An Efficient Synthesis of Spiro[indoline-3,9′-xanthene]trione Derivatives Catalyzed by Magnesium Perchlorate
Abstract
:1. Introduction
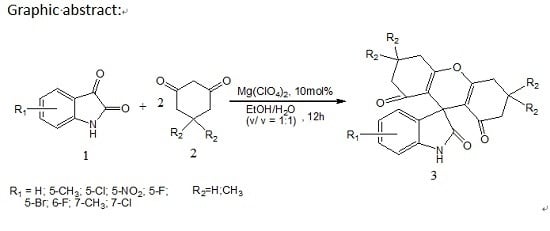
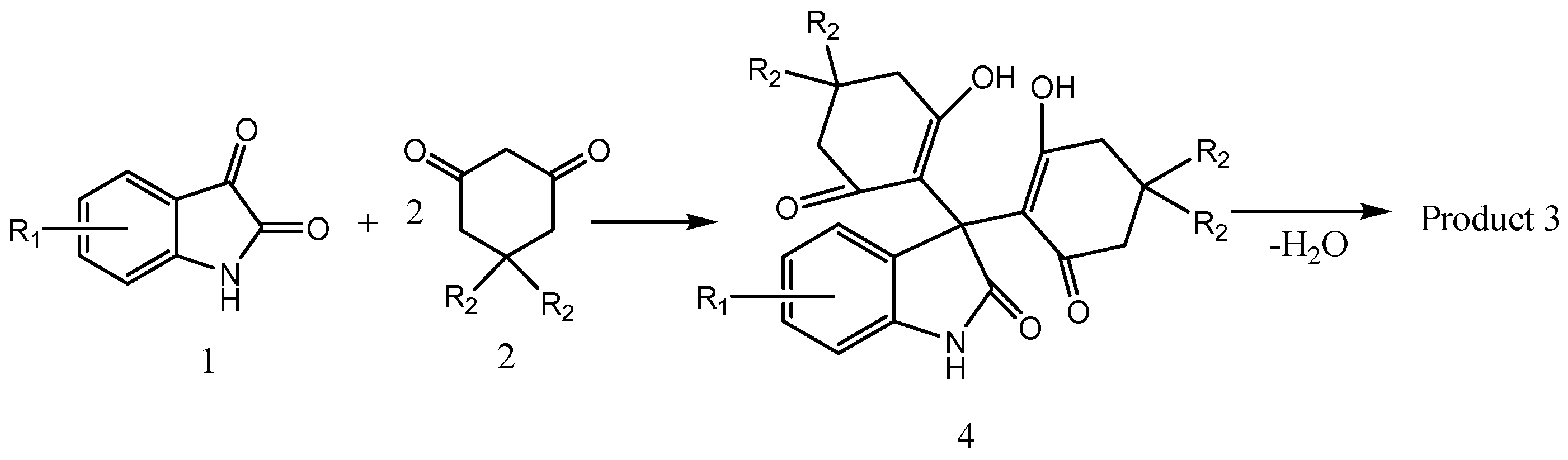
2. Results and Discussion
3. Experimental Section
General Procedure for the Synthesis of Spiro[indoline-3,9′-xanthene]trione (3)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, M.-Z.; Chen, Q.; Yang, G.-F. A review on recent developments of indole-containing antiviral agents. Eur. J. Med. Chem. 2015, 89, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Feng, J.-H.; Fang, L. A novel monoterpenoidindole alkaloid with anticancer activity from Melodinuskhasianus. Bioorg. Med. Chem. Lett. 2017, 27, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Perron, F.; Albizati, K.F. Chemistry of spiroketals. Chem. Rev. 1989, 89, 1617–1661. [Google Scholar] [CrossRef]
- Santos, M.M.M. Recent advances in the synthesis of biologically active spirooxindoles. Tetrahedron 2014, 70, 9735–9757. [Google Scholar] [CrossRef]
- Creasey, W.A. Pharmacology, Biochemistry, and Clinical Applications of the Monoterpenoid Alkaloids. In Chemistry of Heterocyclic Compounds; Saxton, J.E., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1983; Volume 25. [Google Scholar] [CrossRef]
- Kang, T.H.; Matsumoto, K.; Murakami, Y.; Takayama, H.; Kitajima, M.; Aimi, N.; Watanabe, H. Pteropodine and isopteropodine positively modulate the function of rat muscarinic M1 and 5-HT2 receptors expressed in Xenopus oocyte. Eur. J. Pharmacol. 2002, 444, 39–45. [Google Scholar] [CrossRef]
- Yu, S.; Qin, D.; Shangary, S.; Chen, J.; Wang, G.; Ding, K.; McEachern, D.; Qiu, S.; Nikolovska-Coleska, Z.; Miller, R.; et al. Potent and orally active small molecule inhibitors of the MDM2-P53 interaction. J. Med. Chem. 2009, 52, 7970–7973. [Google Scholar] [CrossRef] [PubMed]
- Kasralikar, H.M.; Jadhavar, S.C.; Bhusare, S.R. Synthesis and molecular docking studies of oxochromenylxanthenone and indolylxanthenone derivatives as anti-HIV-1 RT inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 3882–3886. [Google Scholar] [CrossRef] [PubMed]
- Evangelinou, O.; Hatzidimitriou, A.G.; Velali, E. Mixed-ligand copper (I) halide complexes bearing 4,5-bis(diphenylphosphano)-9,9-dimethyl-xanthene and N-methylbenzothiazole-2-thione: Synthesis, structures, luminescence and antibacterial activity mediated by DNA and membrane damage. Polyhedron 2014, 72, 122–129. [Google Scholar] [CrossRef]
- Jamison, J.M.; Krabill, K.; Hatwalkar, A.; Jamison, E.; Tsai, C. Potentiation of the antiviral activity of poly r(A-U) by xanthene dyes. Cell Biol. Int. Rep. 1990, 14, 1075–1084. [Google Scholar] [CrossRef]
- Ion, R.M.; Frackowiak, D.; Planner, A.; Wiktorowicz, K. The incorporation of various porphyrins into blood cells measured via flow cytometry, absorption and emission spectroscopy. Acta Biochim. Pol. 1998, 45, 833–845. [Google Scholar] [PubMed]
- Stefanović, G.; Pavičić-Woss, M.; Lorenc, L.; Mihailović, M.L. Condensation of isatin with diketones. Tetrahedron 1959, 6, 97–102. [Google Scholar] [CrossRef]
