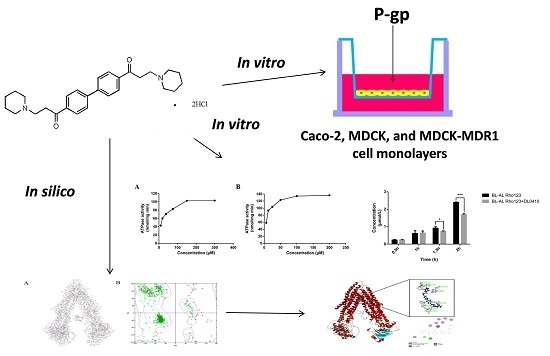
Effects of P-Glycoprotein on the Transport of DL0410, a Potential Multifunctional Anti-Alzheimer Agent
Abstract
:1. Introduction
2. Results
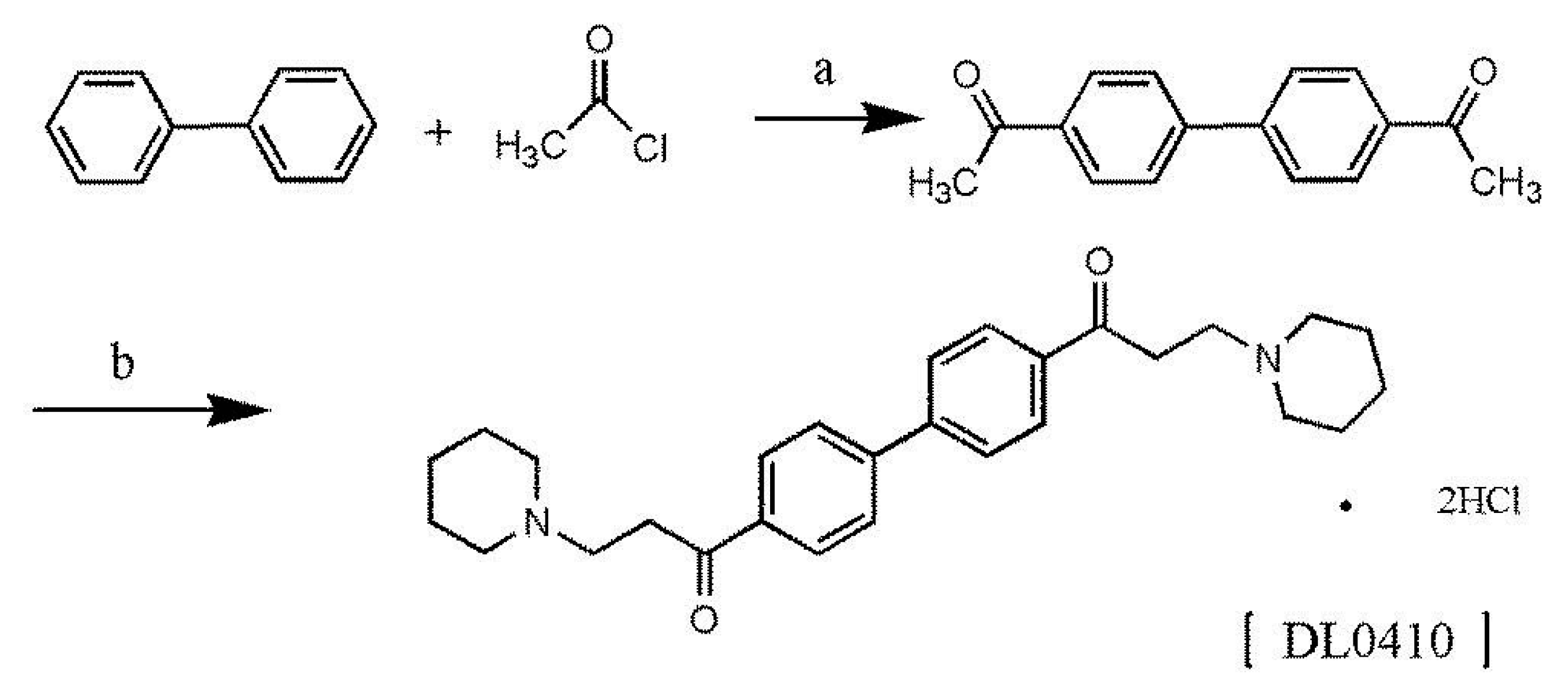
2.1. Synthesis of 1,1’-([1,1’-Biphenyl]-4,4’-diyl)bis(3-(piperidin-1-yl)propan-1-one)dihydrochloride (DL0410)
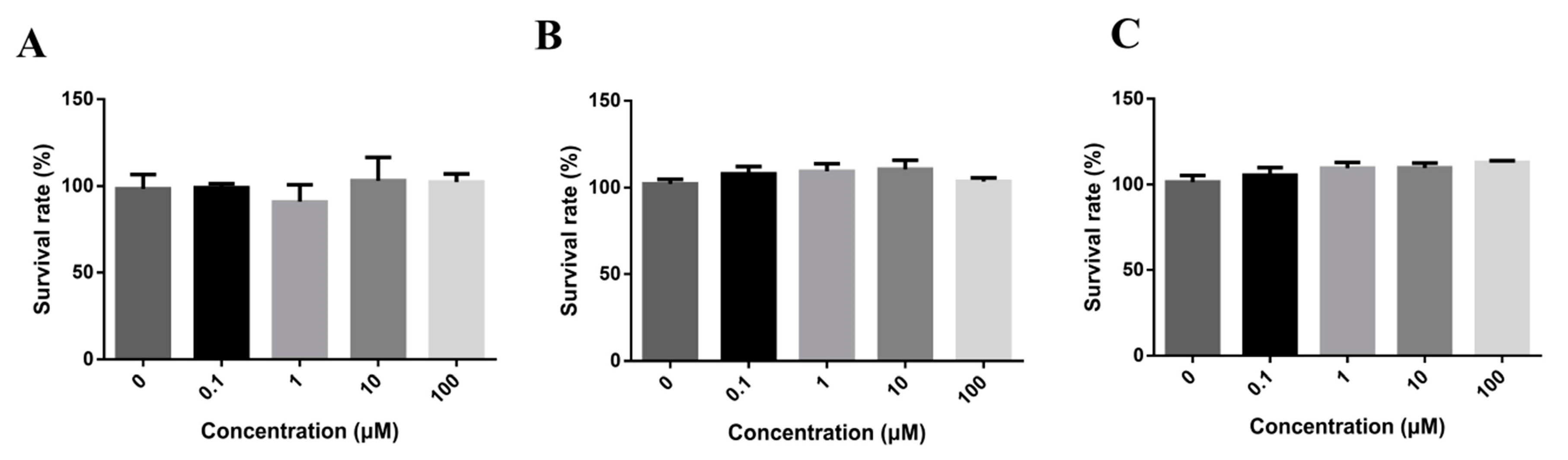
2.2. DL0410 Cytotoxicity Profile
2.3. Transcellular Transport of DL0410 across Caco-2 Cell Monolayer
2.4. Transcellular Transport of DL0410 across MDCK, and MDCK-MDR1 Cell Monolayers
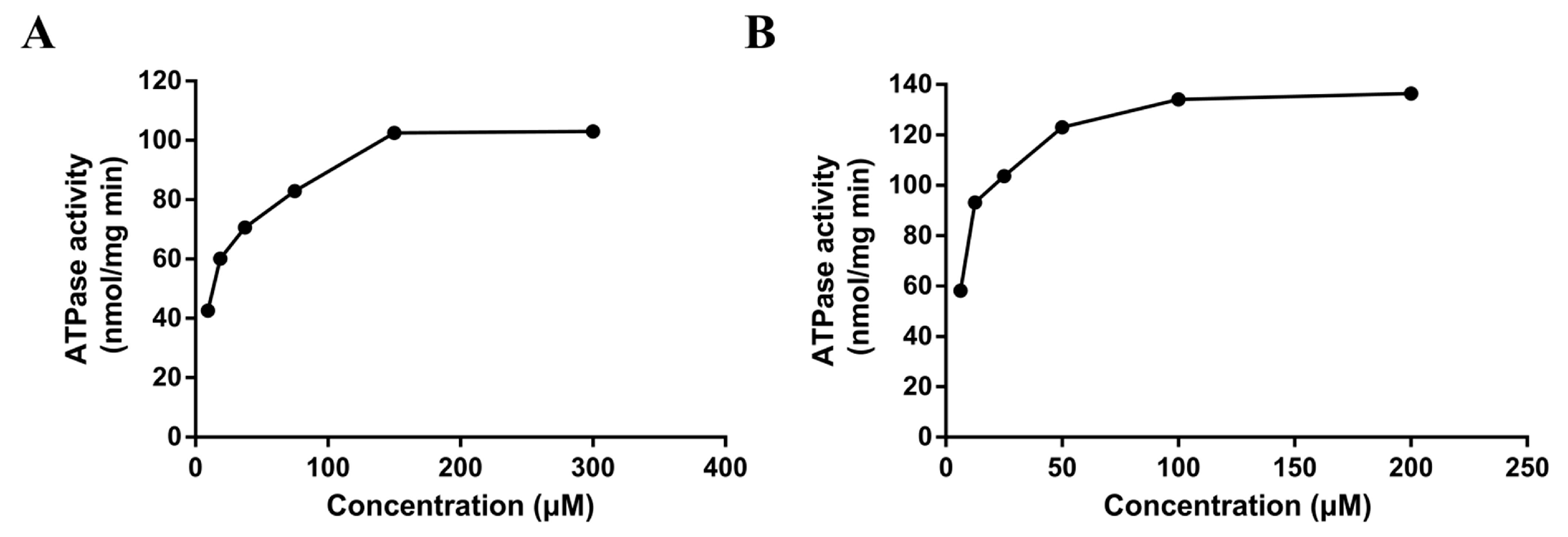
2.5. Stimulation of P-gp ATPase by DL0410
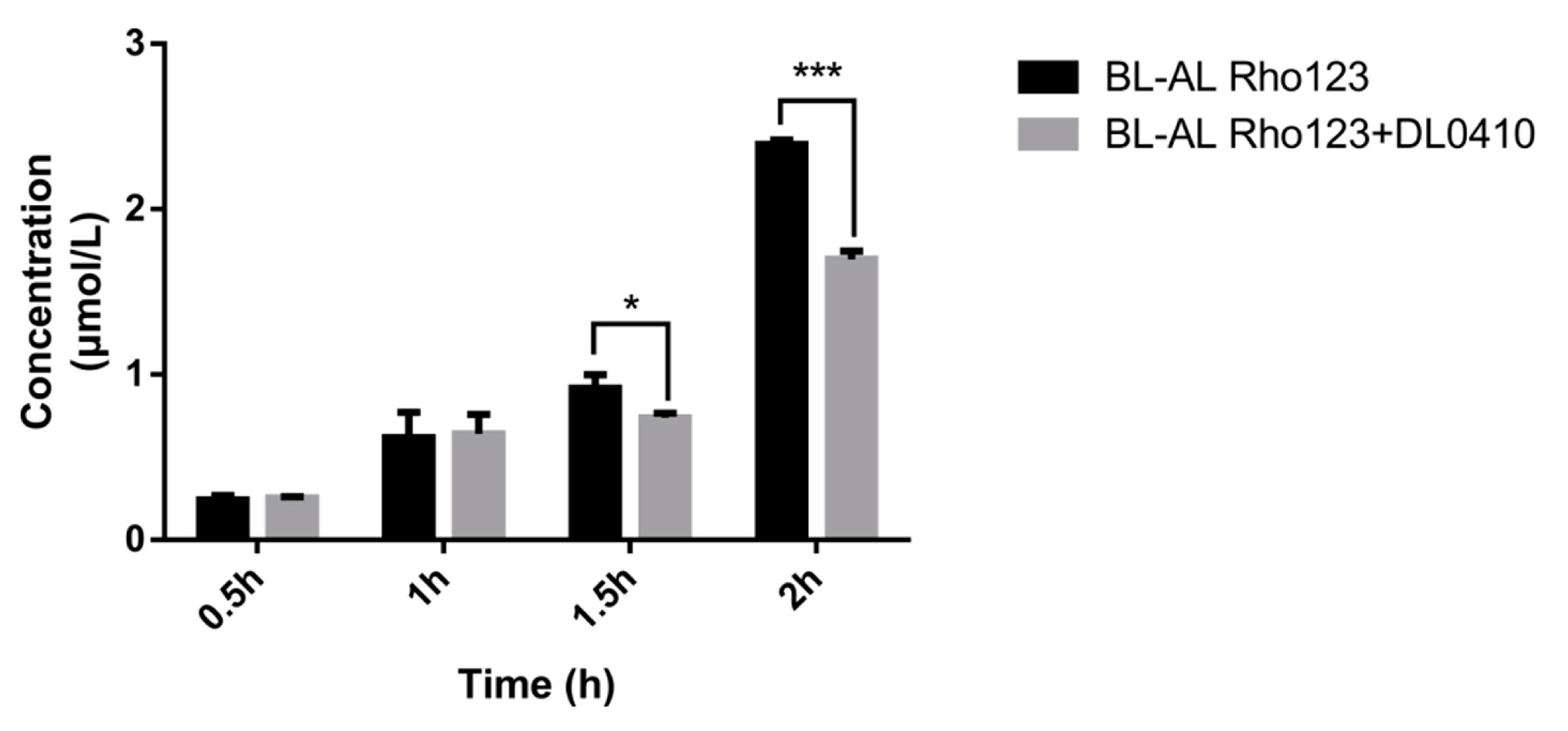
2.6. Effects of DL0410 on Rho123 Transport in MDCK-MDR1 Cell
2.7. Homology Modelling of MDR1
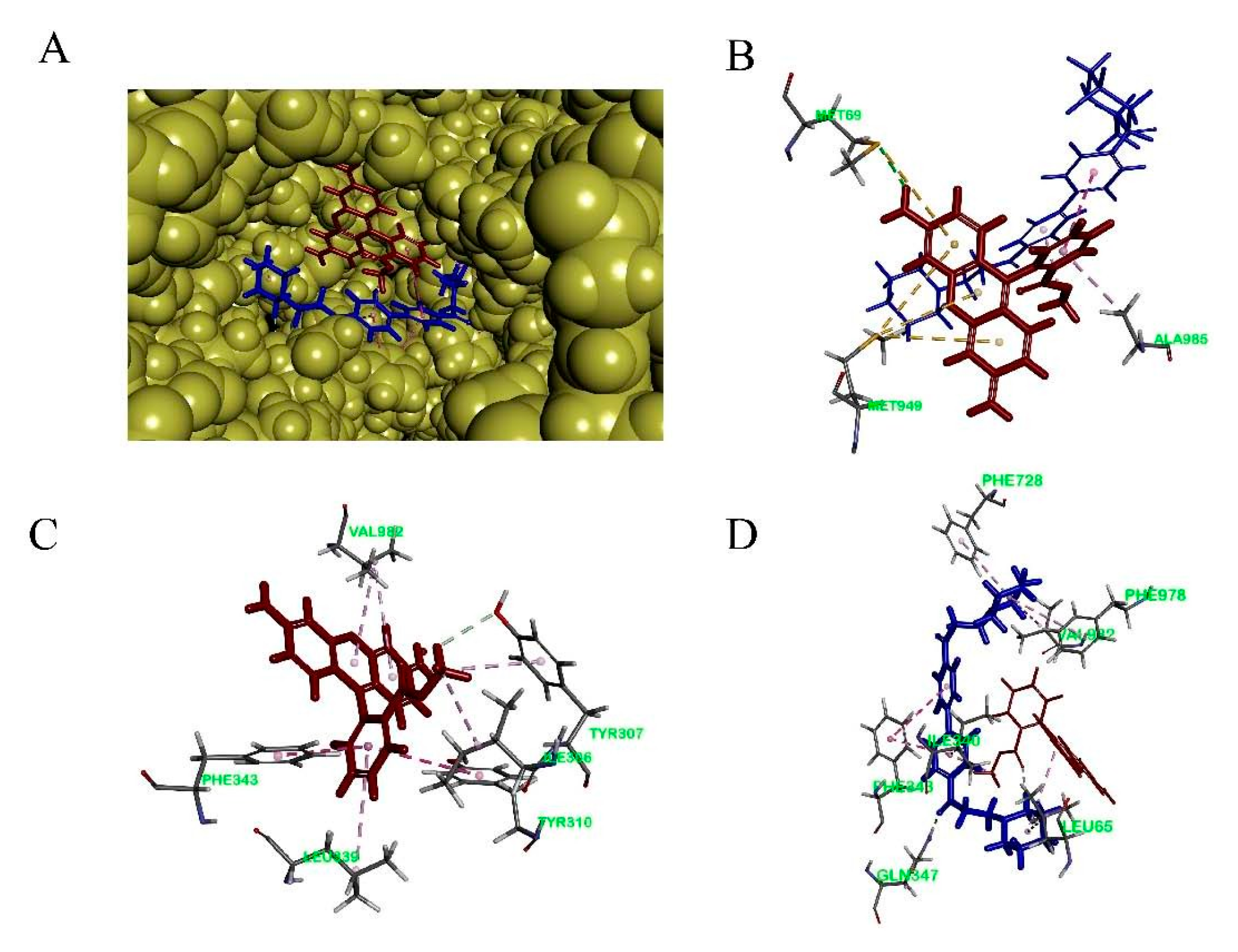
2.8. DL0410 Docking to the Drug Binding Pocket of P-gp
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis of DL0410
4.3. Cell Culture
4.4. Cytotoxicity Study In Vitro
4.5. Transport Assays
4.6. Determination of P-gp ATPase Activity
4.7. Liquid Chromatography/Mass Spectral Analysis
4.8. Homology Modeling
4.9. Molecular Docking
