Study of the Interactions of Bovine Serum Albumin with the New Anti-Inflammatory Agent 4-(1,3-Dioxo-1,3-dihydro-2H-isoindol-2-yl)-N′-[(4-ethoxy-phenyl)methylidene]benzohydrazide Using a Multi-Spectroscopic Approach and Molecular Docking
Abstract
:1. Introduction
2. Results and Discussion
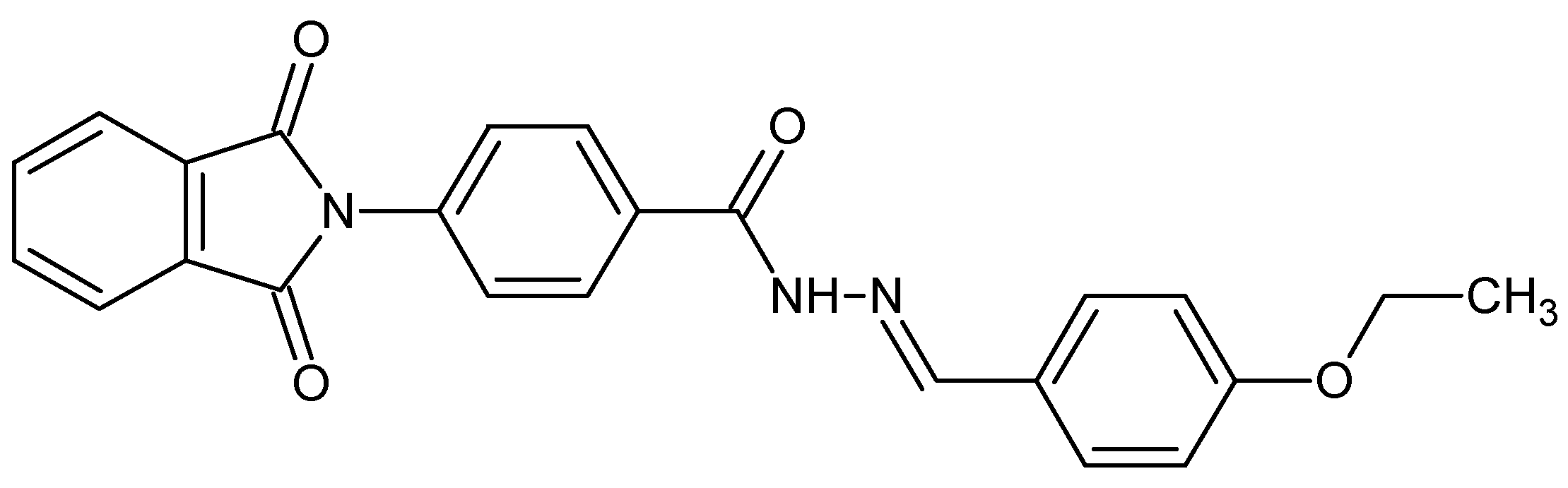
2.1. Study of Conformational Changes in BSA with UV-Visible Spectroscopy
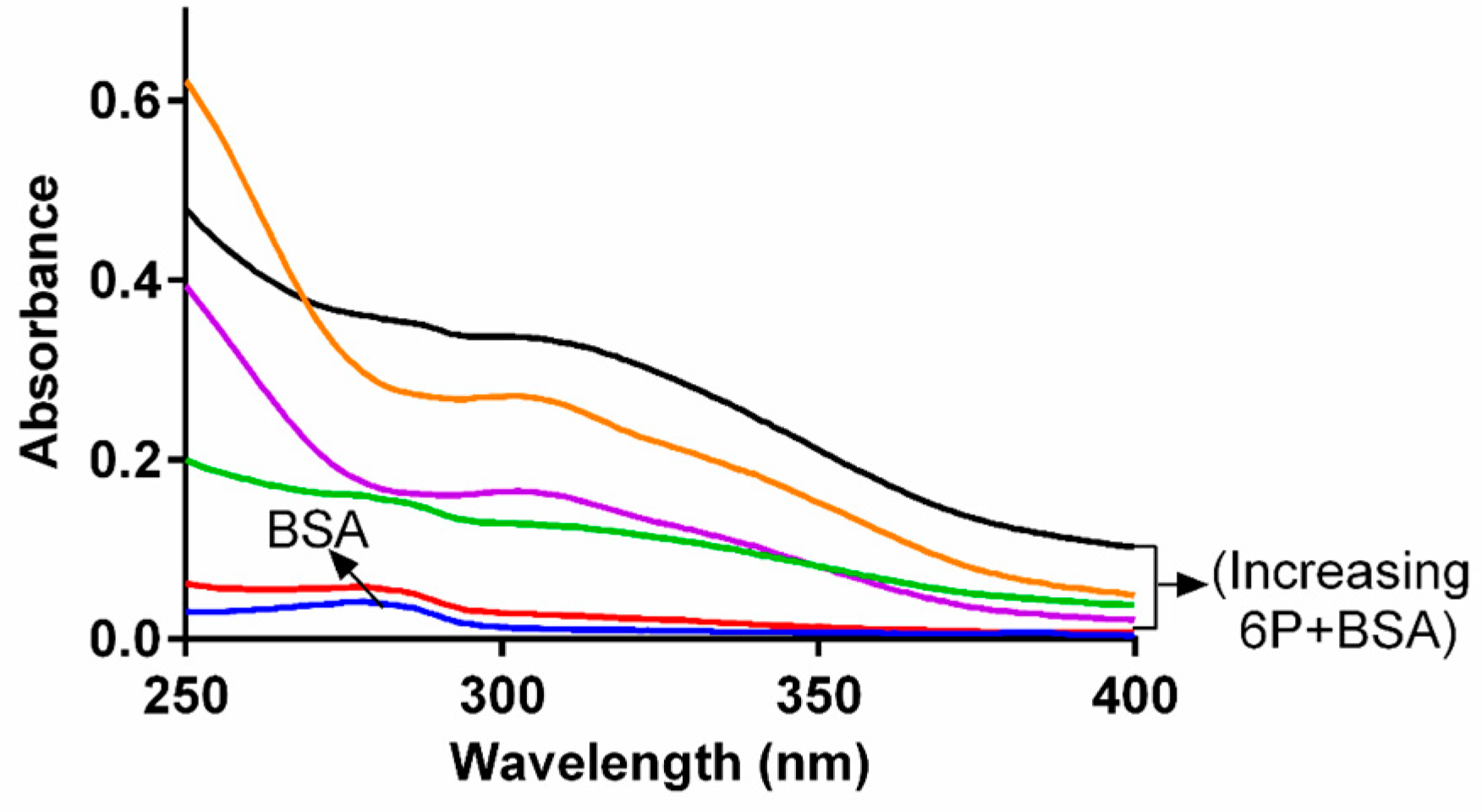
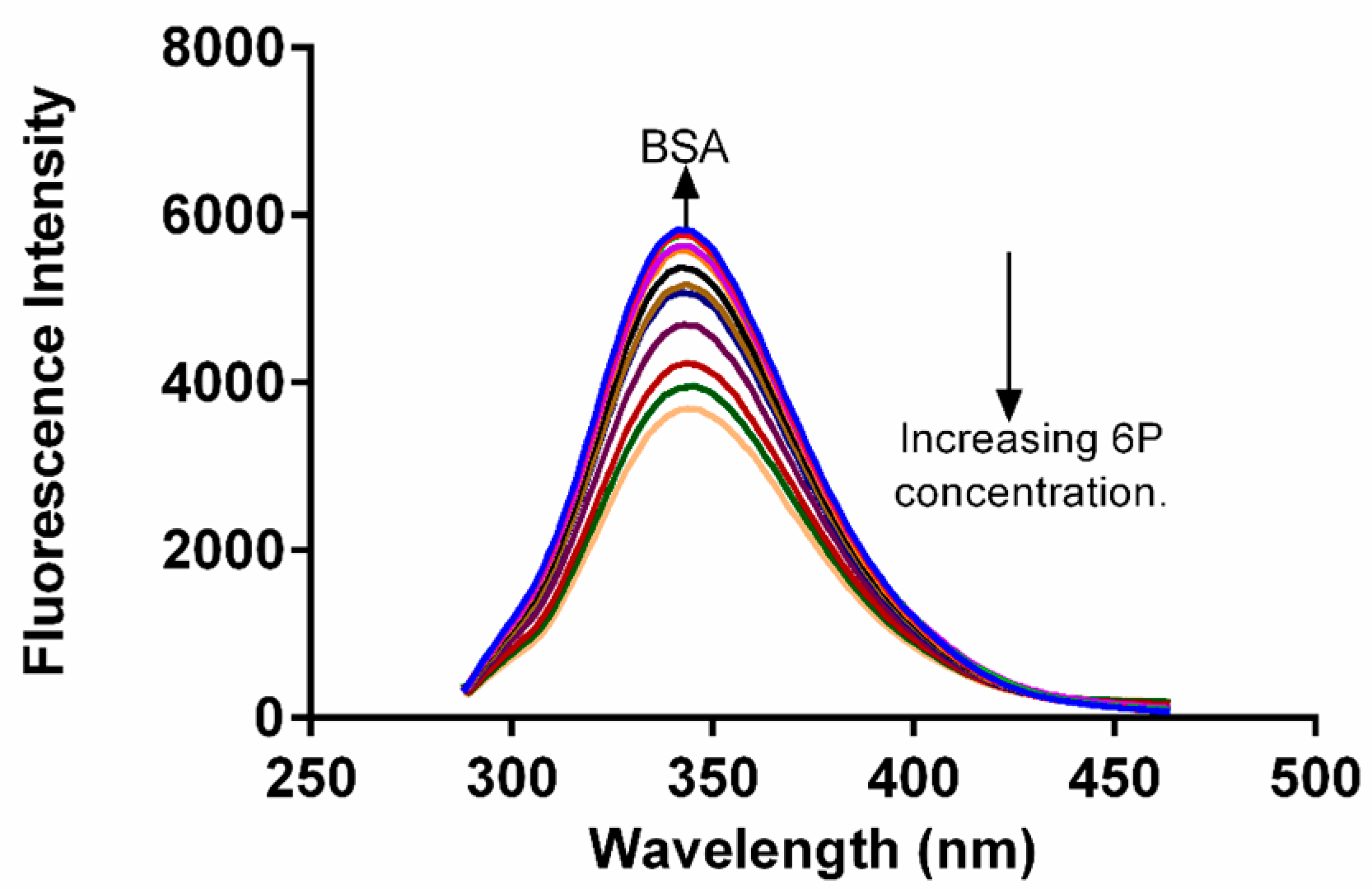
2.2. Fluorescence Quenching of BSA
2.3. Analysis of Fluorescence Quenching and Mechanism
2.4. Binding Constant and Binding Modes
2.5. Thermodynamic Parameters and Binding Forces
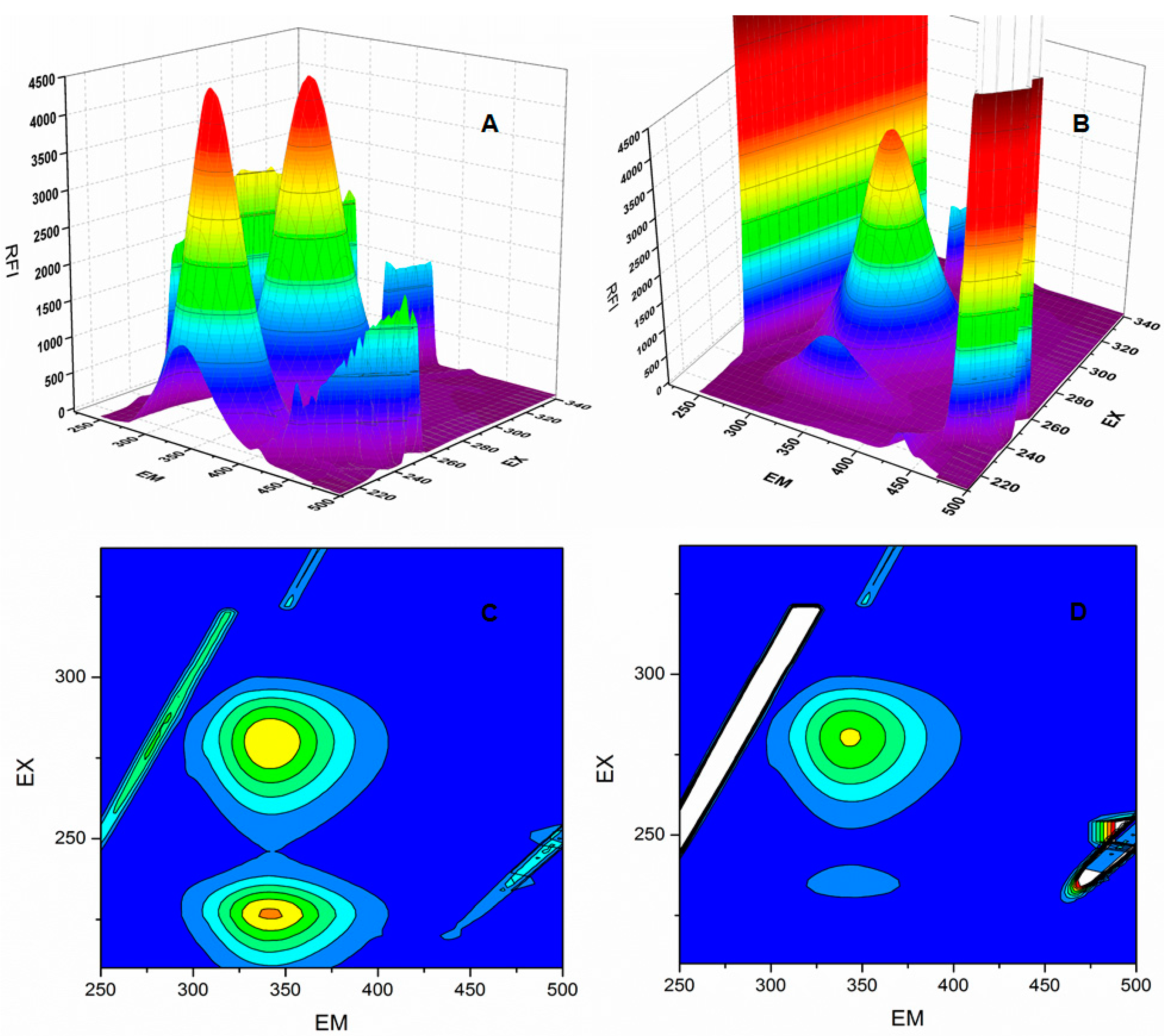
2.6. Synchronous Fluorescence Spectroscopy of BSA and 6P Complex
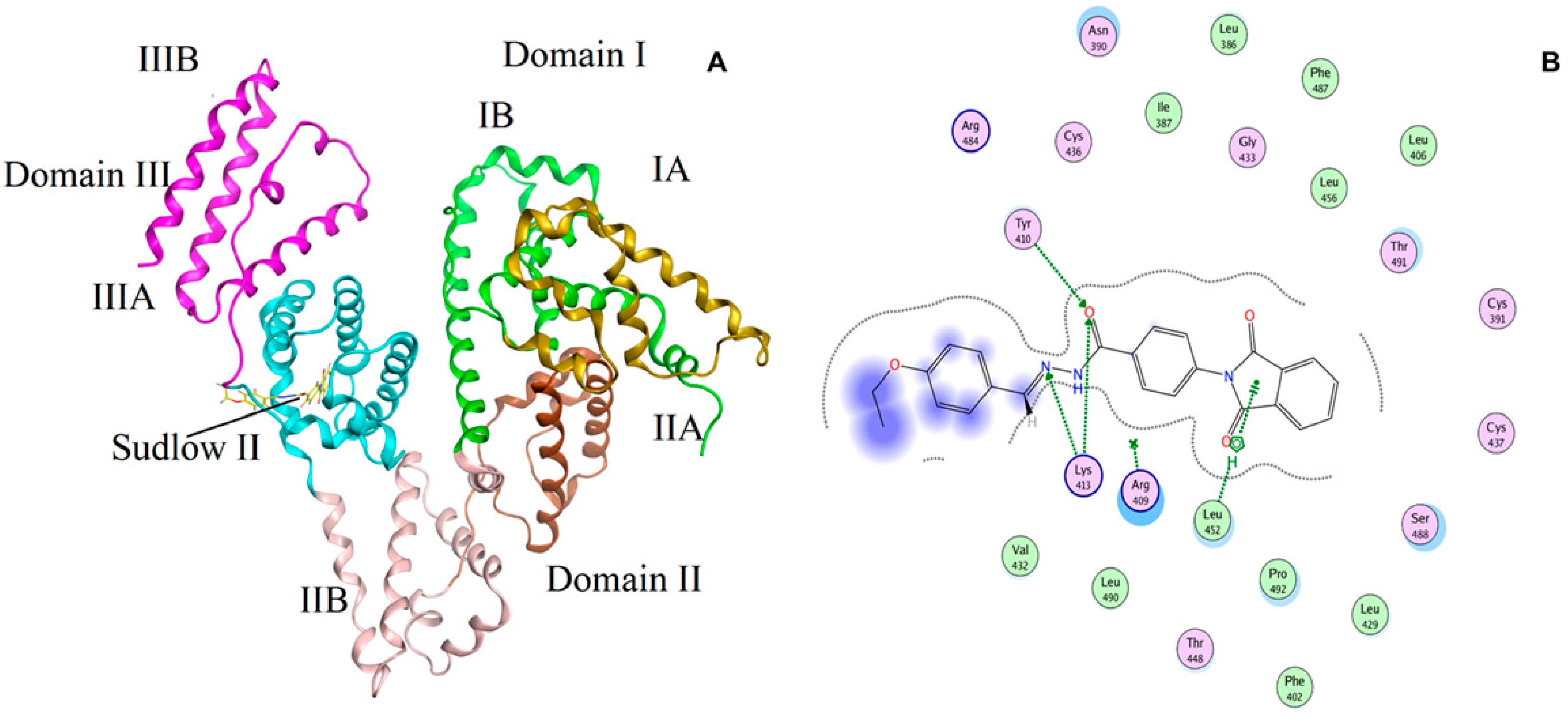
2.7. Molecular Simulation Studies
3. Materials and Methods
3.1. Chemical and Reagents
3.2. UV Spectra Measurements
3.3. Fluorescence Measurements
3.4. Synchronous Fluorescence (SF) Measurement
3.5. Molecular Docking
4. Conclusions
Acknowledgments
Author contributions
Conflicts of Interest
References
- Sampaio, E.P.; Sarno, E.N.; Galilly, R.; Cohn, Z.A.; Kaplan, G. Thalidomide selectively inhibits tumor necrosis factor a production by stimulated human monocytes. J. Exp. Med. 1991, 173, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Melchert, M.; List, A. The