- Sarkar, P.; Mukhopadhyay, C. p-tert-Butylcalix[8]arene: An effective nano-ranged organocatalyst for the syntheses of xanthenes and acridines. Curr. Organocatal. 2016, 3, 205–215. [Google Scholar] [CrossRef]
- Baradarani, M.M.; Khoshsirat, S.; Moharampour, M.; Ebrahimisaatlo, B.; Rashidi, A.; Różycka-Sokołowska, E.; Joule, J.A. Spiro[4H-pyran-3,3′-oxindoles] derived from 1,2,3,4-tetrahydroquinoline-Part 2. J. Heterocycl. Chem. 2017, 54, 944–951. [Google Scholar] [CrossRef]
- Ahadi, S.; Khavast, H.R.; Bazgir, A. A clean synthesis of spiro[indoline-3,9′-xanthene]trione derivatives. Chem. Pharm. Bull. 2008, 56, 1328–1330. [Google Scholar] [CrossRef] [PubMed]
- Kothandapani, J.; Ganesan, A.; Mani, G.K.; Kulandaisamy, A.J.; Rayappan, J.B.; Ganesan, S.S. Zinc oxide surface: A versatile nanoplatform for solvent-free synthesis of diverse isatin derivatives. Tetrahedron Lett. 2016, 57, 3472–3475. [Google Scholar] [CrossRef]
- Das, P.; Dutta, A.; Bhaumik, A.; Mukhopadhyay, C. Heterogeneous ditopic ZnFe2O4 catalyzed synthesis of 4H-pyrans: Further conversion to 1,4-DHPs and report of functional group interconversion from amide to ester. Green Chem. 2014, 16, 1426–1435. [Google Scholar] [CrossRef]
- Karimi-Jaberi, Z.; Jaafarizadeh, A. One-pot, three-component reaction of dimedone, amines, and isatin in the presence of tris(hydrogensulfato) boron: Synthesis of pyrroloacridine derivatives. Res. Chem. Intermed. 2015, 41, 4913–4918. [Google Scholar] [CrossRef]
- Suman, R.; Bhaumik, A.; Pramanik, M.; Butcher, R.J.; Yildirim, S.O. Binary conjugate Bronsted-Lewis acid supported on mesoporous silica nanoparticles for the domino addition/elimination/addition and addition/elimination/addition/cyclization cascade. Catal. Commun. 2014, 43, 173–178. [Google Scholar]
- Liang, B.; Kalidindi, S.; Porco, J.A., Jr.; Stephenson, C.R.J. Multicomponent reaction discovery: Three-component synthesis of spirooxindoles. Org. Lett. 2010, 12, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, S.; Chakraborti, A.K. An extremely efficient three-component reaction of aldehydes/ketones, amines, and phosphites (Kabachnik-Fields Reaction) for the synthesis of α-aminophosphonates catalyzed by magnesium perchlorate. J. Org. Chem. 2007, 72, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.A.; Moradgholi. Mg(ClO4)2, an efficient catalyst for synthesis of β-amino alcohols by ring opening of epoxides with amines under solvent-free conditions. Synth. Commun. 2011, 41, 594–602. [Google Scholar] [CrossRef]
- Khatik, G.L.; Kumar, R.; Chakraborti, A.K. Magnesium perchlorate as a new and highly efficient catalyst for the synthesis of 2,3-dihydro-1,5-benzothiazepines. Synthesis 2007, 4, 541–546. [Google Scholar] [CrossRef]
- Kamble, V.T.; Ekhe, V.R.; Joshi, N.S.; Biradar, A.V. Magnesium perchlorate: An efficient catalyst for one-pot synthesis of pyrano- and furanoquinolines. Synlett 2007, 9, 1379–1382. [Google Scholar] [CrossRef]
