Extracts Obtained from Pterocarpus angolensis DC and Ziziphus mucronata Exhibit Antiplasmodial Activity and Inhibit Heat Shock Protein 70 (Hsp70) Function
Abstract
:1. Introduction
2. Results
2.1. Z. mucronata and P. angolensis Extracts Contain Phenolic Compounds
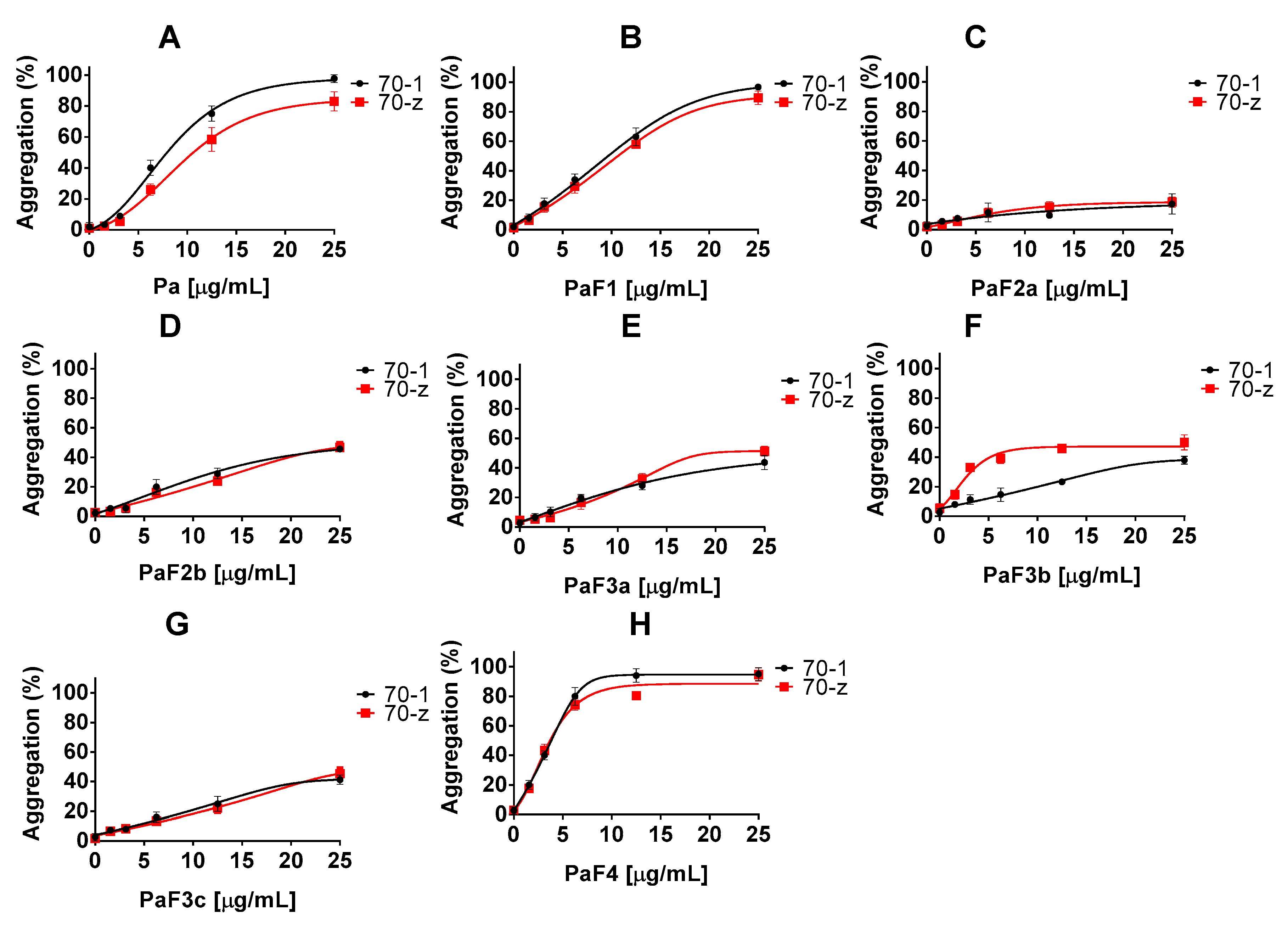
2.2. Z. mucronata and P. angolensis Extracts Suppress the Chaperone Function of Both PfHsp70-1 and PfHsp70-z
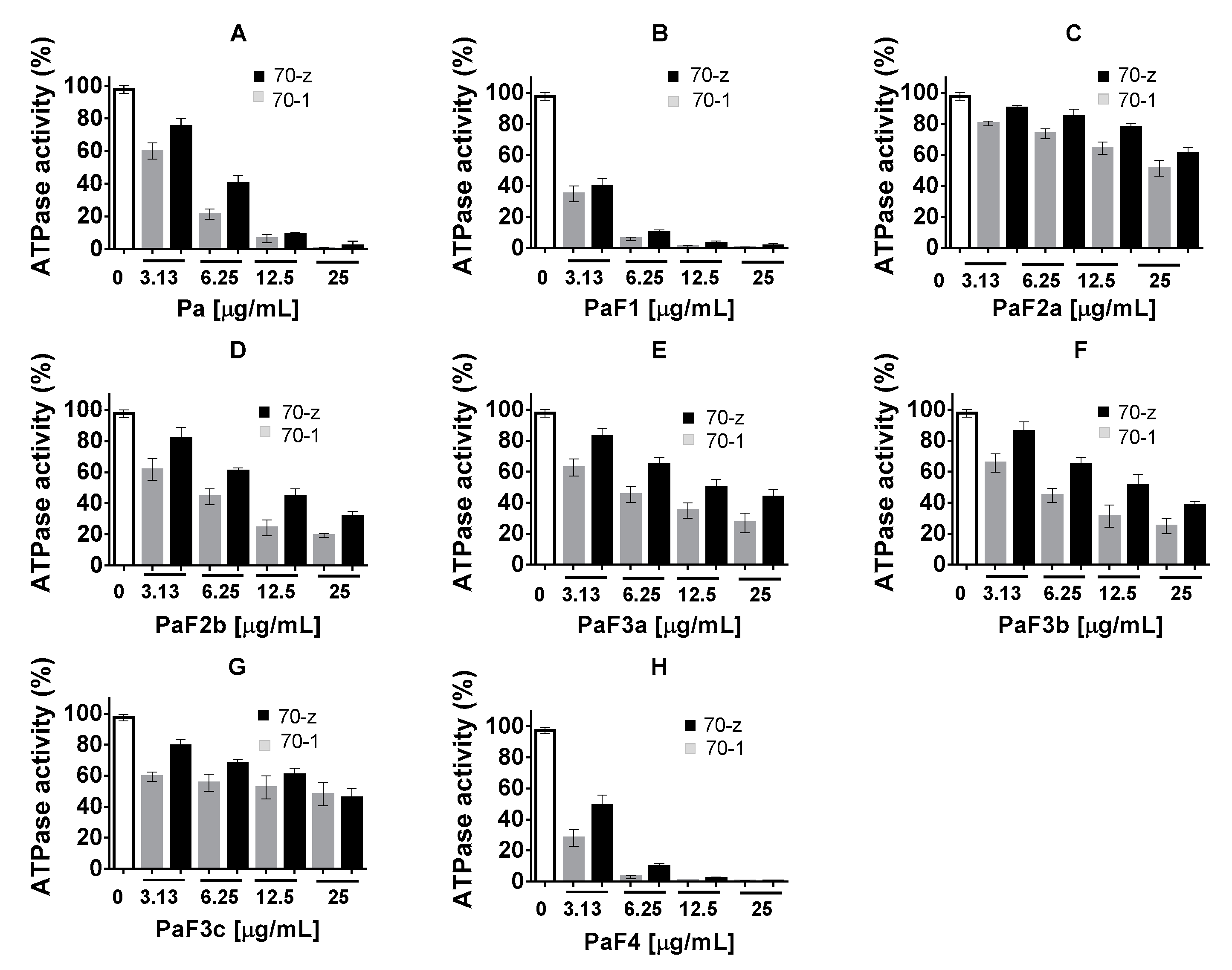
2.3. Select P. angolensis and Z. mucronata Extracts Inhibit PfHsp70-1 and PfHsp70-z ATPase Activity
2.4. Z. mucronata and P. angolensis Extracts Inhibit Growth of Plasmodium falciparum Parasites
3. Discussion
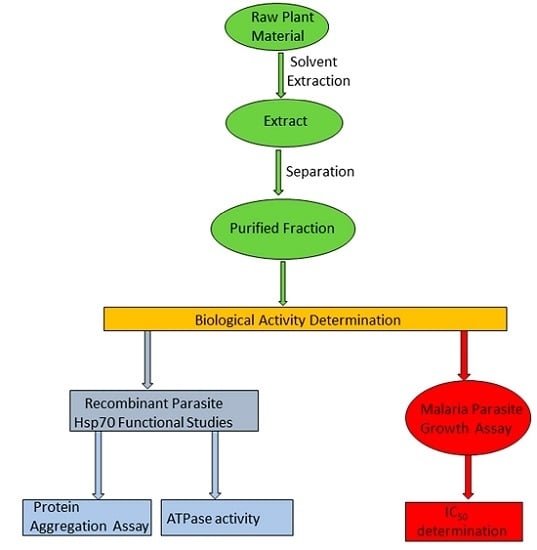
4. Materials and Methods
4.1. Materials
4.2. Fractionation of Pterocarpus angolensis and Ziziphus mucronata Stem Bark Extracts
4.3. Quantification of Phenolics in Pterocarpus angolensis and Ziziphus mucronata Extracts
4.4. Expression and Purification of Recombinant Proteins
4.5. Analysis of the Effect of P. angolensis and Z. mucronata Extracts on the Chaperone Function of PfHsp70-1 and PfHsp70-z
4.6. Investigation of the Effects of P. angolensis and Z. mucronata Extracts on Basal Hsp70 ATPase Activity
4.7. Investigation of the Effects of P. angolensis and Z. mucronata Extracts on P. falciparum Parasite Growth
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. World Malaria Report. Available online: http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/ (accessed on 27 May 2017).
- Hartl, F.U. Heat shock proteins in protein folding and membrane translocation. Semin. Immunol. 1991, 3, 5–16. [Google Scholar] [PubMed]