thalidomide saga. Int. J. Biochem. Cell Biol. 2007, 39, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.K. Properties of thalidomide and its analogues: Implications for anticancer therapy. AAPS J. 2005, 7, E14–E19. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y. Thalidomide as a multi-template for development of biologically active compounds. Arch. Der Pharm. 2008, 341, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Doherty, S.D.; Hsu, S. Innovative uses of thalidomide. Dermatol. Clin. 2010, 28, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, E.; Muià, C.; Di Paola, R.; Genovese, T.; De Sarro, A.; Cuzzocrea, S. Thalidomide treatment reduces colon injury induced by experimental colitis. Shock 2005, 23, 556–564. [Google Scholar] [PubMed]
- Amirshahrokhi, K. Anti-inflammatory effect of thalidomide in paraquat-induced pulmonary injury in mice. Int. Immunopharmacol. 2013, 17, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Goli, V. Does thalidomide have an analgesic effect? Current status and future directions. Curr. Pain Headache Rep. 2007, 11, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.C.; Fernandes, E.S.; Quintão, N.L.; Campos, M.M.; Calixto, J.B. Relevance of tumour necrosis factor-α for the inflammatory and nociceptive responses evoked by carrageenan in the mouse paw. Br. J. Pharmacol. 2006, 148, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Niwayama, S.; Turk, B.E.; Liu, J.O. Potent inhibition of tumor necrosis factor-α production by tetrafluorothalidomide and tetrafluorophthalimides. J. Med. Chem. 1996, 39, 3044–3045. [Google Scholar] [CrossRef] [PubMed]
- Muller, G.W.; Corral, L.G.; Shire, M.G.; Wang, H.; Moreira, A.; Kaplan, G.; Stirling, D.I. Structural modifications of thalidomide produce analogs with enhanced tumor necrosis factor inhibitory activity 1. J. Med. Chem. 1996, 39, 3238–3240. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Al-Omar, M.A.; Ansari, M.A.; Zoheir, K.M.; Imam, F.; Attia, S.M.; Bakheet, S.A.; Nadeem, A.; Korashy, H.M.; Voronkov, A. Design and synthesis of n-arylphthalimides as inhibitors of glucocorticoid-induced tnf receptor-related protein, proinflammatory mediators, and cytokines in carrageenan-induced lung inflammation. J. Med. Chem. 2015, 58, 8850–8867. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.A.; Zargar, S.; Ahmad, A. Ultra-performance liquid chromatography tandem mass spectrometric method development and validation for determination of neratinib in human plasma. S. Afr. J. Chem. 2015, 68, 93–98. [Google Scholar] [CrossRef]
- Wani, T.A.; Samad, A.; Sharma, P.; Tandon, M.; Paliwal, J. Determination of interchangeability of different brands of diclofenac sodium sustained release tablets in healthy subjects using pharmacokinetic end points. Lett. Drug Des. Discov. 2009, 6, 629–636. [Google Scholar] [CrossRef]