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oboudiyat, C.; Glazer, H.; Seifan, A.; Greer, C.; Isaacson, R.S. Alzheimer’s disease. Semin. Neurol. 2013, 33, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Mucke, L. Neuroscience: Alzheimer’s disease. Nature 2009, 461, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Tayeb, H.O.; Yang, H.D.; Price, B.H.; Tarazi, F.I. Pharmacotherapies for Alzheimer’s disease: Beyond cholinesterase inhibitors. Pharmacol. Ther. 2012, 134, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Ghidoni, R.; Galimberti, D.; Squitti, R. Pharmacogenomics in Alzheimer’s disease: A genome-wide association study of response to cholinesterase inhibitors. Neurobiol. Aging 2013, 34, 1711–1717. [Google Scholar]
- Citron, M. Alzheimer’s disease: Strategies for disease modification. Nat. Rev. Drug Discov. 2010, 9, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, G.; Greig, N.H.; Khan, J.A.; Kamal, M.A. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS. Neurol. Disord. Drug Targets 2014, 13, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Raddatz, R.; Tao, M.; Hudkins, R.L. Histamine H3 antagonists for treatment of cognitive deficits in CNS diseases. Curr. Top. Med. Chem. 2010, 10, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhou, W.; Song, J.K.; Feng, Z.Y.; Yang, R.Y.; Wu, S.; Wang, L.; Liu, A.L.; Du, G.H. DL0410, a novel dual cholinesterase inhibitor, protects mouse brains against Aβ-induced neuronal damage via the Akt/JNK