γ-Tocotrienol Inhibits Proliferation and Induces Apoptosis via the Mitochondrial Pathway in Human Cervical Cancer HeLa Cells
Abstract
:1. Introduction
2. Results
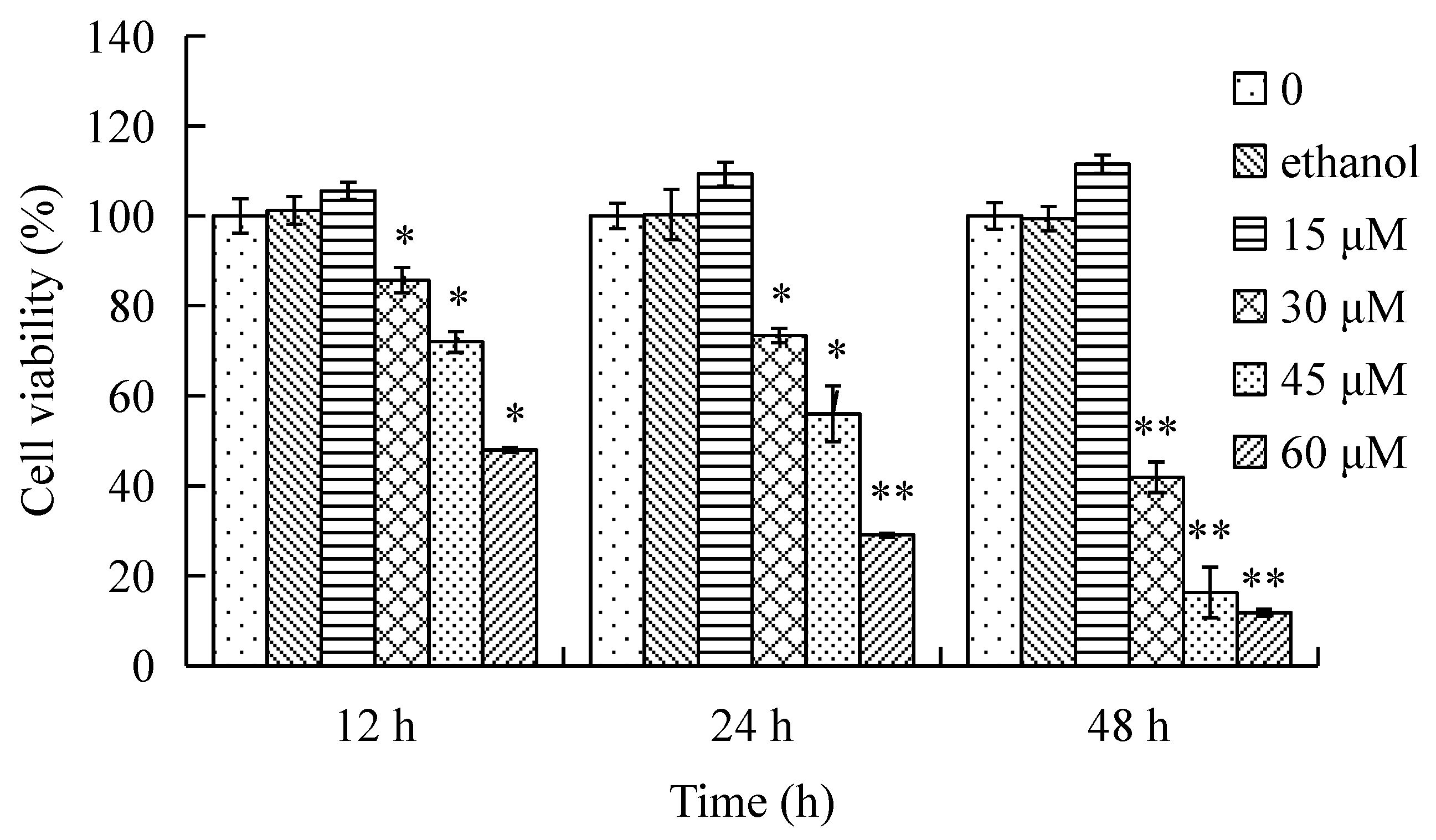
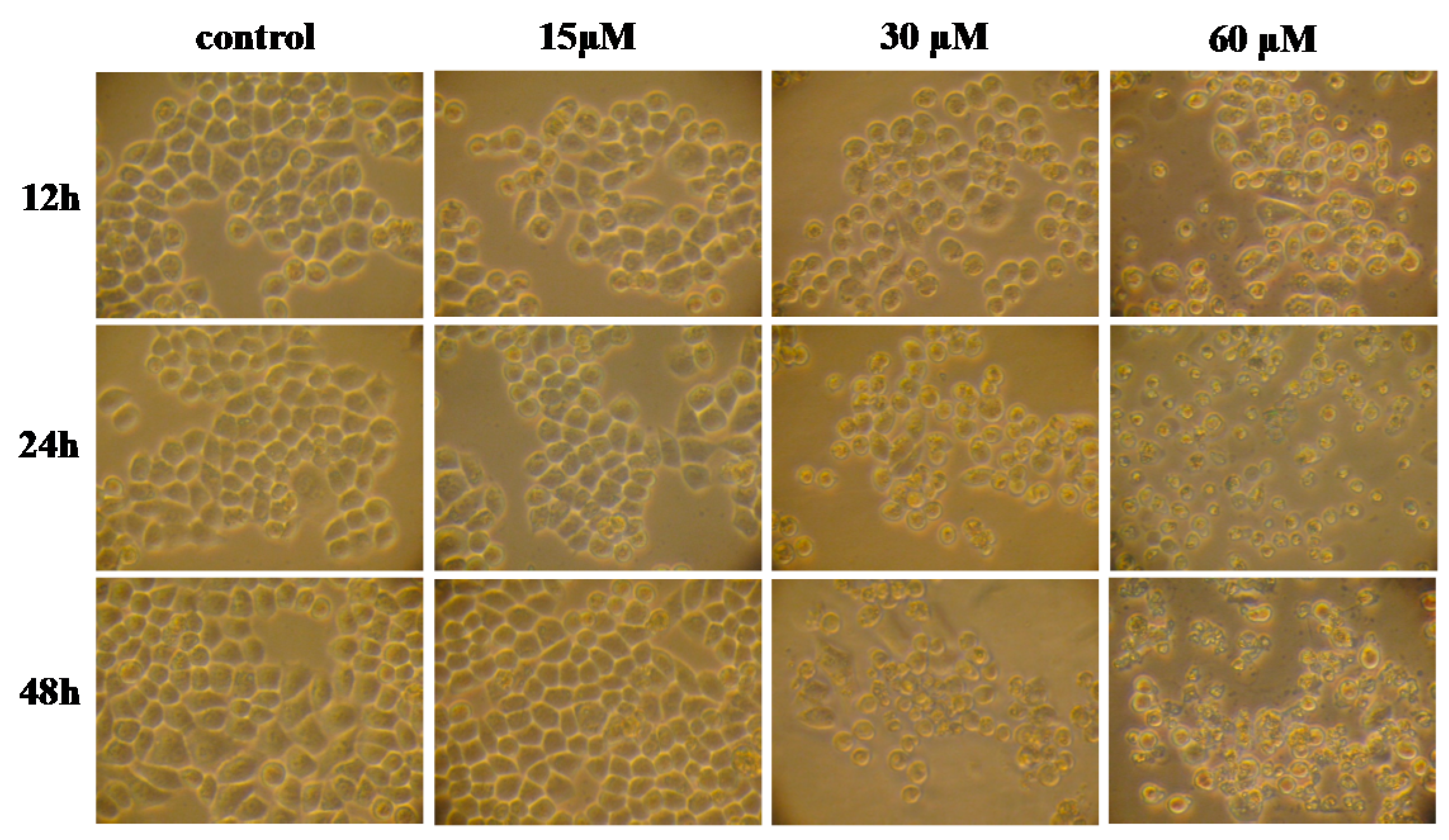
2.1. Effect of γ-Tocotrienol on the Viability of HeLa Cells
2.2. Effect of γ-Tocotrienol on Mitotic Index of HeLa Cells
2.3. Effect of γ-Tocotrienol on Colony Formation in HeLa Cells
2.4. γ-Tocotrienol Induces Cell-cycle Arrest in HeLa Cells
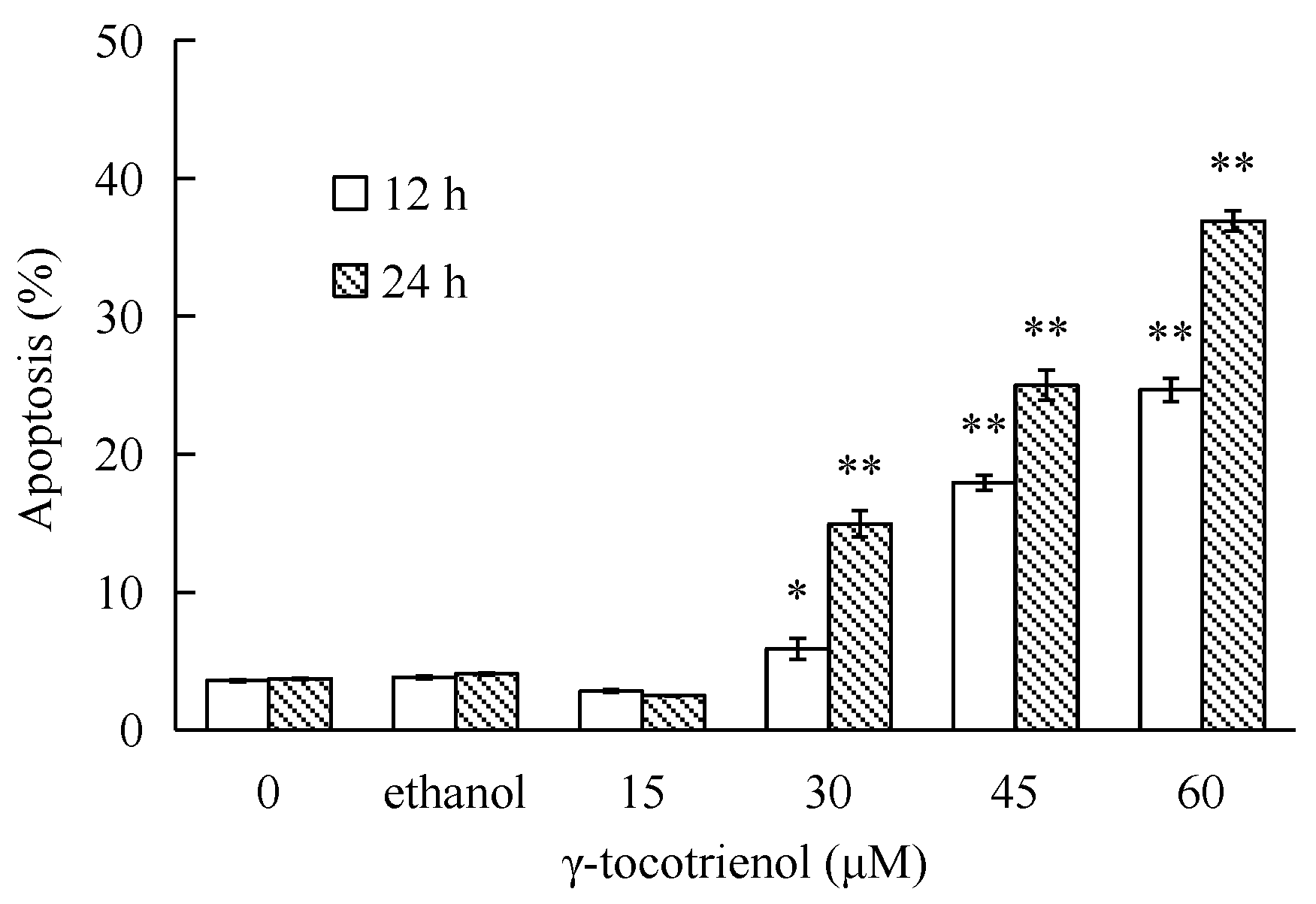
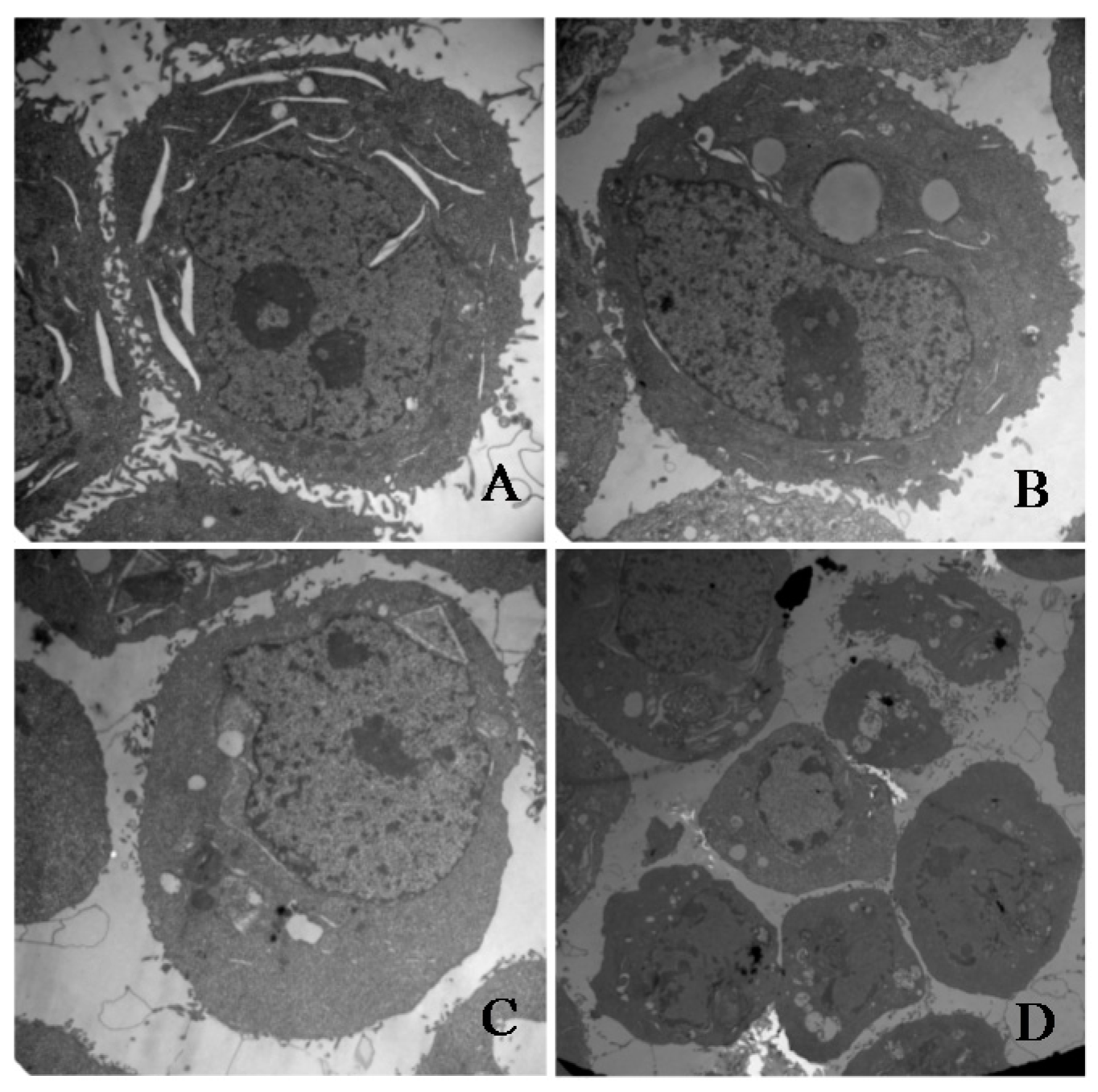
2.5. γ-Tocotrienol Induces Apoptosis in HeLa Cells
2.6. DNA Fragmentation
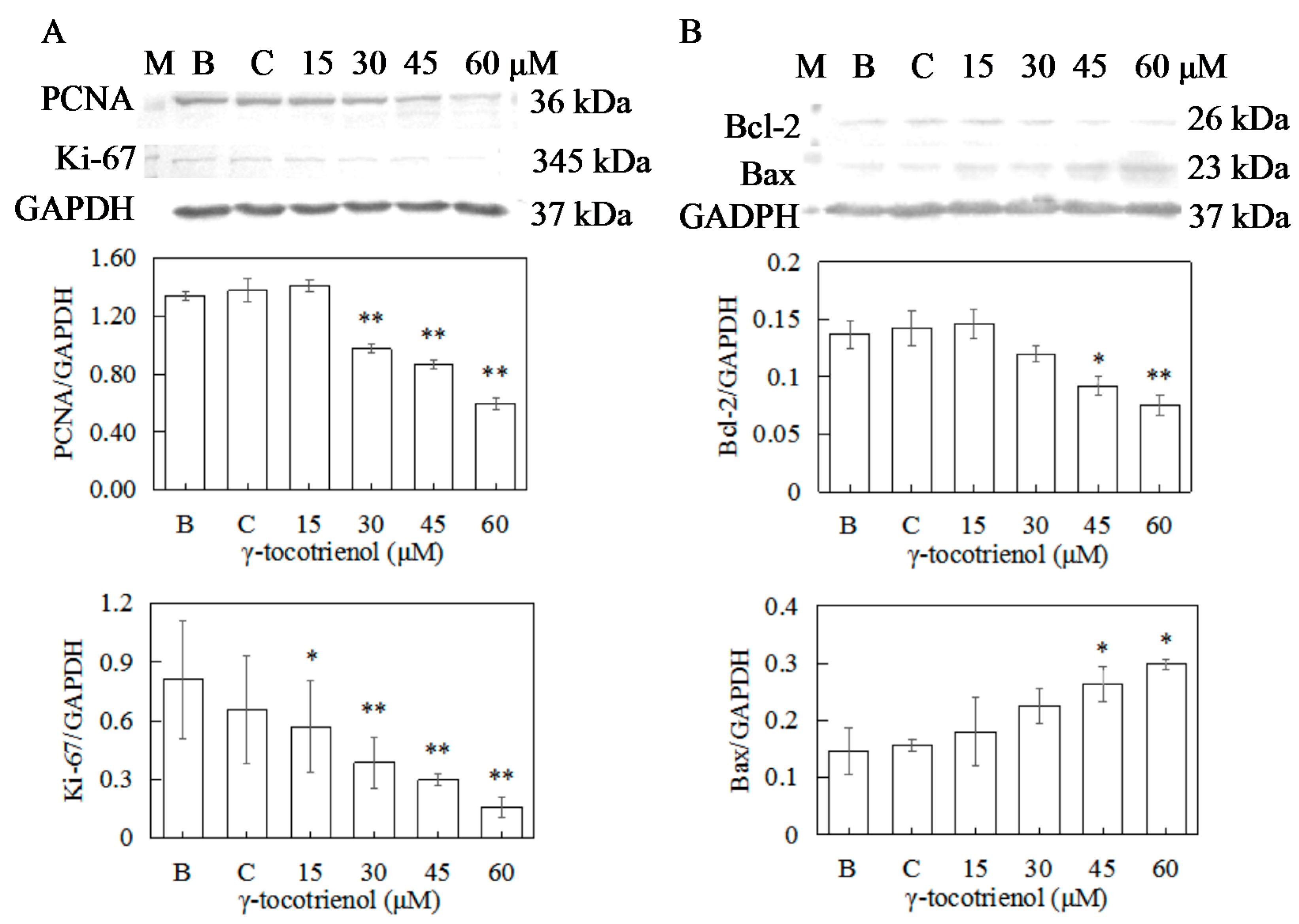
2.7. Effect of γ-Tocotrienol on the Expression of Proteins Involved in Proliferation in HeLa Cells
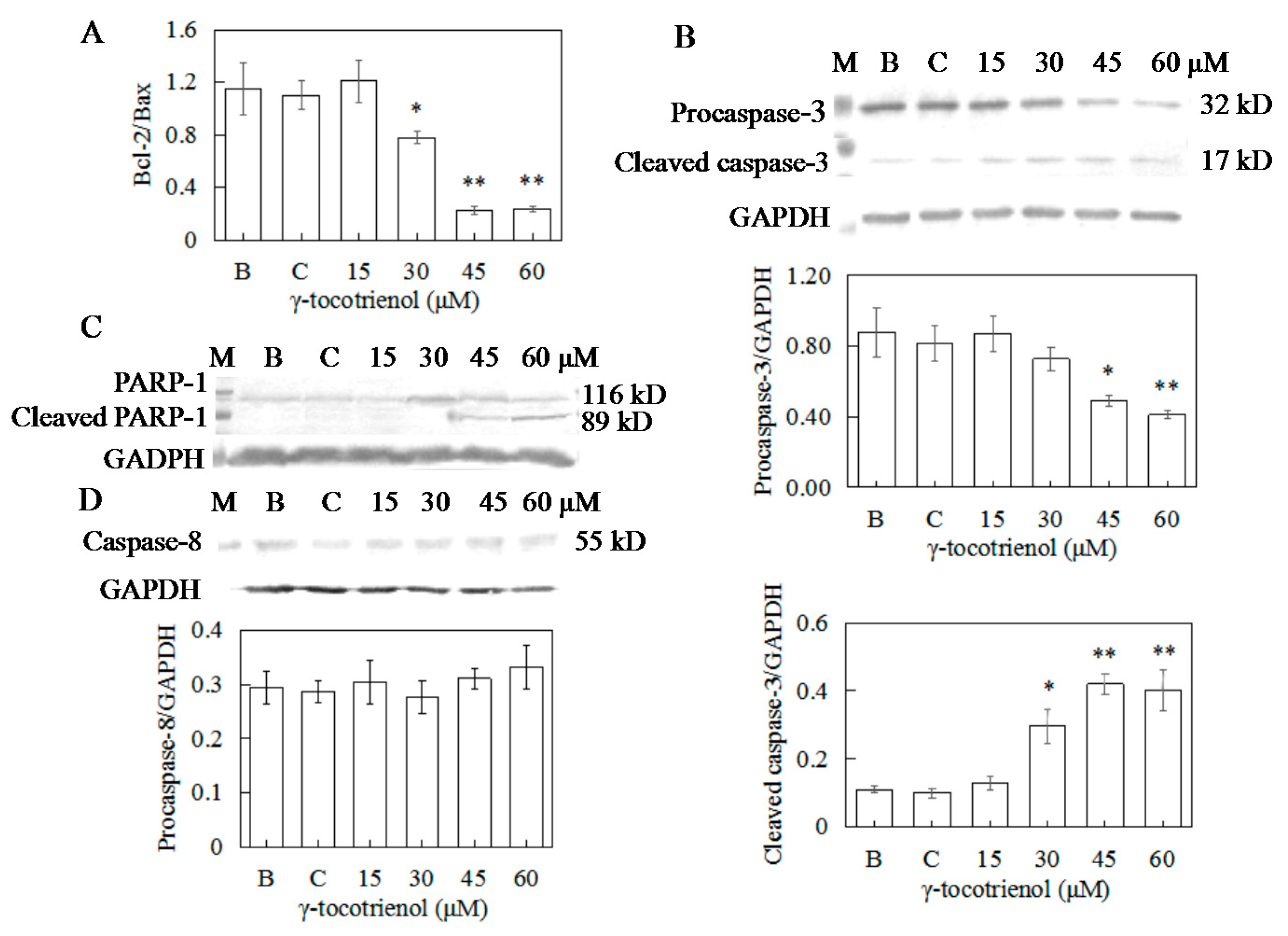
2.8. Effect of γ-Tocotrienol on the Expression of Apoptosis-Related Proteins in HeLa Cells
2.9. Apoptosis of HeLa Cells Induced by γ-Tocotrienol via Mitochondrial Pathway
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability
4.4. Mitotic Index
4.5. Colony Formation
4.6. Flow Cytometric Analysis
4.7. Morphologic Observation of Apoptosis
4.8. Detection of DNA Fragmentation
4.9. Western Blot Analysis
4.10. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Rates Could Increase by 50% to 15 Million by 2020. Available online: http://www.who.int/mediacentre/news/releases/2003/pr27/en/ (accessed on 3 August 2017).
- Doll, R.; Peto, R. The causes of cancer: Quantitative estimates of avoidable risks of cancer in the United States today. J. Natl. Cancer Inst. 1981, 66, 1192–1308. [Google Scholar] [CrossRef]
- Willett, W.C. Balancing life-style and genomics research for disease prevention. Science 2002, 296, 695–698. [Google Scholar] [CrossRef] [PubMed]
- DiMarco-Crook, C.; Xiao, H. Diet-based strategies for cancer chemoprevention: The role of combination regimens using dietary bioactive components. Annu. Rev. Food Sci. Technol. 2015, 6, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J.; Schatzkin, A.; Willett, W.C.; Allen, N.E.; Spencer, E.A.; Travis, R.C. Diet, nutrition and the prevention of cancer. Public Health Nutr. 2004, 7, 187–200. [Google Scholar] [CrossRef]