- Zininga, T.; Shonhai, A. Are heat shock proteins druggable candidates? Am. J. Biochem. Biotechnol. 2014, 10, 211–213. [Google Scholar] [CrossRef]
- Shonhai, A. Role of Hsp70s in development and pathogenicity of plasmodium species. In Heat Shock Proteins of Malaria; Shonhai, A., Blatch, G., Eds.; Springer: New York, NY, USA, 2014; Volume 1, pp. 47–70. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Craig, E. The Hsp70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol. 2010, 11, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, F.; Nicoll, W.S.; Zimmermann, R.; Cheetham, M.E.; Blatch, G.L. Not all J domains are created equal: Implications for the specificity of Hsp40-Hsp70 interactions. Protein Sci. 2005, 14, 1697–1709. [Google Scholar] [CrossRef] [PubMed]
- Dragovic, Z.; Broadley, S.A.; Shomura, Y.; Bracher, A.; Hartl, F.U. Molecular chaperones of the Hsp110 family act as nucleotide exchange factors of Hsp70s. EMBO J. 2006, 25, 2519–2528. [Google Scholar] [CrossRef] [PubMed]
- Shonhai, A.; Boshoff, A.; Blatch, G.L. The structural and functional diversity of Hsp70 proteins from Plasmodium falciparum. Protein Sci. 2007, 16, 1803–1818. [Google Scholar] [CrossRef] [PubMed]
- Shonhai, A. Plasmodial heat shock proteins: Targets for chemotherapy. FEMS Immunol. Med. Microbiol. 2010, 58, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Shonhai, A.; Botha, M.; de Beer, T.A.P.; Boshoff, A.; Blatch, G.L. Structure-function study of Plasmodium falciparum Hsp70 using three-dimensional modelling and in-vitro analyses. Protein Pept. Lett. 2008, 15, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Zininga, T.; Achilonu, I.; Hoppe, H.; Prinsloo, E.; Dirr, H.W.; Shonhai, A. Overexpression, purification and characterization of the Plasmodium falciparum Hsp70-z (PfHsp70-z). PLoS ONE 2015, 10, e0129445. [Google Scholar] [CrossRef] [PubMed]