signaling pathway. Acta Pharmacol. Sin. 2016, 37, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Zhao, G.; Wang, D.M.; Pang, X.C.; Wang, S.B.; Fang, J.S.; Li, C.; Liu, A.L.; Wu, S.; Du, G.H. DL0410 can reverse cognitive impairment, synaptic loss and reduce plaque load in APP/PS1 transgenic mice. Pharmacol. Biochem. Behav. 2015, 139, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.S.; Yang, R.Y.; Gao, L.; Zhou, D.; Yang, S.; Liu, A.L.; Du, G.H. Predictions of BuChE inhibitors using support vector machine and naive Bayesian classification techniques in drug discovery. J. Chem. Inf. Model. 2013, 53, 3009–3020. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.S.; Li, Y.J.; Liu, R.; Pang, X.C.; Li, C.; Yang, R.Y.; He, Y.Y.; Lian, W.W.; Liu, A.L.; Du, G.H. Discovery of multitarget-directed ligands against Alzheimer’s disease through systematic prediction of chemical-protein interactions. J. Chem. Inf. Model. 2015, 55, 149. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.W.; Fang, J.S.; Xu, L.J.; Zhou, W.; Kang, D.; Xiong, W.; Jia, H.; Liu, A.L.; Du, G.H. DL0410 Ameliorates Memory and Cognitive Impairments Induced by Scopolamine via Increasing Cholinergic Neurotransmission in Mice. Molecules 2017, 22, 410. [Google Scholar] [CrossRef] [PubMed]