- Thomasset, S.C.; Berry, D.P.; Garcea, G.; Marczylo, T.; Steward, W.P.; Gescher, A.J. Dietary polyphenolic phytochemicals-promising cancer chemopreventive agents in humans? A review of their clinical properties. Int. J. Cancer 2007, 120, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.T.; Luk, S.U.; Al-Ejeh, F.; Khanna, K.K. Tocotrienol as a potential anticancer agent. Carcinogenesis 2011, 33, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Rink, C.; Roy, S. Tocotrienols: The emerging face of natural vitamin E. Vitam. Horm. 2007, 76, 203–261. [Google Scholar] [PubMed]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Nesaretnam, K.; Guthrie, N.; Chambers, A.F.; Carroll, K.K. Effect of tocotrienols on the growth of a human breast cancer cell line in culture. Lipids 1995, 30, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Sami, S.A.; Salser, W.A.; Khan, F.A. Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran in percholesterolemic humans. Atherosclerosis 2002, 161, 199–207. [Google Scholar] [CrossRef]
- Khanna, S.; Roy, S.; Ryu, H.; Bahadduri, P.; Swaan, P.W.; Ratan, R.R.; Sen, C.K. Molecular basis of vitamin E action: tocotrienol modulates 12-lipoxygenase, a keymediatorof glutamate-induced neurodegeneration. J. Biol. Chem. 2003, 278, 43508–43515. [Google Scholar] [CrossRef]
- He, L.; Mo, H.; Hadisusilo, S.; Qureshi, A.A.; Elson, C.E. Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J. Nutr. 1997, 127, 668–674. [Google Scholar] [PubMed]
- Liu, H.K.; Wang, Q.; Li, Y.; Sun, W.G.; Liu, J.R.; Yang, Y.M.; Xu, W.L.; Sun, X.R.; Chen, B.Q. Inhibitory effects of γ-tocotrienol on invasion and metastasis of human gastric adenocarcinoma SGC-7901 cells. J. Nutr. Biochem. 2010, 21, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Simmons-Menchaca, M.; Gapor, A.; Sanders, B.G.; Kline, K. Induction of apoptosis in human breast cancer cells by tocopherols and tocotrienols. Nutr. Cancer 1999, 33, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, P.W.; McIntyre, B.S.; Gapor, A.; Briski, K.P. Vitamin E inhibition of normal mammary epithelial cell growth is associated with a reduction in protein kinase C(alpha) activation. Cell Prolif. 