- Zininga, T.; Makumire, S.; Gitau, G.W.; Njunge, J.M.; Pooe, O.J.; Klimek, H.; Scheurr, R.; Raifer, R.; Prinsloo, E.; Przyborski, J.M.; et al. Plasmodium falciparum Hop (PfHop) interacts with the Hsp70 chaperone in a nucleotide-dependent fashion and exhibits ligand selectivity. PLoS ONE 2015, 10, e0135326. [Google Scholar] [CrossRef] [PubMed]
- Zininga, T.; Achilonu, I.; Hoppe, H.; Prinsloo, E.; Dirr, H.W.; Shonhai, A. Plasmodium falciparum Hsp70-z (Hsp110c) exhibits independent chaperone activity and interacts with Hsp70–1 in a nucleotide dependent fashion. Cell Stress Chaperones 2016, 21, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Zininga, T.; Pooe, O.J.; Makhado, P.B.; Ramatsui, L.; Achilinou, I.; Prinsloo, E.; Hoppe, H.; Dirr, H.; Shonhai, A. Polymyxin B inhibits the chaperone activity of Plasmodium falciparum Hsp70. Cell Stress Chaperones 2017. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.P.; Chandra, B.R.; Bhattacharya, A.; Akhouri, R.R.; Singh, S.K.; Sharma, A. Hyper-expansion of asparagines correlates with an abundance of proteins with prion-like domains in Plasmodium falciparum. Mol. Biochem. Parasitol. 2004, 137, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, R.; Archarya, P.; Chandran, S.; Daily, J.P.; Tatu, U. Chaperone expression profiles correlate with distinct physiological states of Plasmodium falciparum in malaria patients. Malar. J. 