- Glavinas, H.; Krajcsi, P.; Cserepes, J.; Sarkadi, B. The role of ABC transporters in drugresistance, metabolism and toxicity. Curr. Drug Deliv. 2004, 1, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Loscher, W.; Potschka, H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat. Rev. Neurosci. 2005, 6, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Pichardo, S.; Devesa, V.; Puerto, M.; Velez, D.; Camean, A.M. Intestinal transport of Cylindrospermopsin using the Caco-2 cell line. Toxicol. In Vitro 2017, 38, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Mi, J.; Yang, S.; Zhao, M.; Li, Y.; Sheng, L. Effects of P-glycoprotein on the intestine and blood-brain barrier transport of YZG-331, a promising sedative-hypnotic compound. Eur. J. Pharmacol. 2016, 791, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Subhani, S.; Jayaraman, A.; Jamil, K. Homology modelling and molecular docking of MDR1 with chemotherapeutic agents in non-small cell lung cancer. Biomed. Pharmacother. 2015, 71, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, O.; Saeed, M.E.; Valoti, M.; Frosini, M.; Sgaragli, G.; Efferth, T. Interactions of human P-glycoprotein transport substrates and inhibitors at the drug binding domain: Functional and molecular docking analyses. Biochem. Pharmacol. 2016, 104, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Ayrton, A.; Morgan, P. Role of transport proteins in drug absorption, distribution and excretion. Xenobiotica 2001, 31, 469–497. [Google Scholar] [CrossRef] [PubMed]
