Isolation and Purification of Three Ecdysteroids from the Stems of Diploclisia glaucescens by High-Speed Countercurrent Chromatography and Their Anti-Inflammatory Activities In Vitro
Abstract
:1. Introduction
2. Results and Discussion
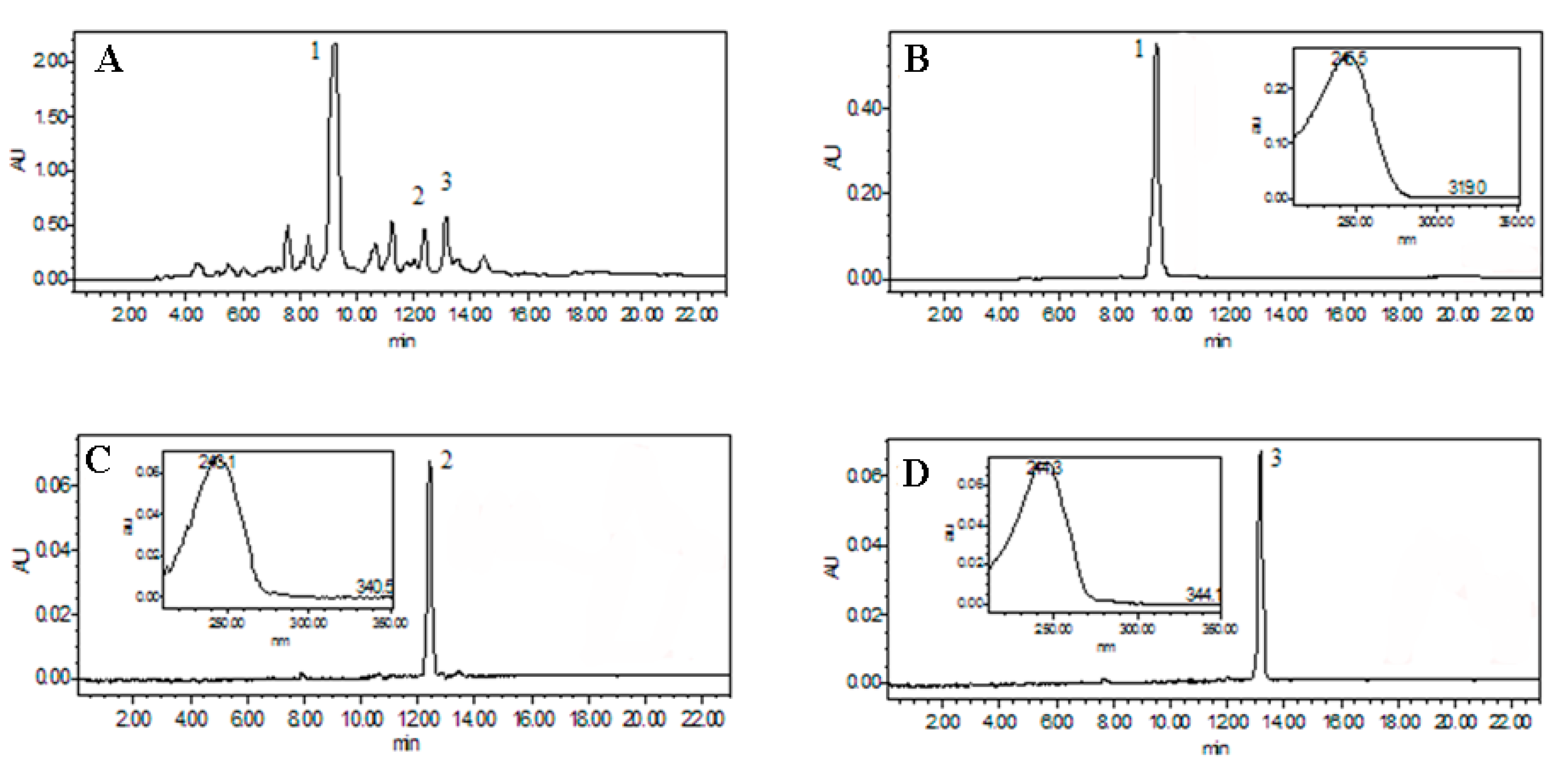
2.1. Optimization of HPLC Conditions
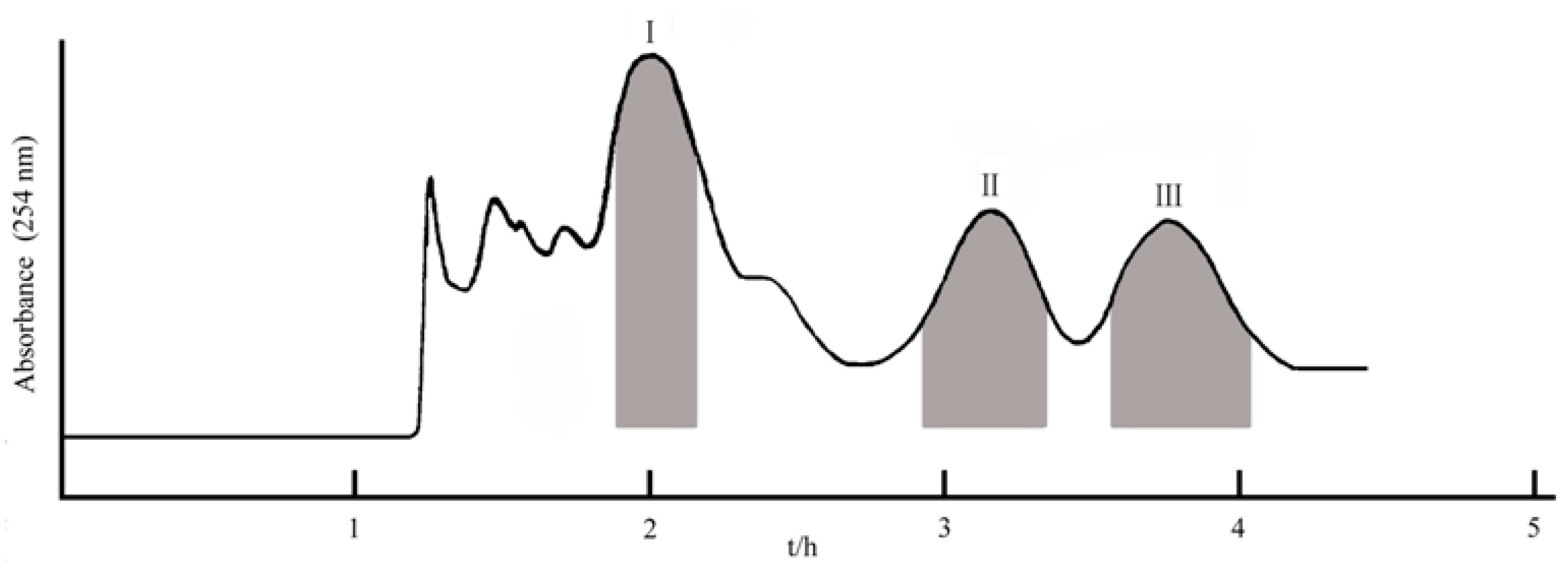
2.2. Selection of HSCCC Separation Conditions
2.3. Purification of Three Ecdysteroids by HSCCC
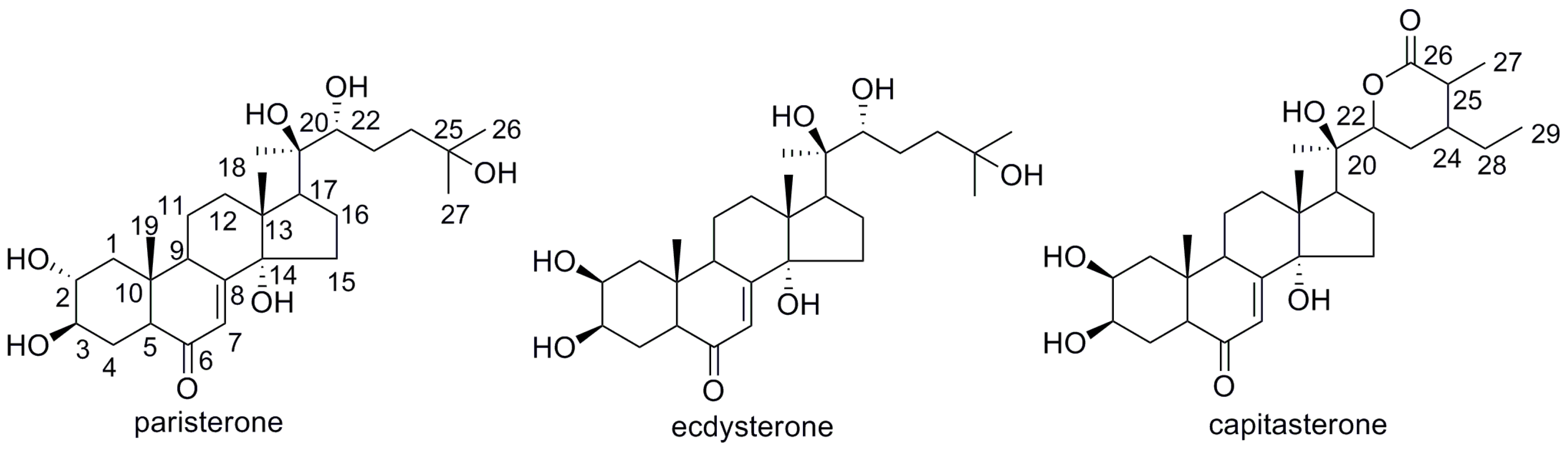
2.4. Structure Identification of the Separated Compounds
2.5. Anti-Inflammatory Activities of Three Ecdysteroids
3. Materials and Methods
3.1. Apparatus
3.2. Reagents and Materials
3.3. Preparation of Crude Sample
3.4. Selection of Two-Phase Solvent System
3.5. HSCCC Separation Procedure
3.6. HPLC Analysis and Identification of HSCCC Fractions
3.7. Assay for Anti-Inflammatory Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nanjing University of Chinese Medicine. Dictionary of Chinese Herbs, 2nd ed.; Shanghai Science and Technology Press: Shanghai, China, 2006; p. 1072. [Google Scholar]
- Jayasinghe, L.; Jayasooriya, C.P.; Hara, N.; Fujimoto, Y. A pyridine ring-containing ecdysteroid from Diploclisia glaucescens. Tetrahedron Lett. 2003, 44, 8769–8771. [Google Scholar] [CrossRef]
- Jayasinghe, L.; Kumarihamy, B.M.M.; Arundathie, B.G.S.; Dissanayake, L.; Hara, N.; Fujimoto, Y. A new ecdysteroid, 2-deoxy-5β,20-dihydroxyecdysone from the fruits of Diploclisia glaucescens. Steroids 2003, 68, 447–450. [Google Scholar] [CrossRef]