2010, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Shonhai, A.; Maier, A.G.; Przyborski, J.M.; Blatch, G.L. Intracellular protozoan parasites of humans: The role of molecular chaperones in development and pathogenesis. Protein Pept. Lett. 2011, 18, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Samie, A.; Housein, A.; Lall, N.; Meyer, J.J.M. Crude extracts of, and purified compounds from Pterocarpus angolensis, and the essential oil of Lippia javanica: Their in vitro cytotoxicities and activities against selected bacteria and Entamoeba histolytica. Ann. Trop. Med. Parasitol. 2009, 103, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Luseba, D.; Elgorashi, E.; Ntloedibe, D.; van Staden, J. Antibacterial, anti-inflammatory and mutagenic effects of some medicinal plants used in South Africa for the treatment of wounds and retained placenta in livestock. S. Afr. J. Bot. 2007, 73, 378–383. [Google Scholar] [CrossRef]
- Mulaudzi, R.B.; Ndhlala, A.R.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Antimicrobial properties and phenolic contents of medicinal plants used by the Venda people for conditions related to venereal disease. J. Ethnopharmacol. 2011, 135, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Sigidi, M.; Anokwuru, C.; Zininga, T.; Tshisikhawe, M.P.; Shonhai, A.; Ramaite, I.; van der Venter, M.; Traoré, A.N.; Potgieter, N. Comparative in vitro cytotoxic, anti-inflammatory and anti-microbiological activities of two indigenous Venda medicinal plants. Trans. Med. Commun. 2016, 1, 9. [Google Scholar] [CrossRef]
- Red List of South African Plants: Ziziphus Mucronata. Available online: http://redlist.sanbi.org/species.php?species=4058-4 (accessed on 2 June 2017).