2001, 34, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Loo, G. Disruption of mitochondria during tocotrienol-induced apoptosis in MDA-MB-231 human breast cancer cells. Biochem. Pharmacol. 2004, 67, 315–324. [Google Scholar] [CrossRef]
- Mc Intyre, B.S.; Briski, K.P.; Gapor, A.; Sylvester, P.W. Antiproliferative and apoptoticeffects of tocopherols and tocotrienols on preneoplastic and neoplastic mousemammary epithelial cells. Proc. Soc. Exp. Biol. Med. 2000, 224, 292–301. [Google Scholar] [CrossRef]
- Mc Intyre, B.S.; Briski, K.P.; Tirmenstein, M.A.; Fariss, M.W.; Gapor, A.; Sylvester, P.W. Antiproliferative and apoptotic effects of tocopherols and tocotrienols onnormal mouse mammary epithelial cells. Lipids 2000, 35, 171–180. [Google Scholar] [CrossRef]
- Wali, V.B.; Bachawal, S.V.; Sylvester, P.W. Endoplasmic reticulum stress mediates gamma-tocotrienol-induced apoptosis in mammary tumor cells. Apoptosis 2009, 14, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Sylvester, P.W. γ-tocotrienol inhibits neoplastic mammary epithelial cell proliferation by decreasing Akt and nuclear factor kappaB activity. Exp. Biol. Med. 2005, 230, 235–241. [Google Scholar] [CrossRef]
- Abdul Rahman, A.; Jamal, A.R.; Harun, R.; Mohd Mokhtar, N.; Wan Ngah, W.Z. Gamma-tocotrienol and hydroxy-chavicol synergistically inhibits growth and induces apoptosis of human glioma cells. BMC Complement. Altern. Med. 2014, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.L.; Liu, J.R.; Liu, H.K.; Qi, G.Y.; Sun, X.R.; Sun, W.G.; Chen, B.Q. Inhibition of proliferation and induction of apoptosis by gamma-tocotrienol in human colon carcinoma HT-29 cells. Nutrition 2009, 25, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Yusof, K.M.; Makpol, S.; Jamal, R.; Harun, R.; Mokhtar, N.; Wan Ngah, W.Z. γ-Tocotrienol and 6-Gingerol in Combination Synergistically Induce Cytotoxicity and Apoptosis in HT-29 and SW837 Human Colorectal Cancer Cells. Molecules 2015, 20, 10280–10297. [Google Scholar] [CrossRef]