- Wani, T.A.; Bakheit, A.H.; Zargar, S.; Hamidaddin, M.A.; Darwish, I.A. Spectrophotometric and molecular modelling studies on in vitro interaction of tyrosine kinase inhibitor linifanib with bovine serum albumin. PLoS ONE 2017, 12, e0176015. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Bhat, M.A.; Raish, M.; Ezzeldin, E. Determination of (4-(1, 3-dioxo-1,3-dihydro-2H-isoindol-2-yl)-N′-[(4-ethoxyphenyl)methylidene]benzohydrazide, a novel anti-inflammatory agent, in biological fluids by UPLC-MS/MS: Assay development, validation and in vitro metabolic stability study. Biomed. Chromatogr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Khorsand Ahmadi, S.; Mahmoodian Moghadam, M.; Mokaberi, P.; Reza Saberi, M.; Chamani, J. A comparison study of the interaction between β-lactoglobulin and retinol at two different conditions: Spectroscopic and molecular modeling approaches. J. Biomol. Struct. Dyn. 2015, 33, 1880–1898. [Google Scholar] [CrossRef] [PubMed]
- Marouzi, S.; Rad, A.S.; Beigoli, S.; Baghaee, P.T.; Darban, R.A.; Chamani, J. Study on effect of lomefloxacin on human holo-transferrin in the presence of essential and nonessential amino acids: Spectroscopic and molecular modeling approaches. Int. J. Biol. Macromol. 