- Venter, F.; Venter, J.A. Making the Most of Indigenous Trees; Briza publications: Pretoria, South Africa, 1996; p. 300. [Google Scholar]
- Hutchings, A.; Haxton-Scott, A.; Lewis, G; Cunningham, A. Zulu Medicinal Plants—An Inventory Pietermaritizburg; University of Natal Press: Pietermaritzburg, South Africa, 1996. [Google Scholar]
- Luthuli, S.D.; Chili, M.M.; Revaprasadu, N.; Shonhai, A. Cysteine-capped gold nanoparticles suppress aggregation of proteins exposed to heat stress. IUBMB Life 2013, 65, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Makler, M.T.; Hinrichs, D.J. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am. J. Trop. Med. Hyg. 1993, 48, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Komlaga, G.; Cojean, S.; Dickson, R.A.; Beniddir, M.A.; Suyyagh-Albouz, S.; Mensah, M.L.K.; Loiseau, P.M. Antiplasmodial activity of selected medicinal plants used to treat malaria in Ghana. J. Parasitol. Res. 2016, 115, 3185–3195. [Google Scholar] [CrossRef] [PubMed]
- Peatey, C.L.; Leroy, D.; Gardiner, D.L.; Trenholme, K.R. Anti-malarial drugs: How effective are they against Plasmodium falciparum gametocytes? Malar. J. 2012, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Rahardja, F.; Tjokropranoto, R.; Widowati, W.; Lusiana, S.; Suhendra, A.; Tjahjani, S.; Budiman, I.; Maesaroh, M.; Fauziah, N. Phytochemical assay, potential of antimalarial and antioxidant activities of green tea extract and its fraction. J. Biomed. Eng. 2015, 1, 1–6. [Google Scholar]
- Lim, S.S.; Kim, H.-S.; Lee, D.-U. In vitro antimalarial activity of flavonoids and chalcones. Bull. Korean Chem. Soc. 2007, 28, 2495–2497. [Google Scholar]
- Bankole, A.E.; Adekunle, A.A.; Sowemimo, A.A.; Umebese, C.E.; Abiodun, O.; Gbotosho, G.O. Phytochemical screening and in vivo antimalarial activity of extracts from three medicinal plants used in malaria treatment in Nigeria. Parasitol. Res. 2016, 115, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 546–582, PMCID:PMC88925. [Google Scholar] [PubMed]
- Howarth, J.; Lloyd, D.G. Redox systems as conduits for antimalarial compounds. J. Antimicrob. Chemoth. 2001, 47, 122–124. [Google Scholar] [CrossRef]
- Keller, C.C.; Kremsner, P.G.; Hittner, J.B.; Misukonis, M.A.; Weinberg, J.B.; Perkins, D.J. Elevated nitric oxide production in children with malarial anemia: Hemozoin-induced nitricoxide synthase type 2 transcripts and nitric oxide in blood mononuclear cells. Infect. Immun. 2004, 72, 4868–4873. [Google Scholar] [CrossRef] [PubMed]
- Anokwuru, C.P.; Sigidi, M.T.; Zininga, T.; Tshisikhawe, M.P.; Shonhai, A.; Ramaite, I.D.I.; Traoré, A.N.; Potgieter, N. Phenolic contents, antioxidant activity and spectroscopic characteristics of Pterocarpus angolensis DC stem bark fractions. Indian J. Tradit. Know. 2017, 16, 400–406. [Google Scholar]
- ICH Q2(R1) Validation of Analytical Procedures: Text and Methodology; ICH Harmonised Tripartite Guideline; ICH Secretariat: Switzerland, Geneva, 2005; Available online: http://www.gmp-compliance.org/guidelines/gmp-guideline/ich-q2r1-validation-of-analytical-procedures-text-and-methodology (accessed on 28 June 2017).