- Gschwind, L.; Rollason, V.; Daali, Y.; Bonnabry, P.; Dayer, P.; Desmeules, J.A. Role of P-glycoprotein in the uptake/efflux transport of oral vitamin K antagonists and rivaroxaban through the Caco-2 cell model. Basic Clin. Pharmacol. Toxicol. 2013, 113, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Yamashita, F.; Tsujimoto, M.; Ohtani, H.; Sawada, Y. Effects of citrus juices on the function of human organic anion transporting polypeptide OATP-B (OATP2B1). Drug Metab. Dispos. 2006, 34, 577–582. [Google Scholar]
- Luo, S.H.; Wang, Z.; Kansara, V.; Pal, D.; Mitra, A.K. Activity of a sodium-dependent vitamin C transporter (SVCT) in MDCK-MDR1 cells and mechanism of ascorbate uptake. Int. J. Pharm. 2008, 358, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Xu, C.; O’Neal, S.; Bi, H.C.; Huang, M.; Zheng, W.; Zeng, S. Roles of P-glycoprotein and multidrug resistance protein in transporting para-aminosalicylic acid and its N-acetylated metabolite in mice brain. Acta Pharmacol. Sin. 2014, 35, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Long, L.M.; Henze, H.R. Synthesis of ketone derivatives of bipheny by the Friedel-Crafts reaction. J. Am. Chem. Soc. 1941, 63, 1939–1940. [Google Scholar] [CrossRef]