- Jiang, Q.; Rao, X.; Kim, C.Y.; Freiser, H.; Zhang, Q.; Jiang, Z.; Li, G. Gamma-tocotrienol induces apoptosis and autophagy in prostate cancer cells by increasing intracellular dihydrosphingosine and dihydroceramide. Int. J. Cancer 2012, 130, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.E.; Whaley, S.G.; Phillips, R.; Aggarwal, B.B.; Stimmel, J.B.; Leesnitzer, L.; Blanchard, S.G.; Stone, W.L.; Muenyi, Christian; Krishnan, K. Gamma tocotrienol and prostate cancer: the regulation of two independent pathways to potentiate cell growth inhibition and apoptosis. J. Oil Palm Res. 2008, 33–43. [Google Scholar]
- Sakai, M.; Okabe, M.; Tachibana, H.; Yamada, K. Apoptosis induction by γ-tocotrienol in human hepatoma Hep3B cells. J. Nutr. Biochem. 2006, 17, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Kani, K.; Momota, Y.; Harada, M.; Yamamura, Y.; Aota, K.; Yamanoi, T.; Takano, H.; Motegi, K.; Azuma, M. γ-tocotrienol Enhances the Chemosensitivity of Human oral Cancer Cells to Docetaxel Through the Downregulation of the Expression of NF-kappa B-regulated Anti-apoptotic Gene Products. Int. J. Oncol. 2013, 42, 75–82. [Google Scholar] [PubMed]
- Sun, W.; Wang, Q.; Chen, B.; Liu, J.; Liu, H.; Xu, W. γ-tocotrienol- induced apoptosis in human gastric cancer SGC-7901 cells is associated with a suppression in mitogen-activated protein kinase signalling. Br. J. Nutr. 2008, 99, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xu, W.; Liu, H.; Liu, J.; Wang, Q.; Zhou, J.; Dong, F.; Chen, B. gamma-Tocotrienol induces mitochondria-mediated apoptosis in human gastric adenocarcinoma SGC-7901 cells. J. Nutr. Biochem. 2009, 20, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Ng, L.T. Tocotrienols inhibited growth and induced apoptosis in human HeLa cells through the cell cycle signaling pathway. Integr. Cancer Ther. 2010, 9, 66–72. [Google Scholar]