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson Educational Limited: Edinburgh Gate, UK; pp. 110–151.
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1997, 193, 673–675. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Samples | Compounds [mg/g Dry Weight]/(±Standard Deviation; n = 3) | ||||||
|---|---|---|---|---|---|---|---|
| Protocatechin | Catechin | Gallic Acid | Caffeic Acid | Rutin | Epicatechin | Taxifolin | |
| ZF1 | ND | - | - | - | - | - | - |
| ZF2 | 0.04 (±0.003) | 2.25 (±0.12) | - | 0.29 (±0.02) | 0.02 (±0.001) | 5.34 (±0.08) | 0.16 (±0.01) |
| ZF3 | 0.26 (±0.02) | 1.71 (±0.21) | - | 2.72 (±0.20) | - | 4.06 (±0.30) | 0.09 (±0.01) |
| ZF4 | 0.46 (±0.03) | 0.72 (±0.16) | 0.41 (±0.03) | - | - | 1.47 (±0.02) | 0.04 (±0.002) |
| ZF5 | 0.58 (±0.04) | 0.23 (±0.04) | 0.18 (±0.02) | - | - | 0.41 (±0.01) | 0.01 (±0.001) |
| PaF1 | ND | - | - | - | - | - | - |
| PaF2a | ND | - | -- | - | - | 15.1 (±0.23) | 0.02 (±0.001) |
| PaF2b | ND | - | - | - | 0.19 (±0.001) | - | |
| PaF3a | ND | - | - | - | - | 0.02 (±0.003) | - |
| PaF3b | ND | - | - | - | - | 0.41 (±0.006) | - |
| PaF4 | ND | - | - | - | - | 0.05 (±0.0001) | - |
| Relative Aggregation% (±Standard Deviation; n = 3) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | DMSO | Pa | PaF1 | PaF2a | PaF2b | PaF3a | PaF3b | PaF3c | PaF4 | |
| PfHsp70-1 | 1.2 (±0.9) | 1.3 (±0.5) | 2.3 (±0.5) | 1.2 (±0.9) | 1.4 (±0.3) | 3.3 (±0.4) | 1.3 (±0.5) | 1.6 (±0.9) | 1.9 (±0.7) | 2.2 (±0.8) |
| PfHsp70-z | 1.1 (±0.8) | 2.2 (±0.2) | 1.2 (±0.4) | 1.9 (±0.8) | 3.2 (±0.5) | 1.3 (±0.2) | 1.2 (±0.8) | 2.0 (±0.6) | 1.2 (±0.2) | 1.7 (±0.8) |
| BSA | 1.6 (±1.2) | 1.2 (±0.2) | 1.5 (±0.9) | 1.6 (±0.4) | 1.3 (±0.5) | 1.2 (±0.9) | 1.9 (±0.7) | 2.7 (±1.4) | 3.9 (±2.7) | 1.6 (±1.2) |
| MDH | 98.0 (±1.2) | 91.6 (±1.2) | 86.6 (±1.2) | 97.2 (±0.9) | 91.6 (±1.2) | 90.2 (±0.9) | 89.6 (±1.2) | 95.2 (±0.9) | 98.6 (±1.2) | 92.2 (±0.9) |
| Buffer | DMSO | Zm | ZmF1 | ZmF2 | ZmF3 | ZmF4 | ZmF5 | |||
| PfHsp70-1 | 1.2 (±0.9) | 1.3 (±0.5) | 1.6 (±0.2) | 1.4 (±0.3) | 1.5 (±0.6) | 1.2 (±0.9) | 1.9 (±0.3) | 1.7 (±0.7) | ||
| PfHsp70-z | 1.1 (±0.8) | 1.2 (±0.8) | 1.7 (±0.4) | 1.6 (±0.8) | 1.9 (±0.3) | 1.7 (±0.1) | 1.5 (±0.2) | 1.8 (±0.8) | ||