- Boulton, D.W.; DeVane, C.L.; Liston, H.L.; Markowitz, J.S. In vitro P-glycoprotein affinity for atypical and conventional antipsychotics. Life Sci. 2002, 71, 163–169. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, H.; Tempczykrussell, A.; Haubold, K.; Santorico, S.A.; Shokati, T.; Christians, U. Genetic and structure-function studies of missense mutations in human endothelial lipase. PLoS ONE 2013, 8, e55716. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the compound DL0410 is available from the authors. |


| Conditions | PappA→B (10−6 cm/s) | PappB→A (10−6 cm/s) | ER |
|---|---|---|---|
| 100 μM | 1.280 ± 0.017 | 2.384 ± 0.138 | 1.858 |
| 30 μM | 0.841 ± 0.098 | 3.450 ± 0.619 | 4.103 |
| 10 μM | 0.725 ± 0.184 | 3.275 ± 0.251 | 4.518 |
| 30 μM + verapamil (50 μM) | 1.464 ± 0.201 | 1.949 ± 0.271 | 1.331 |
| Conditions | MDCK | MDCK-MDR1 | NER | ||||
|---|---|---|---|---|---|---|---|
| Papp (10−6 cm/s) | ER | Papp (10−6 cm/s) | ER | ||||
| A→B | B→A | A→B | B→A | ||||
| 100 μM | 0.950 ± 0.116 | 0.924 ± 0.005 | 0.973 | 0.321 ± 0.039 | 1.733 ± 0.105 | 5.406 | 5.556 |
| 30 μM | 0.740 ± 0.023 | 0.871 ± 0.068 | 1.177 | 0.310 ± 0.021 | 3.205 ± 0.061 | 10.323 | 8.770 |
| 10 μM | 0.502 ± 0.103 | 0.708 ± 0.114 | 1.410 | 0.297 ± 0.111 | 4.434 ± 0.376 | 14.929 | 10.588 |