- Jayasinghe, L.; Jayasooriya, C.P.; Oyama, K.; Fujimoto, Y. 3-Deoxy-1β,20-dihydroxyecdysone from the leaves of Diploclisia glaucescens. Steroids 2002, 67, 555–558. [Google Scholar] [CrossRef]
- Gorelick-Feldman, J.; Maclean, D.; Ilic, N.; Poulev, A.; Lila, M.A.; Cheng, D.; Raskin, I. Phytoecdysteroids increase protein synthesis in skeletal muscle cells. J. Agric. Food Chem. 2008, 56, 3532–3537. [Google Scholar] [CrossRef] [PubMed]
- Kizelsztein, P.; Govorko, D.; Komarnytsky, S.; Evans, A.; Wang, Z.; Cefalu, W.T.; Raskin, I. 20-Hydroxyecdysone decreases weight and hyperglycemia in a diet-induced obesity mice model. Am. J. Physiol. Endocrinol. Metab. 2009, 296, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Dinan, L. Phytoecdysteroids: Biological aspects. Phytochemistry 2001, 57, 325–339. [Google Scholar] [CrossRef]
- Hornok, S.; Kováts, D.; Flaisz, B.; Csörgő, T.; Könczöl, Á.; Tibor Balogh, G.; Csorba, A.; Hunyadi, A. An unexpected advantage of insectivorism: Insect moulting hormones ingested by song birds affect their ticks. Sci. Rep. 2016, 6, 23390. [Google Scholar] [CrossRef] [PubMed]
- Issaadi, H.M.; Tsai, Y.C.; Chang, F.R.; Hunyadi, A. Centrifugal partition chromatography in the isolation of minor ecdysteroids from Cyanotisarachnoidea. J. Chromatogr. A 2017, 1054, 44–49. [Google Scholar]
- Miller, R.W.; Clardy, J.; Kozlowski, J.; Mikolajcak, K.L.; Plattner, R.D.; Powell, R.G.; Smith, C.R.; Weisleder, D.; Zheng, Q.T. Phytoecdysteroids of Diploclisia glaucescens. Planta Med. 1985, 51, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Bandara, B.M.; Jayasinghe, L.; Karunaratne, V.; Wannigama, G.P.; Bokel, M.; Kraus, W.; Sotheeswaran, S. Ecdysterone from stem of Diploclisia glaucescens. Phytochemistry 1989, 1075, 1073–1075. [Google Scholar] [CrossRef]
