Isolation, Purification and Structural Characterization of Two Novel Water-Soluble Polysaccharides from Anredera cordifolia
Abstract
:1. Introduction
2. Results and Discussion
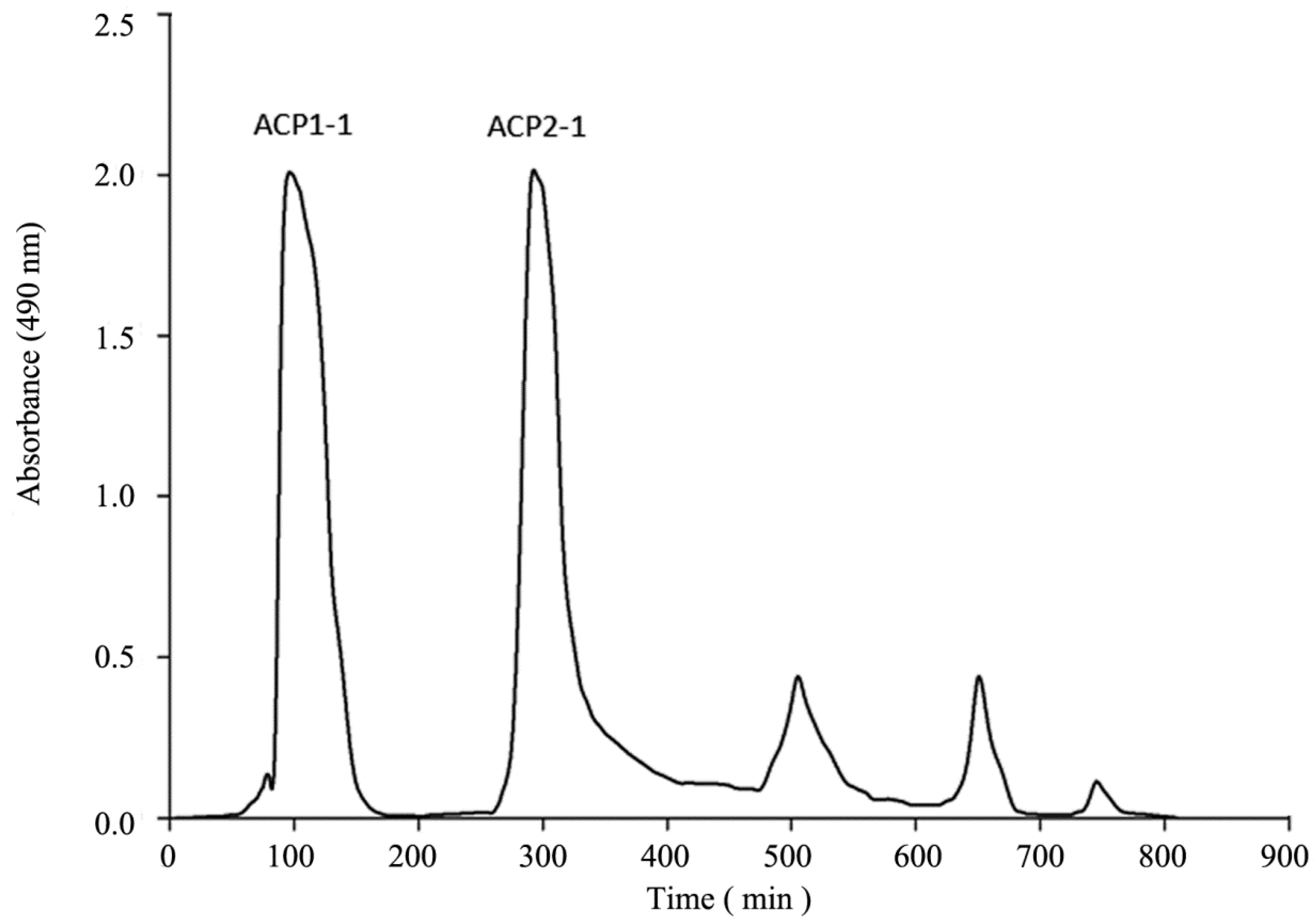
2.1. Isolation and Purification of Polysaccharides
2.2. Homogeneity and Molecular Weights of ACP1-1 and ACP2-1
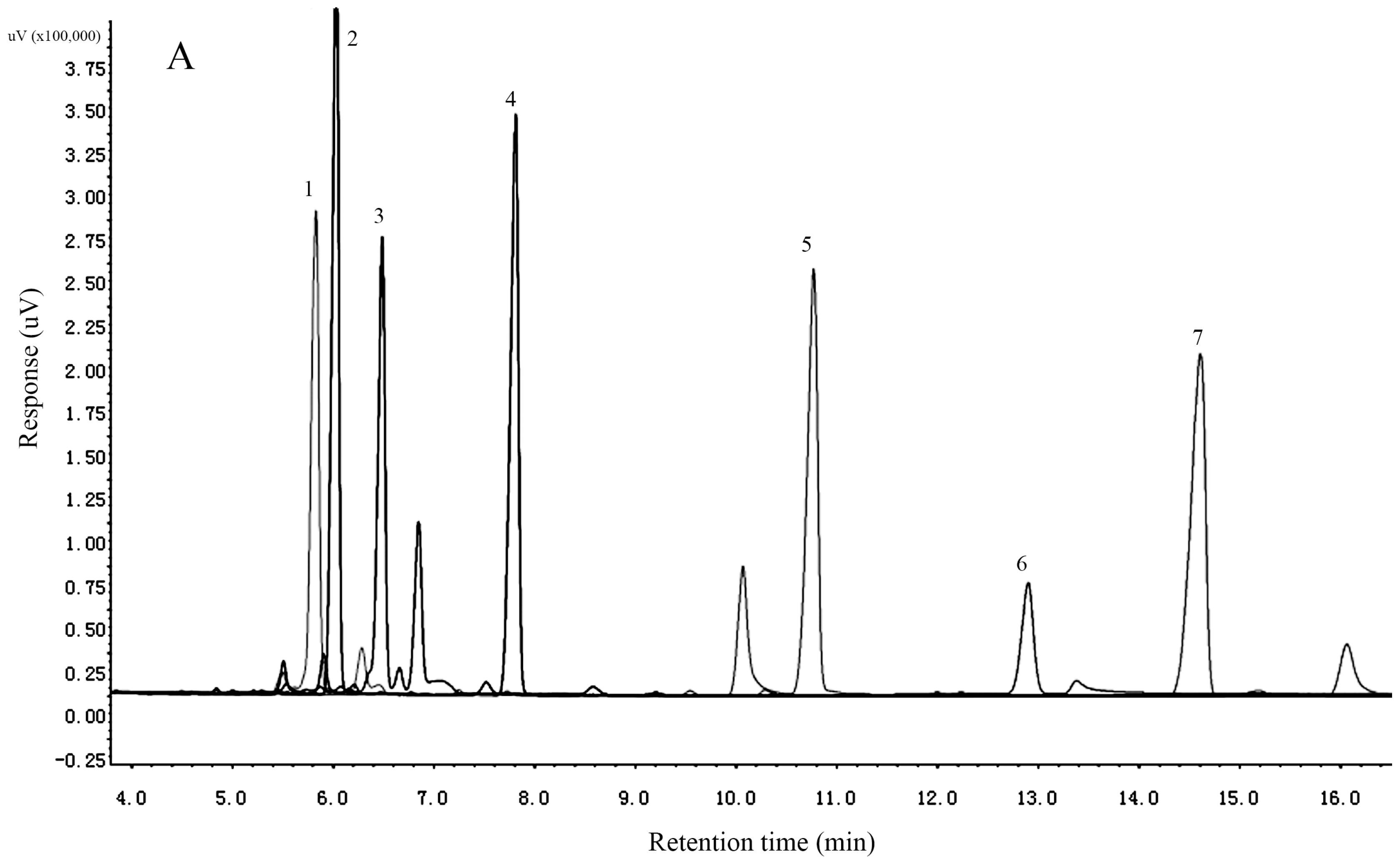
2.3. Determination of the Monosaccharide Composition
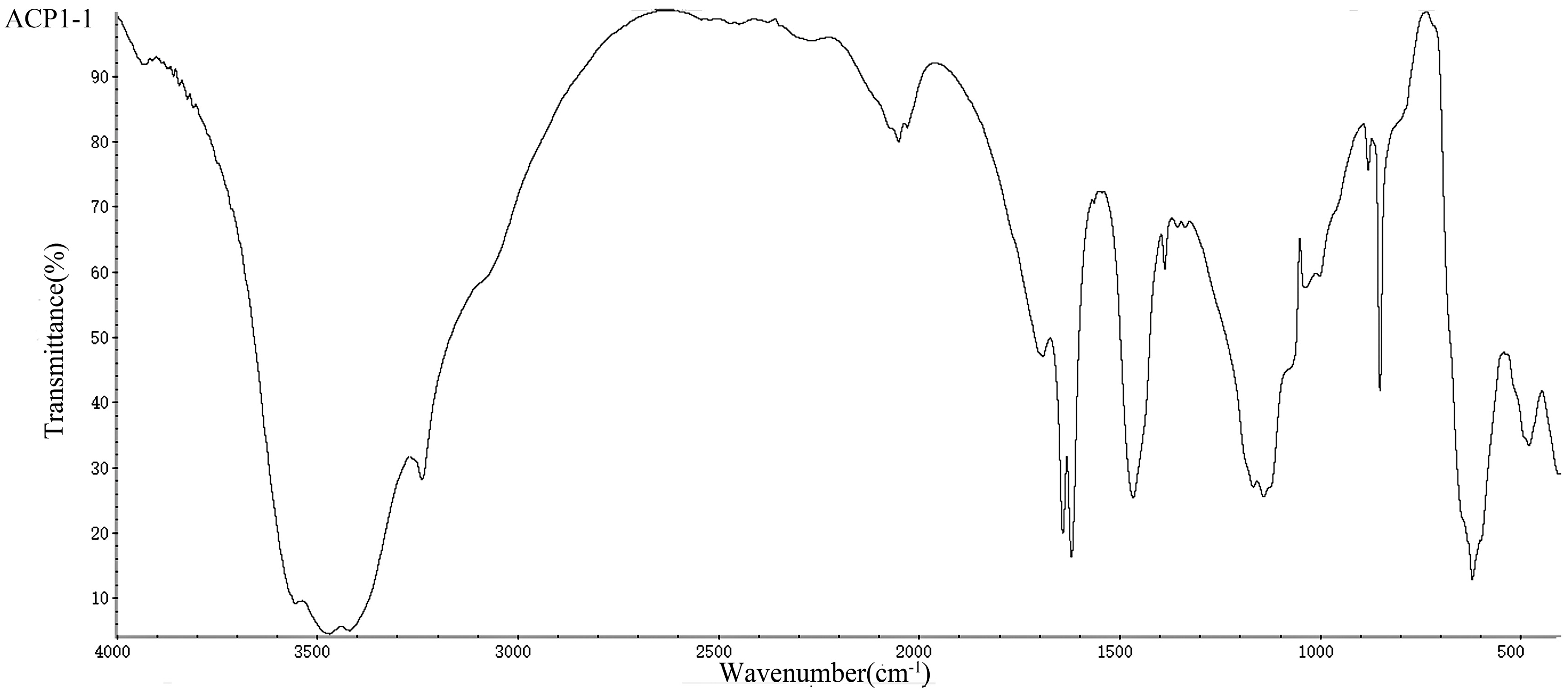
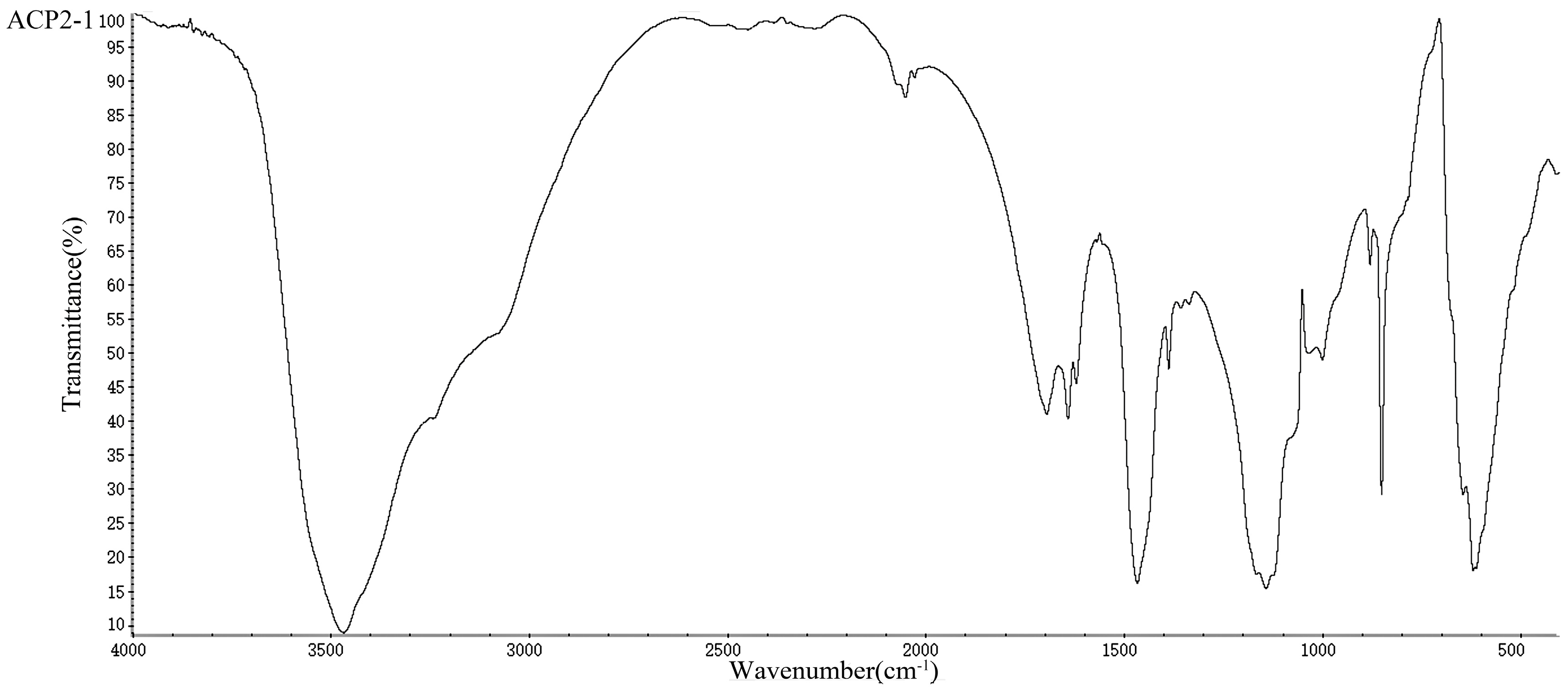
2.4. UV/Vis and FT-IR Analysis of the Polysaccharides
2.5. Periodate Oxidation
2.6. Methylation Analysis
3. Materials and Methods
3.1. Plant Materials, Chemicals, and Instrumentation
3.2. Isolation and Purification of Polysaccharides
3.3. Determination of Homogeneity and Relative Molecular Weights
3.4. Monosaccharide Composition of ACP1-1 and ACP2-1
3.5. UV/Vis and FT-IR Analysis
3.6. Periodate Oxidation
3.7. Methylation Analysis
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoang, M.H.; Kim, J.Y.; Ji, H.L.; You, S.G.; Lee, S.J. Antioxidative, hypolipidemic, and anti-inflammatory activities of sulfated polysaccharides from Monostroma nitidum. Food Sci. Biotechnol. 2015, 24, 199–205. [Google Scholar] [CrossRef]
- Boual, Z.; Pierre, G.; Delattre, C.; Benaoun, F.; Petit, E.; Gardarin, C.; Michaud, P.; Hadj, M.D.O.E. Mediterranean semi-arid plant Astragalus armatus as a source of bioactive galactomannan. Bioact. Carbohydr. Diet. Fibre 2015, 5, 10–18. [Google Scholar] [CrossRef]
- Cui, J.; Gu, X.; Wang, F.; Ouyang, J.; Wang, J. Purification and structural characterization of an alpha-glucosidase inhibitory polysaccharide from apricot (Armeniaca sibirica L. Lam.) pulp. Carbohydr. Polym. 2015, 121, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Mao, W.; Yan, M.; Liu, X.; Xia, Z.; Wang, S.; Xiao, B.; Chen, C.; Zhang, L.; Cao, S. Structural characterization and anticoagulant activity of a sulfated polysaccharide from the green alga Codium divaricatum. Carbohydr. Polym. 