- Comitato, R.; Guantario, B.; Leoni, G.; Nesaretnam, K.; Ronci, M.B.; Canali, R.; Virgili, F. Tocotrienols induce endoplasmic reticulum stress and apoptosis in cervical cancer cells. Genes Nutr. 2016, 11, 32. [Google Scholar] [CrossRef]
- Budihardjo, I.; Oliver, H.; Lutter, M.; Luo, X.; Wang, X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 1999, 15, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Cheikh, A.; El Majjaoui, S.; Ismaili, N.; Cheikh, Z.; Bouajaj, J.; Nejjari, C.; El Hassani, A.; Cherrah, Y.; Benjaafar, N. Evaluation of the cost of cervical cancer at the National Institute of Oncology, Rabat. Pan. Afr. Med. J. 2016, 23, 209. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P. Cervical cancer: Can it be prevented? World J. Clin. Oncol. 2014, 5, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, R.V.; Nagini, S. Cancer chemoprevention by dietary phytochemicals: Promises and pitfalls. Curr. Pharm. Biotechnol. 2012, 13, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Bode, A.M.; Dong, Z. Molecular targets of phytochemicals for cancer prevention. Nat. Rev. Cancer 2011, 11, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Hasani, N.A.; Yusoff, P.A.; Bak, K.; Mt, A.G.; Wan Ngah, W.Z. The Possible Mechanism of Action of Palm oil Gamma-tocotrienol and Alpha-tocopherol on the Cervical Carcinoma CaSki Cell Apoptosis. Biomed. Res. 2008, 19, 194–200. [Google Scholar]
- Wada, S.; Satomi, Y.; Murakoshi, M.; Noguchi, N.; Yoshikawa, T.; Nishino, H. Tumor suppressive effects of tocotrienol in vivo and in vitro. Cancer Lett. 2005, 229, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.V.; Parajuli, P.; Sylvester, P.W. γ-tocotrienol-induced autophagy in malignant mammary cancer cells. Exp. Biol. Med. 2014, 239, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Li, D.M.; Ma, Y.; He, N.; Gu, Q.; Wang, F.S.; Jiang, S.Q.; Chen, B.Q.; Liu, J.R. γ-tocotrienol induces paraptosis-like cell death in human colon carcinoma SW620 cells. PLoS ONE 2013, 8, e57779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.H.; Xiao, H.; Jin, H.Y.; Koo, P.T.; Tsang, D.J.; Yang, C.S. Synergistic Actions of Atorvastatin with Gamma-tocotrienol and Celecoxib Against Human Colon Cancer HT29 and HCT116 Cells. Int. J. Cancer 2010, 126, 852–863. [Google Scholar] [PubMed]