- Fang, L.; Liu, Y.Q.; Yang, B.; Wang, X.; Huang, L.Q. Separation of alkaloids from herbs using high-speed counter-current chromatography. J. Sep. Sci. 2011, 34, 2545–2558. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.A.; Fisher, D. Role of counter-current chromatography in the modernisation of Chinese herbal medicines. J. Chromatogr. A 2009, 1216, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Xu, X.J.; Xie, Z.Y.; Xie, Z.S.; Yang, M.; Yang, D.P. Isolation and purification of 20-hydroxyecdysone from Radix SerratulaeChinensis by high-speed counter-current chromatography. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 1909–1916. [Google Scholar] [CrossRef]
- Sinh, S.B.; Thanur, R.S. Structure and stereochemistry of paristerone, a novel phytoecdysteroids from the tubers of Paris polyphylla. Tetrahedron 1982, 38, 2189–2194. [Google Scholar] [CrossRef]
- Takemoto, T.; Nomoto, K.; Hikino, Y. Structure of capitasterone, a novel C29 insect-moulting substance from Cyathulacapitata. Tetrahedron Lett. 1968, 9, 4929–4932. [Google Scholar] [CrossRef]
- Dai, S.J.; Mi, Z.M.; Ma, Z.B.; Li, S.; Chen, R.Y.; Yu, D.Q. Bioactive diels-alder type adducts from the stem bark of morus macroura. Planta Med. 2004, 70, 758–763. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds paristerone, ecdysterone, and capitasteroneare available from the authors. |