2015, 121, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Xu, H.; Xie, L.; Sun, J.; Sun, T.; Wu, X.; Fu, Q. Purification, characterization and in vitro anticoagulant activity of polysaccharides from Gentiana scabra Bunge roots. Carbohydr. Polym. 2016, 140, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Nadour, M.; Laroche, C.; Pierre, G.; Delattre, C.; Moulti-Mati, F.; Michaud, P. Structural Characterization and Biological Activities of Polysaccharides from Olive Mill Wastewater. Appl. Biochem. Biotechnol. 2015, 177, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.Y.; Liu, X.C.; Fang, X.N.; Sun, H.Q.; Yang, X.Y.; Zhang, Y.M. Structural characterization and anti-tumor activity of polysaccharide produced by Hirsutella sinensis. Int. J. Biol. Macromol. 2016, 82, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Heiss, W.D. Antioxidant and immunostimulatory activities in vitro of polysaccharides from pomegrante peels. J. Chem. Soc. Pak. 2015, 37, 86–91. [Google Scholar]
- Wang, R.; Chen, P.; Jia, F.; Tang, J.; Ma, F. Optimization of polysaccharides from Panax japonicus C.A. Meyer by RSM and its anti-oxidant activity. Int. J. Biol. Macromol. 2012, 50, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, S.; Xiong, Q.; Jiang, C.; Lai, X. Extraction, characterization and biological activities of polysaccharides from Amomum villosum. Carbohydr. Polym. 2013, 95, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, B.S.; Barsett, H. Bioactive Pectic Polysaccharides; Springer: Berlin/Heidelberg, Germany, 2005; pp. 69–101. [Google Scholar]
- Lefsih, K.; Delattre, C.; Pierre, G.; Michaud, P.; Aminabhavi, T.M.; Dahmoune, F.; Madani, K. Extraction, characterization and gelling behavior enhancement of pectins from the cladodes of Opuntia ficus indica. Int. J. Biol. Macromol. 2016, 82, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Petera, B.; Delattre, C.; Pierre, G.; Wadouachi, A.; Elboutachfaiti, R.; Engel, E.; Poughon, L.; Michaud, P.; Fenoradosoa, T.A. Characterization of arabinogalactan-rich mucilage from Cereus triangularis cladodes. Carbohydr. Polym. 