- McCormick, D.; Chong, H.; Hobbs, C.; Datta, C.; Hall, P.A. Detection of the Ki-67 antigen in fixed and wax-embedded sections with the monoclonal antibody MIB1. Histopathology 1993, 22, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Barnouti, Z.P.; Owtad, P.; Shen, G.; Petocz, P.; Darendeliler, M.A. The biological mechanisms of PCNA and BMP in TMJ adaptive remodeling. Angle Orthod. 2011, 81, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Liu, T.Y.; Huang, S.P.; Ho, C.T.; Huang, T.C. Differentiation and Apoptosis Induction by Lovastatin and γ-tocotrienol in HL-60 cells via Ras/ERK/NF-κB and Ras/Akt/NF-κB Signaling Dependent Down-regulation of Glyoxalase 1 and HMG-CoA reductase. Cell. Signal. 2015, 27, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Elson, C.E. Apoptosis and Cell-cycle Arrest in Human and Murine Tumor Cells are Initiated by Isoprenoids. J. Nutr. 1999, 129, 804–813. [Google Scholar] [PubMed]
- Sun, S.Y.; Hail, N., Jr.; Lotan, R. Apoptosis as a novel target for cancer chemoprevention chemoprevention. J. Natl. Cancer Inst. 2004, 96, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, S.J. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999, 59, 1693s–1700s. [Google Scholar] [CrossRef]
- Cohen, G.M. Caspases: The executioners of apoptosis. Biochem. J. 1997, 326, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ola, M.S.; Nawaz, M.; Ahsan, H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol. Cell. Biochem. 2011, 351, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y.; Shimizu, S. Bcl-2 family: Life-or-death switch. FEBS Lett. 2000, 466, 6–10. [Google Scholar] [CrossRef]
- Communal, C.; Sumandea, M.; de Tombe, P.; Narula, J.; Solaro, R.J.; Hajjar, R.J. Functional consequences of caspase activation in cardiac myocytes. Proc. Natl. Acad. Sci. USA. 2002, 99, 6252–6256. [Google Scholar] [CrossRef]