2017, 97, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, Y.; Ohta, D.; Hachiya, K.; Mitsui, Y.; Takeda, K. Fluorescence behavior of tryptophan residues of bovine and human serum albumins in ionic surfactant solutions: A comparative study of the two and one tryptophan (s) of bovine and human albumins. J. Protein Chem. 1996, 15, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Lackowicz, J. Principle of Fluorescence Spectroscopy; Springer Science and Business Media: Spring Street, NY, USA, 2006; Volume 13, pp. 278–327. [Google Scholar]
- Lakowicz, J.R.; Weber, G. Quenching of fluorescence by oxygen. Probe for structural fluctuations in macromolecules. Biochemistry 1973, 12, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.J.; Liu, Y.; Wang, J.B.; Xiao, X.H.; Qu, S.S. Study of the interaction between monoammonium glycyrrhizinate and bovine serum albumin. J. Pharm. Biomed. Anal. 2004, 36, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, N.; Hadidi, S.; Feizi, F. Study on the interaction of antiviral drug ‘Tenofovir’ with human serumalbumin by spectral and molecular modeling methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 138, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.h.; Pan, D.Q.; Jiang, M.; Liu, T. T.; Wang, Q. In vitro study on binding interaction of quinapril with bovine serum albumin (BSA) using multi-spectroscopic and molecular docking methods. J. Biomol. Struct. Dyn. 2016, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Liu, G.; Kokot, S. Fluorescence spectrometric study on the interactions of isoprocarb and sodium 2-isopropylphenate with bovine serum albumin. Talanta 2008, 76, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Albert, D.H.; Tapang, P.; Magoc, T.J.; Pease, L.J.; Reuter, D.R.; Wei, R.Q.; Li, J.; Guo, J.; Bousquet, P.F.; Ghoreishi-Haack, N.S. Preclinical activity of abt-869, a multitargeted receptor tyrosine kinase inhibitor. Mol. Cancer Ther. 2006, 5, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Meti, M.D.; Nandibewoor, S.T.; Joshi, S.D.; More, U.A.; Chimatadar, S.A. Multi-spectroscopic investigation of the binding interaction of fosfomycin with bovine serum albumin. J. Pharm. Anal. 2015, 5, 249–255. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound 4-(1,3-Dioxo-1,3-dihydro-2H-isoindol-2-yl)-N′-[(4-ethoxy-phenyl)methylidene]benzohydrazine are available from the authors. |


| T (K) | R | Ksv (L·mol–1) | Kq (L·mol−1·s−1) |
|---|---|---|---|
| 298 | 0.9934 | 2.73 × 104 | 2.73 × 1012 |
| 303 | 0.9957 | 3.02 × 104 | 3.02 × 1012 |
| 308 | 0.9913 | 3.20 × 104 | 3.20 × 1012 |
| T (K) | R | Kb (L·mol−1) | n | ∆G (kJ·mol−1) | ∆H (kJ·mol−1) | ∆S (J·mol−1·K−1) |
|---|---|---|---|---|---|---|
