Anti-Anxiety Effect of (−)-Syringaresnol-4-O-β-d-apiofuranosyl-(1→2)-β-d-glucopyranoside from Albizzia julibrissin Durazz (Leguminosae)
Abstract
:1. Introduction
2. Results
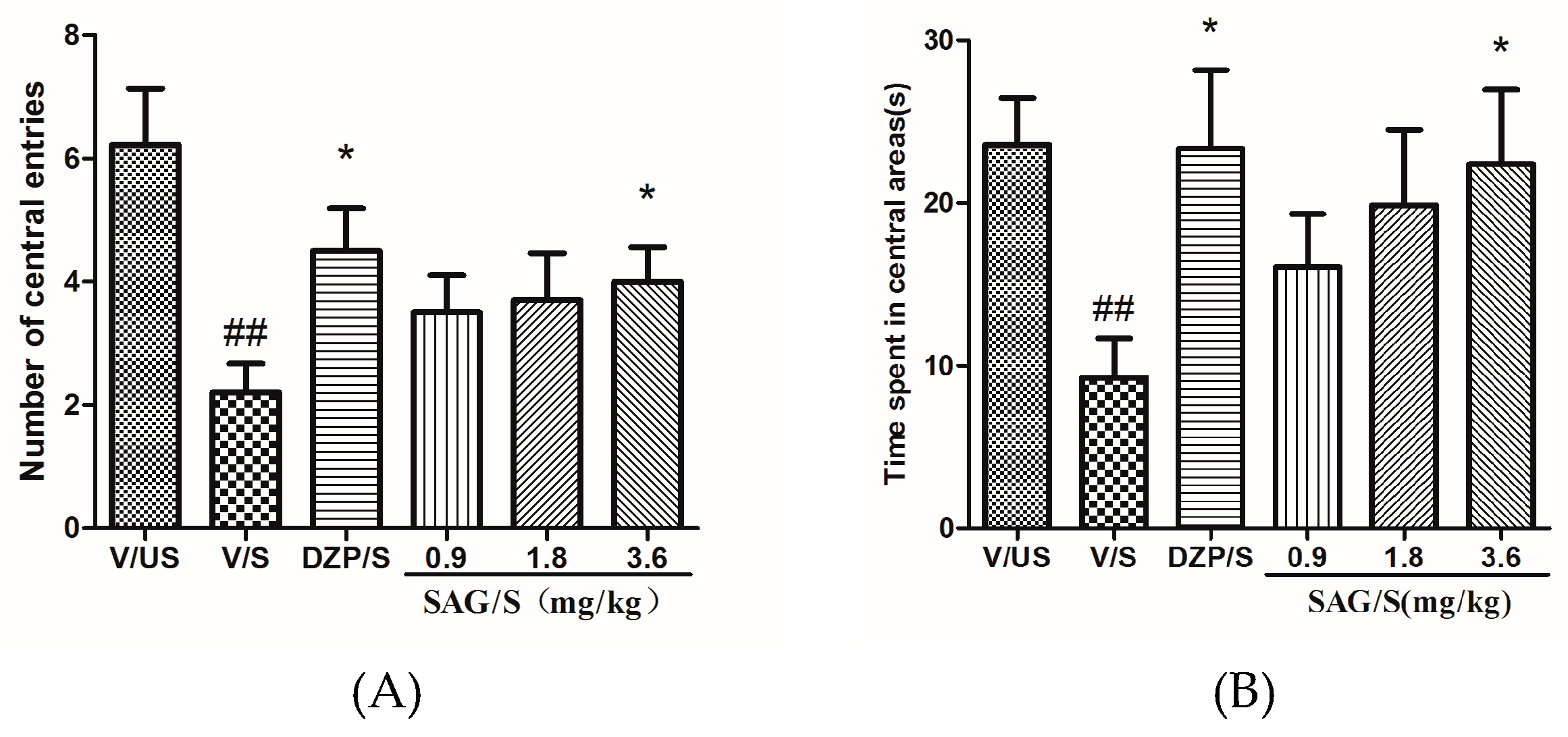
2.1. Effect of SAG in the Open Field Test
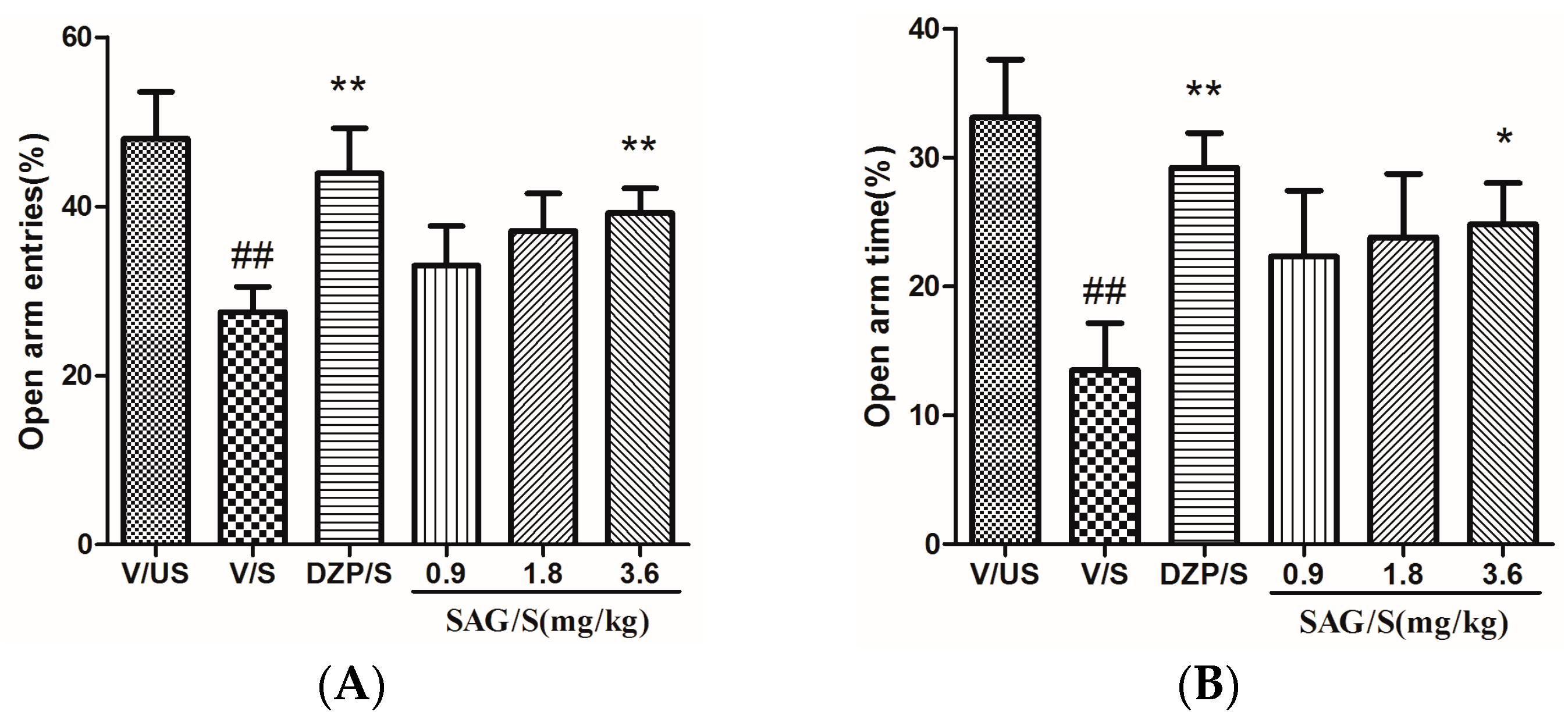
2.2. Effect of SAG in the Elevated Plus Maze