| 30 μM + verapamil (50 μM) | 0.794 ± 0.057 | 0.761 ± 0.022 | 0.958 | 1.055 ± 0.0414 | 0.314 ± 0.056 | 0.298 | 0.311 |
| Compound | -CDCOKER ENERGY | -CDCOKER INTERACTION_ENENGY |
|---|---|---|
| Verapamil | 7.396 | 37.663 |
| Epirubicin | 13.898 | 39.036 |
| Rho123 | 26.988 | 30.668 |
| Dl0410 | 5.500 | 36.275 |
| Rho123(in the presence of DL0410) | 11.324 | 24.717 |
| Dl0410(in the presence of Rho123) | 12.830 | 45.560 |
| Dl0410(in the presence of verapamil) | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, X.; Wang, L.; Kang, D.; Zhao, Y.; Wu, S.; Liu, A.-L.; Du, G.-H. Effects of P-Glycoprotein on the Transport of DL0410, a Potential Multifunctional Anti-Alzheimer Agent. Molecules 2017, 22, 1246. https://doi.org/10.3390/molecules22081246
Pang X, Wang L, Kang D, Zhao Y, Wu S, Liu A-L, Du G-H. Effects of P-Glycoprotein on the Transport of DL0410, a Potential Multifunctional Anti-Alzheimer Agent. Molecules. 2017; 22(8):1246. https://doi.org/10.3390/molecules22081246
Chicago/Turabian StylePang, Xiaocong, Lin Wang, De Kang, Ying Zhao, Song Wu, Ai-Lin Liu, and Guan-Hua Du. 2017. "Effects of P-Glycoprotein on the Transport of DL0410, a Potential Multifunctional Anti-Alzheimer Agent" Molecules 22, no. 8: 1246. https://doi.org/10.3390/molecules22081246