- Wajant, H. The Fas signaling pathway: More than a paradigm. Science 2002, 296, 1635–1636. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.G.; Choi, E.J. Apoptotic signaling pathways: Caspases and stress-activated protein kinases. BMB Rep. 2002, 35, 24–27. [Google Scholar] [CrossRef]
- Kantari, C.; Walczak, H. Caspase-8 and bid: Caught in the act between death receptors and mitochondria. BBA-Mol. Cell Res. 2011, 1813, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Sylte, M.J.; Corbeil, L.B.; Inzana, T.J.; Czuprynski, C.J. Haemophilus somnus induces apoptosis in bovine endothelial cells in vitro. Infect. Immun. 2001, 69, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Yin, H.Q.; Kim, Y.H.; Li, G.Y.; Lee, B.H. Apoptosis inducing effects of 6-methoxydihydrosanguinarine in HT29 colon carcinoma cells. Arch. Pharm. Res. 2004, 27, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |



| Groups (μM) | No. of Cancer Cells in Mitosis (Mean ± SD) | Mitotic Index (%) | Inhibition (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 12 h | 24 h | 48 h | 12 h | 24 h | 48 h | 12 h | 24 h | 48 h | |
| 0 | 214.00 ± 4.20 | 256.00 ± 3.70 | 293.00 ± 3.50 | 21.40 | 25.60 | 29.30 | − | − | − |
| ethanol | 216.00 ± 6.50 | 252.00 ± 4.50 | 287.00 ± 3.80 | 21.60 | 25.20 | 28.70 | −0.90 | 1.60 | 2.00 |
| 15 | 219.00 ± 2.50 | 268.00 ± 3.60 | 313.00 ± 3.20 | 21.90 | 26.80 | 31.30 | −2.30 | −4.70 | −6.80 |
| 30 | 195.60 ± 6.20 | 222.40 ± 2.90 | 236.00 ± 5.30 | 19.60 | 22.20 | 23.60 * | 8.40 | 13.10 | 19.50 |
| 45 | 159.00 ± 5.30 | 182.00 ± 4.20 | 80.00 ± 6.70 | 15.90 * | 18.20 * | 0.80 ** | 25.70 | 28.90 | 72.70 |
| 60 | 137.00 ± 5.00 | 102.00 ± 6.30 | 61.00 ± 5.90 | 13.70 * | 10.20 * | 0.60 ** | 36.00 | 60.20 | 79.20 |
| Groups (μM) | No. of Cancer Cells Colonies (Mean ± SD) | Colony Formation (%) | Colony Inhibition (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 12 h | 24 h | 48 h | 12 h | 24 h | 48 h | 12 h | 24 h | 48 h | |
| 0 | 76.30 ± 2.90 | 79.80 ± 4.30 | 84.30 ± 3.80 | 38.15 | 39.90 | 42.15 | − | − | − |
| ethanol | 76.00± 1.00 | 77.00 ± 3.00 | 83.50 ± 3.00 | 38.00 | 38.50 | 41.75 | 0.40 | 3.50 | −0.90 |
