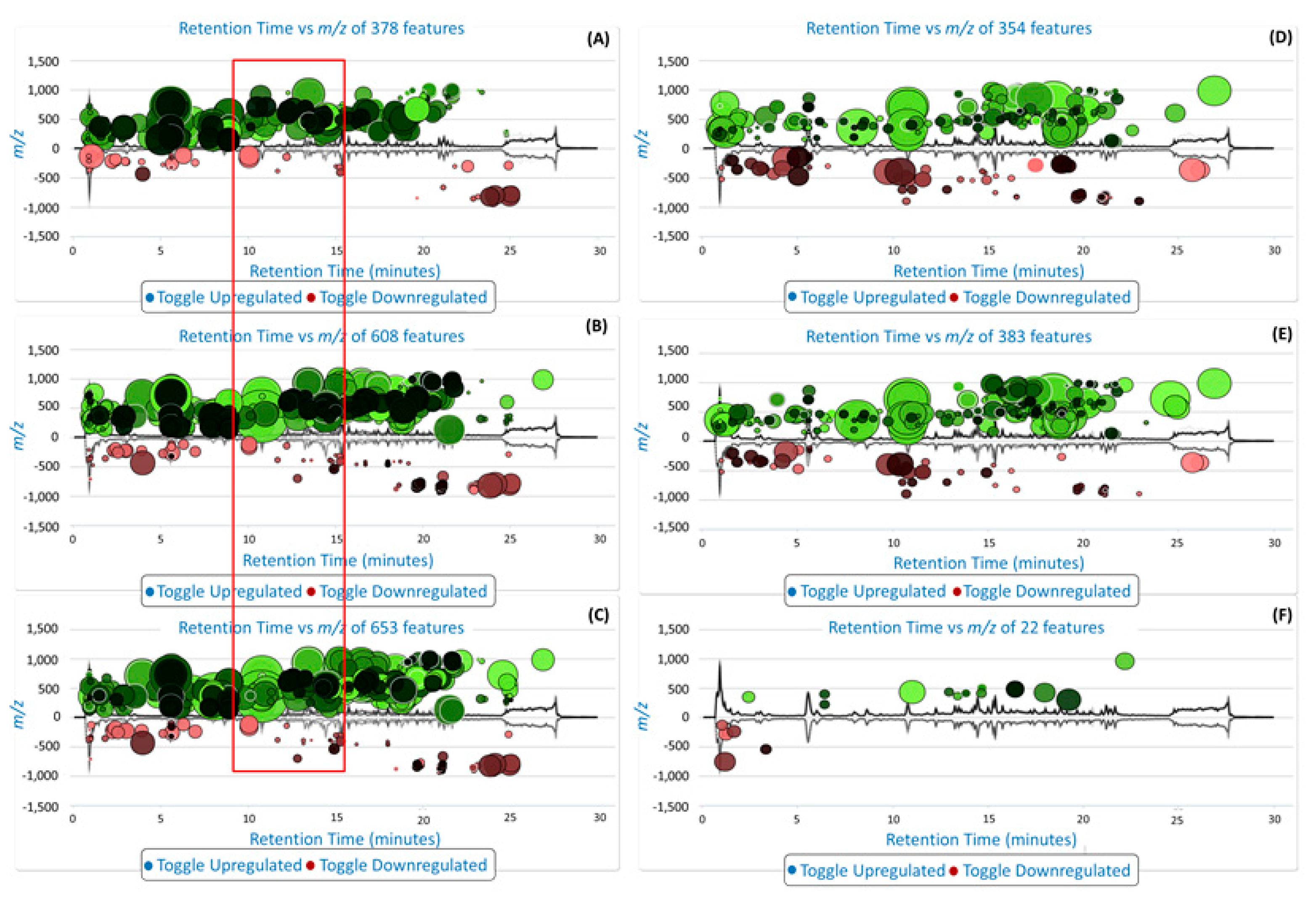
| Molecule Number | Rt (min) | Compound Name | Negative Ionization (m/z) | Negative Ionization MS/MS | Reference |
|---|---|---|---|---|---|
| 1 | 2.87 | 3-O-caffeoylquinic acid * | 353.0860 | 353→191 #, 179,135 | [20] |
| 2 | 5.56 | 5-O-caffeoylquinic acid * | 353.0887 | 353→191 # | [20,21] |
| 3 | 6.00 | 4-O-caffeoylquinic acid * | 353.0825 | 353→191, 179, 173 # | [20,22] |
| 4 | 7.99 | cis-5-O-caffeoylquinic acid | 353.0806 | 353→191 # | [20,23] |
| 5 | 8.14 | cis-N-caffeoyl-l-DOPA (cis-clovamide) | 358.0879 | 358→222, 178 #, 161 | [24,25] |
| 6 | 8.64 | 3-O-p-coumaroylquinic acid | 337.0873 | 337→191 #, 163 | [20,22] |
| 7 | 10.13 | 3-O-feruloylquinic acid | 367.1044 | 367→191 #, 193 | [20] |
| 8 | 10.74 | trans-N-caffeoyl-l-DOPA (clovamide) | 358.0871 | 358→222, 178 #, 161 | [24,25] |
| 9 | 10.82 | 5-O-p-coumaroylquinic acid | 337.0901 | 337→191 # | [20,22] |
| 10 | 12.23 | quercetin-3-O-hexoside-O-pentoside | 595.1245 | 595→301, 300 #, 271, 255, 243 | [26] |
| 11 | 12.41 | quercetin-3-O-pentoside-O-hexoside | 595.1288 | 595→301, 300 #, 271, 255 | [27] |
| 12 | 12.64 | quercetin-3-O-hexoside-O-pentoside-O-malonyl | 681.1300 | 681→637, 595, 301, 300 #, 271, 255 | MS/MS spectra |
| 13 | 12.81 | N-caffeoyl-l-tyrosine (deoxyclovamide) | 342.0944 | 342→342 #, 206 | MS/MS spectra |
| 14 | 13.24 | quercetin-3-O-hexoside-O-pentoside-O-acetyl | 637.1335 | 637→595, 301, 300 #, 271, 255, 243 | MS/MS spectra |
| 15 | 13.42 | quercetin-3-O-galactoside | 463.0857 # | 463→301, 300, 271, 255, 243 | [26,28,29] |
| 16 | 13.60 | 5-O-feruloylquinic acid | 367.1008 # | 367→191 | [20] |
| 17 | 13.71 | quercetin-3-O-glucoside (isoquercetin) * | 463.0835 | 463→301, 300 #, 271, 255, 243 | [26,28,29] |
| 18 | 14.04 | quercetin-3-O-galactoside-O-acetyl | 505.0955 | 505→463, 301, 300 #, 271, 255, 243 | MS/MS spectra |