2015, 127, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wu, Q.; Di, X.; Li, S.; Barba, F.J.; Koubaa, M.; Roohinejad, S.; Xiong, X.; He, J. Multistage recovery process of seaweed pigments: Investigation of ultrasound assisted extraction and ultra-filtration performances. Food Bioprod. Process. 2017, 104, 40–47. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, T.; He, J.; Barba, F.J.; Cravotto, G.; Koubaa, M. Ultrasound-Assisted Extraction, Centrifugation and Ultrafiltration: Multistage Process for Polyphenol Recovery from Purple Sweet Potatoes. Molecules 2016, 21, 1584. [Google Scholar] [CrossRef] [PubMed]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. Extraction assisted by pulsed electric energy as a potential tool for green and sustainable recovery of nutritionally valuable compounds from mango peels. Food Chem. 2016, 192, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Vivian-Smith, G.; Lawson, B.E.; Turnbull, I.; Downey, P.O. The biology of Australian weeds. 46. Anredera cordifolia (Ten.) Steenis. Plant Prot. Q. 2007, 22, 2–10. [Google Scholar]
- Hu, H.; Liang, H.; Wu, Y. Isolation, purification and structural characterization of polysaccharide from Acanthopanax brachypus. Carbohydr. Polym. 2015, 127, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Laksmitawati, D.R.; Widyastuti, A.; Karami, N.; Afifah, E.; Rihibiha, D.D.; Nufus, H.; Widowati, W. Anti-inflammatory effects of Anredera cordifolia and Piper crocatum extracts on lipopolysaccharide-stimulated macrophage cell line. Bangladesh J. Pharmacol. 2017, 12, 35–40. [Google Scholar] [CrossRef]
- Xie, J.H.; Jin, M.L.; Morris, G.A.; Zha, X.Q.; Chen, H.Q.; Yi, Y.; Li, J.E.; Wang, Z.J.; Gao, J.; Nie, S.P. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. 1), S60. [Google Scholar] [CrossRef] [PubMed]
- Bohn, J.A.; Bemiller, J.N. (1→3)-β-d-Glucans as biological response modifiers: A review of structure-functional activity relationships. Carbohydr. Polym. 1995, 28, 3–14. [Google Scholar] [CrossRef]