| Solvent System (v/v) | K | ||
|---|---|---|---|
| I | II | III | |
| ethyl acetate–n-butanol–water (3:1:3) | 2.83 | 6.12 | 10.25 |
| ethyl acetate–ethanol–water (3:1:3) | 0.44 | 0.70 | 0.96 |
| ethyl acetate–n-butanol–ethanol–water (3:0.4:0.6:3) | 1.06 | 2.96 | 4.08 |
| ethyl acetate–n-butanol–ethanol–water (3:0.3:0.7:3) | 0.93 | 1.77 | 2.65 |
| ethyl acetate–n-butanol–ethanol–water (3:0.2:0.8:3) | 0.76 | 1.41 | 2.20 |
| Compounds a | IC50-Values (μM) |
|---|---|
| I | 1.51 |
| II | 2.62 |
| III | 11.68 |
| Ginkgolide B a | 2.35 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, L.; Li, J.; Zhou, J.; Wang, X.; Guo, L. Isolation and Purification of Three Ecdysteroids from the Stems of Diploclisia glaucescens by High-Speed Countercurrent Chromatography and Their Anti-Inflammatory Activities In Vitro. Molecules 2017, 22, 1310. https://doi.org/10.3390/molecules22081310
Fang L, Li J, Zhou J, Wang X, Guo L. Isolation and Purification of Three Ecdysteroids from the Stems of Diploclisia glaucescens by High-Speed Countercurrent Chromatography and Their Anti-Inflammatory Activities In Vitro. Molecules. 2017; 22(8):1310. https://doi.org/10.3390/molecules22081310
Chicago/Turabian StyleFang, Lei, Jialian Li, Jie Zhou, Xiao Wang, and Lanping Guo. 2017. "Isolation and Purification of Three Ecdysteroids from the Stems of Diploclisia glaucescens by High-Speed Countercurrent Chromatography and Their Anti-Inflammatory Activities In Vitro" Molecules 22, no. 8: 1310. https://doi.org/10.3390/molecules22081310