- Alinezhad, H.; Tajbakhsh, M.; Tehrani, S.S. Selective monobromination of 1,3-diones with N-bromosaccharin/Mg(ClO4)2 system in solution and under solvent-free conditions. Bull. Korean Chem. Soc. 2011, 32, 1543–1546. [Google Scholar] [CrossRef]
- Wu, C.-L.; Shen, R.-P.; Chen, J.-H.; Hu, C.Q. An efficient method for multicomponent synthesis of spiro[4H-pyran-oxindole] derivatives catalyzed by magnesium perchlorate. Bull. Korean Chem. Soc. 2013, 34, 2431–2435. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Chankeshwara, S.V. Magnesium perchlorate. In e-EROS Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Mendiratta, S.K.; Dotson, R.L.; Brooker, R.T. Perchloric acid and perchlorates. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Poriel, C.; Cocherel, N.; Rault-Berthelot, J.; Vignau, L.; Jeannin, O. Incorporation of Spiroxantheneunits in blue-emitting oligophenyleneframeworks: Anew molecular design for OLED applications. Chem. Eur. J. 2011, 17, 12631–12645. [Google Scholar] [CrossRef] [PubMed]
- Cocherel, N.; Poriel, C.; Vignau, L.; Bergamini, J.F.; Rault-Berthelot, J. DiSpiroXanthene-IndenoFluorene: A new blue emitter for nondopedorganiclight emitting diode applications. Org. Lett. 2010, 12, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.C.; Jain, R.; Arora, S. Synthesis and mass fragmentation of fluorinated spiro[3H-indole-3,9′-9H-Xanthene]-1′,2,8′-(1H,2′H,5′H)-triones. J. Fluor. Chem. 1989, 42, 149–162. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Entry | Solvent/T (°C) | Time (h) | Yield b (%) |
|---|---|---|---|
| 1 | PhCH3(reflux) | 24 | Trace |
| 2 | CH2Cl2(reflux) | 24 | Trace |
| 3 | CH3OH(reflux) | 12 | 64 |
| 4 | C2H5OH(reflux) | 12 | 71 |
| 5 | C2H5OH/H2O(1/1)(80) | 10 | 83 |
| 6 | H2O(80) | 12 | 77 |
| 7 | CH3CN(reflux) | 12 | 60 |
| 8 | THF(reflux) | 12 | 58 |
| Product | R1 | R2 | Time (h) | Yield a (%) |
|---|---|---|---|---|
| 3a | H | CH3 | 10 | 83 |
| 3b | 5-Me | CH3 | 12 | 75 |
| 3c | 5-Cl | CH3 | 10 | 79 |
| 3d | 5-NO2 | CH3 | 10 | 81 |
| 3e | 7-Me | CH3 | 12 | 71 |
| 3f | 7-Cl | CH3 | 10 | 78 |
| 3g | H | H | 10 | 80 |
| 3h | 5-Me | H | 12 | 69 |
| 3i | 5-Cl | H | 10 | 82 |
| 3j | 5-F | CH3 | 10 | 83 |
| 3k | 5-Br | H | 10 | 71 |
| 3l | 5-Br | CH3 | 10 | 78 |
| 3m | 6-F | CH3 | 10 | 85 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Lv, C.; Liang, J.; Jin, J.; Wang, L.; Wu, C.; Shen, R. An Efficient Synthesis of Spiro[indoline-3,9′-xanthene]trione Derivatives Catalyzed by Magnesium Perchlorate. Molecules 2017, 22, 1295. https://doi.org/10.3390/molecules22081295
Chen C, Lv C, Liang J, Jin J, Wang L, Wu C, Shen R. An Efficient Synthesis of Spiro[indoline-3,9′-xanthene]trione Derivatives Catalyzed by Magnesium Perchlorate. Molecules. 2017; 22(8):1295. https://doi.org/10.3390/molecules22081295
Chicago/Turabian StyleChen, Chunfeng, Chunlei Lv, Jianfeng Liang, Jianqing Jin, Lijun Wang, Chunlei Wu, and Runpu Shen. 2017. "An Efficient Synthesis of Spiro[indoline-3,9′-xanthene]trione Derivatives Catalyzed by Magnesium Perchlorate" Molecules 22, no. 8: 1295. https://doi.org/10.3390/molecules22081295