- Muralikrishna, G.; Rao, M.V. Cereal non-cellulosic polysaccharides: Structure and function relationship—An overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Sletmoen, M.; Stokke, B.T. Higher order structure of (1,3)-beta-d-glucans and its influence on their biological activities and complexation abilities. Biopolymers 2008, 89, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.X.; He, X.J.; Zhou, S.D.; Fan, Y.J.; Luo, A.S.; Chun, Z. Purification, composition analysis and antioxidant activity of the polysaccharides from Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 79, 1014–1019. [Google Scholar] [CrossRef]
- Liu, J.; Luo, J.; Sun, Y.; Ye, H.; Lu, Z.; Zeng, X. A simple method for the simultaneous decoloration and deproteinization of crude levan extract from Paenibacillus polymyxa EJS-3 by macroporous resin. Bioresour. Technol. 2010, 101, 6077–6083. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Han, B.; Liu, W.; Zhou, R. Deproteinization and structural characterization of bioactive exopolysaccharides from Ganoderma sinense mycelium. Sep. Sci. Technol. 2016, 51, 359–369. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.; Li, W.; Zhang, H. An effective method for deproteinization of bioactive polysaccharides extracted from lingzhi (Ganoderma atrum). Food Sci. Biotechnol. 2012, 21, 191–198. [Google Scholar] [CrossRef]
- Yang, R.; Meng, D.; Song, Y.; Li, J.; Zhang, Y.; Hu, X.; Ni, Y.; Li, Q. Simultaneous decoloration and deproteinization of crude polysaccharide from pumpkin residues by cross-linked polystyrene macroporous resin. J. Agric. Food Chem. 2012, 60, 8450–8456. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Tanikawa, T.; Hayashi, K.; Asagi, M.; Kasahara, Y.; Hayashi, T. Characterization and biological effects of two polysaccharides isolated from Acanthopanax sciadophylloides. Carbohydr. Polym. 2015, 116, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, F.; Wang, M.; Ma, L.; Ye, H.; Zeng, X. A comparative study of the neutral and acidic polysaccharides from Allium macrostemon bunge. Carbohydr. Polym. 