| 19 | 14.13 | 3,4-di-O-caffeoylquinic acid isomer | 515.1113 | 515→353, 335, 191, 179, 173 # | [20,22] |
| 20 | 14.28 | quercetin-3-O-glucoside-O-acetyl | 505.0970 # | 505→463, 301, 300, 271, 255 | MS/MS spectra |
| 21 | 14.42 | 3,5-di-O-caffeoylquinic acid isomer * | 515.1123 | 515→353, 191 #, 179 | [20,22] |
| 22 | 14.58 | 3,5-di-O-caffeoylquinic acid isomer | 515.1082 | 515→353, 191 #, 179 | [20,22] |
| 23 | 15.00 | quercetin-3-O-rhamnoside (quercetrin) | 447.0850 | 447→301, 300 #, 271, 255, 243 | [28,29] |
| 24 | 15.24 | 4,5-di-O-caffeoylquinic acid * | 515.1099 | 515→353, 191, 179, 173 # | [20,22] |
| 25 | 16.49 | 3,4-di-O-caffeoylquinic acid isomer * | 515.1128 | 515→353, 335, 191, 179, 173 # | [20,22] |
© 2017 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masike, K.; Khoza, B.S.; Steenkamp, P.A.; Smit, E.; Dubery, I.A.; Madala, N.E. A Metabolomics-Guided Exploration of the Phytochemical Constituents of Vernonia fastigiata with the Aid of Pressurized Hot Water Extraction and Liquid Chromatography-Mass Spectrometry. Molecules 2017, 22, 1200. https://doi.org/10.3390/molecules22081200
Masike K, Khoza BS, Steenkamp PA, Smit E, Dubery IA, Madala NE. A Metabolomics-Guided Exploration of the Phytochemical Constituents of Vernonia fastigiata with the Aid of Pressurized Hot Water Extraction and Liquid Chromatography-Mass Spectrometry. Molecules. 2017; 22(8):1200. https://doi.org/10.3390/molecules22081200
Chicago/Turabian StyleMasike, Keabetswe, Bradley S. Khoza, Paul A. Steenkamp, Elize Smit, Ian A. Dubery, and Ntakadzeni E. Madala. 2017. "A Metabolomics-Guided Exploration of the Phytochemical Constituents of Vernonia fastigiata with the Aid of Pressurized Hot Water Extraction and Liquid Chromatography-Mass Spectrometry" Molecules 22, no. 8: 1200. https://doi.org/10.3390/molecules22081200