| 298 | 0.9980 | 2.5 × 105 | 1.21 | −31.01 | −253 | −744 |
| 303 | 0.9955 | 6.2 × 104 | 1.06 | −27.29 | ||
| 308 | 0.9940 | 4.8 × 103 | 0.81 | −22.08 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wani, T.A.; Bakheit, A.H.; Al-Majed, A.-R.A.; Bhat, M.A.; Zargar, S. Study of the Interactions of Bovine Serum Albumin with the New Anti-Inflammatory Agent 4-(1,3-Dioxo-1,3-dihydro-2H-isoindol-2-yl)-N′-[(4-ethoxy-phenyl)methylidene]benzohydrazide Using a Multi-Spectroscopic Approach and Molecular Docking. Molecules 2017, 22, 1258. https://doi.org/10.3390/molecules22081258
Wani TA, Bakheit AH, Al-Majed A-RA, Bhat MA, Zargar S. Study of the Interactions of Bovine Serum Albumin with the New Anti-Inflammatory Agent 4-(1,3-Dioxo-1,3-dihydro-2H-isoindol-2-yl)-N′-[(4-ethoxy-phenyl)methylidene]benzohydrazide Using a Multi-Spectroscopic Approach and Molecular Docking. Molecules. 2017; 22(8):1258. https://doi.org/10.3390/molecules22081258
Chicago/Turabian StyleWani, Tanveer A., Ahmed H. Bakheit, Abdul-Rahman A. Al-Majed, Mashooq A. Bhat, and Seema Zargar. 2017. "Study of the Interactions of Bovine Serum Albumin with the New Anti-Inflammatory Agent 4-(1,3-Dioxo-1,3-dihydro-2H-isoindol-2-yl)-N′-[(4-ethoxy-phenyl)methylidene]benzohydrazide Using a Multi-Spectroscopic Approach and Molecular Docking" Molecules 22, no. 8: 1258. https://doi.org/10.3390/molecules22081258