2015, 117, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Jia, Y.; Wang, L.; Liu, X.; Liu, G.; Li, L.; He, C. Isolation and structural elucidation of a novel homogenous polysaccharide from Tricholoma matsutake. Nat. Prod. Res. 2016, 30, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, L.; Ding, S. Isolation, purification and structure of a new water-soluble polysaccharide from Zizyphus jujuba cv. Jinsixiaozao. Carbohydr. Polym. 2011, 83, 477–482. [Google Scholar] [CrossRef]
- Wang, K.P.; Zhang, Q.L.; Liu, Y.; Wang, J.; Cheng, Y.; Zhang, Y. Structure and inducing tumor cell apoptosis activity of polysaccharides isolated from Lentinus edodes. J. Agric. Food Chem. 2013, 61, 9849–9858. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.K.; Wang, W.Q.; Wu, J.Y. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: A review. J. Funct. Foods 2014, 6, 33–47. [Google Scholar] [CrossRef]
- Kukhta, E.P.; Chirva, V.Y.; Shadrin, G.N.; Stazaeva, L.P. Pectin substances of essential-oil crops II. Isolation and characterization of the pectin of Rosa canina. Chem. Nat. Compd. 1979, 15, 187–188. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Zhu, R.; Yu, J.; Lu, W.; Pan, C.; Yao, W.; Gao, X. Structure characterization, chemical and enzymatic degradation, and chain conformation of an acidic polysaccharide from Lycium barbarum L. Carbohydr. Polym. 2016, 147, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Shakhmatov, E.G.; Udoratina, E.V.; Atukmaev, K.V.; Makarova, E.N. Extraction and structural characteristics of pectic polysaccharides from Abies sibirica L. Carbohydr. Polym. 2015, 123, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yan, C.; Hua, D.; Zhang, D. Isolation, purification, and structural characterization of a novel polysaccharide from Ganoderma capense. Int. J. Biol. Macromol. 2013, 57, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zhao, K.; Huang, Q.; Xu, C.; Shang, P. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (Oliv.) Diels: A review. Carbohydr. Polym. 2012, 89, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Kacuráková, M.; Mathlouthi, M. FTIR and laser-Raman spectra of oligosaccharides in water: Characterization of the glycosidic bond. Carbohydr. Res. 1996, 284, 145–157. [Google Scholar] [CrossRef]