| 15 | 79.00 ± 4.00 | 84.30 ± 3.30 | 90.30 ± 1.80 | 39.50 | 42.30 | 45.15 | −3.50 | −5.60 | −7.10 |
| 30 | 70.50 ± 6.80 | 56.00 ± 3.00 | 41.80 ± 2.30 | 35.25 | 28.00 * | 20.90 * | 7.60 | 29.80 | 50.40 |
| 45 | 34.00 ± 3.00 | 8.50 ± 2.00 | 0.50 ± 0.50 | 17.00 ** | 4.30 ** | 0.25 ** | 55.40 | 89.30 | 99.40 |
| 60 | 0.30 ± 0.40 | 0 | 0 | 0.20 ** | 0 ** | 0 ** | 99.60 | 100 | 100 |
| Group (μM) | Cell Cycle Distribution (%) | Cell Count | ||
|---|---|---|---|---|
| G0/G1 | S | G2/M | × 106 | |
| 0 | 56.27 ± 3.50 | 26.14 ± 2.81 | 17.59 ± 1.10 | 1.67 |
| ethanol | 56.95 ± 2.67 | 23.77 ± 1,83 | 19.28 ± 3.47 | 1.70 |
| 15 | 55.17 ± 4.22 | 24.22 ± 2.58 | 20.61 ± 2.32 | 1.79 |
| 30 | 61.27 ± 2.73 | 19.84 ± 3.21 | 18.89 ± 2.18 | 1.33 |
| 45 | 68.13 ± 3.20 * | 14.90 ± 2.10 * | 16.97 ± 2.51 | 1.04 |
| 60 | 72.03 ± 3.48 * | 8.88 ± 1.71 ** | 19.09 ± 3.21 | 0.82 |
| Group (μM) | Cell Cycle Distribution (%) | Cell Count | ||
|---|---|---|---|---|
| G0/G1 | S | G2/M | × 106 | |
| 0 | 57.57 ± 3.50 | 32.43 ± 2.81 | 10.00 ± 1.10 | 2.13 |
| ethanol | 55.97 ± 2.67 | 34.03 ± 1.83 | 12.81 ± 3.47 | 2.15 |
| 15 | 53.16 ± 4.22 | 36.02 ± 2.58 | 10.08 ± 2.32 | 2.27 |
| 30 | 63.75 ± 2.73 * | 27.14 ± 3.21 * | 9.11 ± 2.18 | 1.49 |
| 45 | 70.31 ± 3.20 * | 19.83 ± 2.10 ** | 9.86 ± 2.51 | 1.19 |
| 60 | 75.87 ± 3.48 * | 15.92 ± 1.71 ** | 8.21 ± 3.21 | 0.62 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Mi, Y.; He, P.; He, S.; Niu, L. γ-Tocotrienol Inhibits Proliferation and Induces Apoptosis via the Mitochondrial Pathway in Human Cervical Cancer HeLa Cells. Molecules 2017, 22, 1299. https://doi.org/10.3390/molecules22081299
Xu W, Mi Y, He P, He S, Niu L. γ-Tocotrienol Inhibits Proliferation and Induces Apoptosis via the Mitochondrial Pathway in Human Cervical Cancer HeLa Cells. Molecules. 2017; 22(8):1299. https://doi.org/10.3390/molecules22081299
Chicago/Turabian StyleXu, Weili, Yaqing Mi, Pan He, Shenghua He, and Lingling Niu. 2017. "γ-Tocotrienol Inhibits Proliferation and Induces Apoptosis via the Mitochondrial Pathway in Human Cervical Cancer HeLa Cells" Molecules 22, no. 8: 1299. https://doi.org/10.3390/molecules22081299