| BSA | 1.6 (±1.2) | 1.3 (±0.2) | 2.0 (±0.9) | 1.7 (±0.4) | 1.5 (±0.5) | 1.6 (±0.9) | 2.0 (±0.7) | 2.2 (±1.7) | ||
| MDH | 98.3 (±1.2) | 90.6 (±1.2) | 97.2 (±0.2) | 89.5 (±0.9) | 93.6 (±0.4) | 91.3 (±0.5) | 95.2 (±0.9) | 92.9 (±0.7) | ||
| IC50 (µg/mL)/[±Standard Deviation] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pa | PaF1 | PaF2a | PaF2b | PaF3a | PaF4 | Zm | ZmF2 | ZmF4 | ZmF5 | |
| PfHsp70-1 | 12.3 (±1.2) | 0.5 (±0.2) | ND | 15.6 (±2.1) | 10.1 (±1.5) | 0.8 (±0.3) | 9.3 (±1.8) | 3.1 (±1.1) | ND (±5.2) | 6.0 (±1.2) |
| PfHsp70-z | 17.5 (±2.1) | 9.0 (±1.1) | 6.5 (±0.2) | ND | 13.7 (±2.0) | 6.1 (±1.0) | 13.8 (±2.1) | 4.3 (±0.4) | 3.2 (±1.0) | 6.4 (±0.3) |
| Compound | IC50 (µg/mL)/[±Standard Deviation; n = 2] |
|---|---|
| Pa | 13.87 (±0.20) |
| PaF1 | 0.7945 (±0.002) |
| PaF4 | 1.961 (±0.01) |
| Zm | 7.4 (±0.3) |
| ZmF2 | 6.404 (±0.02) |
| ZmF5 | 19.9 (±0.3) |
| Chloroquine | 0.008522 (±0.0004) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zininga, T.; Anokwuru, C.P.; Sigidi, M.T.; Tshisikhawe, M.P.; Ramaite, I.I.D.; Traoré, A.N.; Hoppe, H.; Shonhai, A.; Potgieter, N. Extracts Obtained from Pterocarpus angolensis DC and Ziziphus mucronata Exhibit Antiplasmodial Activity and Inhibit Heat Shock Protein 70 (Hsp70) Function. Molecules 2017, 22, 1224. https://doi.org/10.3390/molecules22081224
Zininga T, Anokwuru CP, Sigidi MT, Tshisikhawe MP, Ramaite IID, Traoré AN, Hoppe H, Shonhai A, Potgieter N. Extracts Obtained from Pterocarpus angolensis DC and Ziziphus mucronata Exhibit Antiplasmodial Activity and Inhibit Heat Shock Protein 70 (Hsp70) Function. Molecules. 2017; 22(8):1224. https://doi.org/10.3390/molecules22081224
Chicago/Turabian StyleZininga, Tawanda, Chinedu P. Anokwuru, Muendi T. Sigidi, Milingoni P. Tshisikhawe, Isaiah I. D. Ramaite, Afsatou N. Traoré, Heinrich Hoppe, Addmore Shonhai, and Natasha Potgieter. 2017. "Extracts Obtained from Pterocarpus angolensis DC and Ziziphus mucronata Exhibit Antiplasmodial Activity and Inhibit Heat Shock Protein 70 (Hsp70) Function" Molecules 22, no. 8: 1224. https://doi.org/10.3390/molecules22081224