- Zhao, G.; Kan, J.; Li, Z.; Chen, Z. Structural features and immunological activity of a polysaccharide from Dioscorea opposita Thunb roots. Carbohydr. Polym. 2005, 61, 125–131. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, Y.; Sun, C.; Hu, N. Omar Ishurd, Structural Analysis of an Alkali-Extractable Polysaccharide from the Seeds of Retama raetam ssp. gussonei. J. Nat. Prod. 2006, 69, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ai, L.; Yang, Q.; Liu, Y.; Shan, L. Isolation and structural characterization of a polysaccharide from fruits of Zizyphus jujuba cv. Junzao. Int. J. Biol. Macromol. 2013, 55, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Wang, L.; Chen, C.; Teng, B.; Wang, C.; Xu, Z.; Hu, B.; Zhou, P. Structure characterization of a novel neutral polysaccharide isolated from Ganoderma lucidum fruiting bodies. Food Chem. 2012, 135, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Zhang, X.; Yao, W.B.; Niu, Y.; Gao, X.D. Structure characterization and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L. Carbohydr. Polym. 2010, 80, 1161–1167. [Google Scholar] [CrossRef]
- Qiao, D.; Liu, J.; Ke, C.; Sun, Y.; Ye, H.; Zeng, X. Structural characterization of polysaccharides from Hyriopsis cumingii. Carbohydr. Polym. 2010, 82, 1184–1190. [Google Scholar] [CrossRef]
- Wasser, S. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [PubMed]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure—Activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Wang, X.; Zhang, L.; Pck, C. Advances in lentinan: Isolation, structure, chain conformation and bioactivities. Food Hydrocoll. 2011, 25, 196–206. [Google Scholar] [CrossRef]
- Phillips, G.O.; Steve, W. Cui, Editor, Food Carbohydrates: Chemistry, Physical Properties and Applications, CRC/Taylor and Francis (2005) p. 418, (ISBN: 0-8493-1574). Food Hydrocoll. 2006, 20, 952. [Google Scholar] [CrossRef]
- Xiang, D.; Jie, T.; Mei, C.; Guo, C.X.; Xia, Z.; Jing, Z.; Jie, Z.; Sun, Q.; Su, F.; Yang, Z.R. Structure elucidation and antioxidant activity of a novel polysaccharide isolated from Tricholoma matsutake. Int. J. Biol. Macromol. 2010, 47, 271–275. [Google Scholar]
- Sweet, D.P.; Shapiro, R.H.; Albersheim, P. Quantitative analysis by various g.l.c. response-factor theories for partially methylated and partially ethylated alditol acetates. Carbohydr. Res. 1975, 40, 217–225. [Google Scholar] [CrossRef]
- Li, X.; Zhao, R.; Zhou, H.L.; Wu, D.H. Deproteinization of Polysaccharide from the Stigma Maydis by Sevag Method. Adv. Mater. Res. 2011, 340, 416–420. [Google Scholar] [CrossRef]
- Huang, G.; Chen, Y.; Wang, X. Extraction and deproteinization of pumpkin polysaccharide. Int. J. Food Sci. Nutr. 2011, 62, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Tan, J.; Tan, X.; Peng, D. Preparation of polysaccharides from wax gourd. Int. J. Food Sci. Nutr. 2011, 62, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Gabriela, C.; Norma, S.; Bessio, M.I.; Fernando, F.; Hugo, M. Quantitative determination of pneumococcal capsular polysaccharide serotype 14 using a modification of phenol-sulfuric acid method. J. Microbiol. Methods 2003, 52, 69–73. [Google Scholar]
- Ruijun, W.; Shi, W.; Yijun, X.; Mengwuliji, T.; Lijuan, Z.; Yumin, W. Antitumor effects and immune regulation activities of a purified polysaccharide extracted from Juglan regia. Int. J. Biol. Macromol. 2015, 72, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Needs, P.W.; Selvendran, R.R. An improved methylation procedure for the analysis of complex polysaccharides including resistant starch and a critique of the factors which lead to undermethylation. Phytochem. Anal. 1993, 4, 210–216. [Google Scholar] [CrossRef]
Sample Availability: Samples of the ACP2-1 and ACP2-1 are available from the authors. |

| Effect | Trichloroacetic Acid | Sevag | Hydrochloric Acid |
|---|---|---|---|
| Protein clearance rate/% | 97.73 ± 0.52 | 97.31 ± 0.56 | 55.10 ± 0.43 |
| Polysaccharide loss rate/% | 4.86 ± 0.37 | 49.67 ± 0.26 | 13.53 ± 0.35 |
| Resin | Polarity | Decolorization Rate/% | Polysaccharide Retention Rate/% |
|---|---|---|---|
| D101 | Apolar | 55.24 ± 0.24 | 59.19 ± 0.44 |
| X-5 | Apolar | 72.16 ± 0.47 | 75.24 ± 0.17 |
| AB-8 | Week polar | 59.13 ± 0.32 | 54.43 ± 0.29 |
| HPD-400 | Week polar | 43.86 ± 0.31 | 65.76 ± 0.31 |
| NKA-9 | Polar | 45.59 ± 0.28 | 68.38 ± 0.23 |
| Methylated Sugar | Type of Linkage | Mass Fragments (m/z) | Molar/% |
|---|---|---|---|
| 2,4,6-Me3-Gal | →3-Galp-(1→ | 58, 87, 101, 117, 149, 161, 203 | 5.02 |
| 2,3,6-Me3-Glc | →4)-Glcp-(1→ | 87, 101, 117, 143, 161 | 38.12 |
| 3,6-Me2-Man | →2,4)-Manp-(1→ | 87, 99, 113, 129, 159, 203, 233 | 2.49 |
| 2,3,4,6-Me4-Glc | Glcp-(1→ | 71, 87, 101, 117, 129, 145, 161, 205 | 1.30 |
| 2,4,6-Me3-Man | →3)-Manp-(1→ | 87, 129, 159, 173, 187 | 1.12 |
| 2,3,4-Me3-Gal | →6)-Galp-(1→ | 71, 87, 117, 161 | 1.52 |
| 2,4-Me2-Man | →3,6)-Manp-(1→ | 58, 87, 117, 129, 159, 189 | 13.31 |
| Methylated Sugar | Type of Linkage | Mass Fragments (m/z) | Molar/% |
|---|---|---|---|
| 2,4,6-Me3-Gal | →3)-Galp-(1→ | 71, 87, 101, 117, 129, 161 | 1.10 |
| 2,3,6-Me3-Glc | →4)-Glcp-(1→ | 71, 87, 117, 129, 161, 173 | 9.87 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-P.; Shen, C.-C.; Gao, F.-L.; Wei, H.; Ren, D.-F.; Lu, J. Isolation, Purification and Structural Characterization of Two Novel Water-Soluble Polysaccharides from Anredera cordifolia. Molecules 2017, 22, 1276. https://doi.org/10.3390/molecules22081276
Zhang Z-P, Shen C-C, Gao F-L, Wei H, Ren D-F, Lu J. Isolation, Purification and Structural Characterization of Two Novel Water-Soluble Polysaccharides from Anredera cordifolia. Molecules. 2017; 22(8):1276. https://doi.org/10.3390/molecules22081276
Chicago/Turabian StyleZhang, Zhi-Peng, Can-Can Shen, Fu-Li Gao, Hui Wei, Di-Feng Ren, and Jun Lu. 2017. "Isolation, Purification and Structural Characterization of Two Novel Water-Soluble Polysaccharides from Anredera cordifolia" Molecules 22, no. 8: 1276. https://doi.org/10.3390/molecules22081276