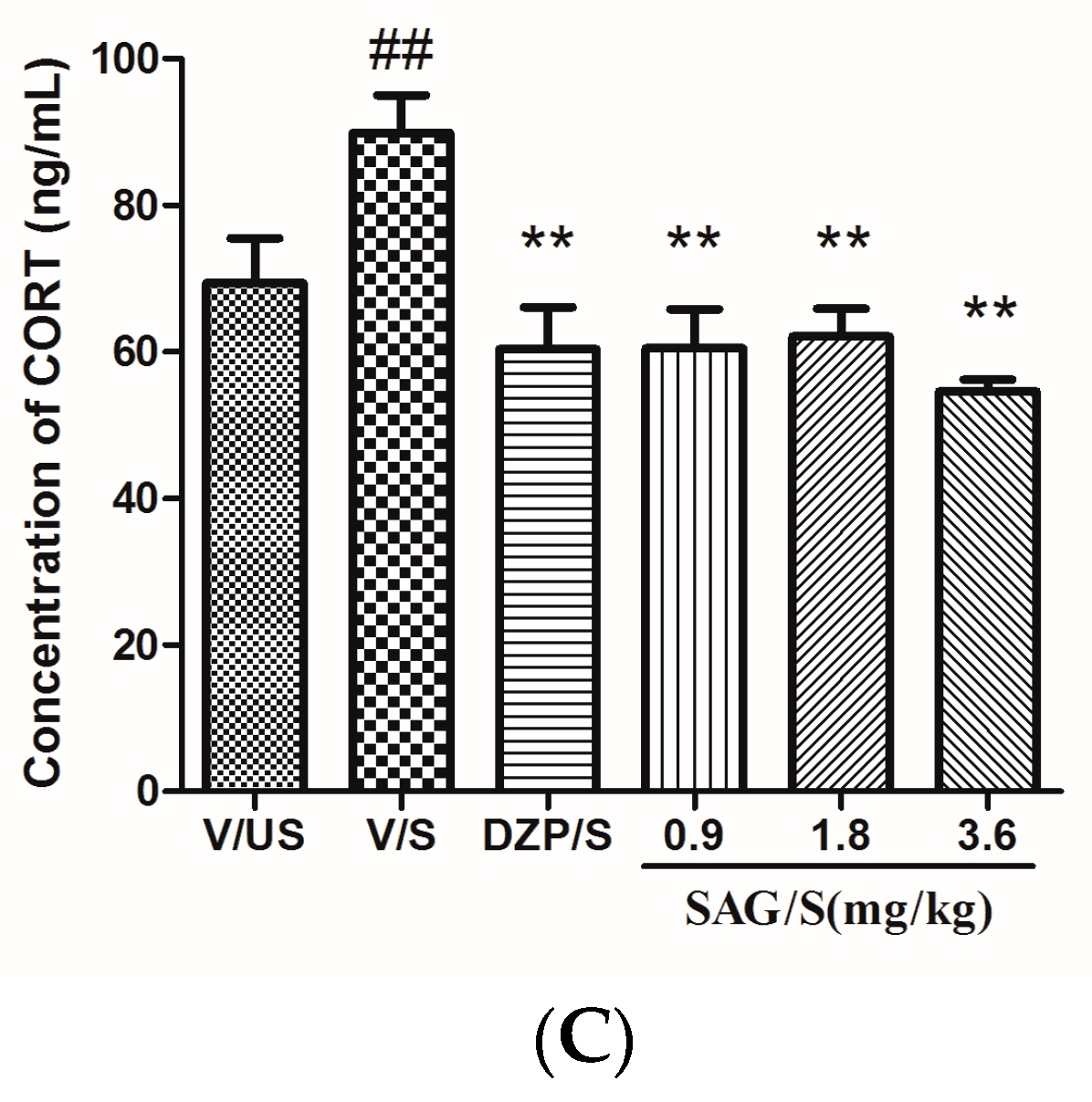
2.3. Effect of SAG on Serum CRF, ACTH, and CORT Levels
2.4. Effect of SAG on Monoamine Neurotransmitters and Their Metabolites
3. Discussion
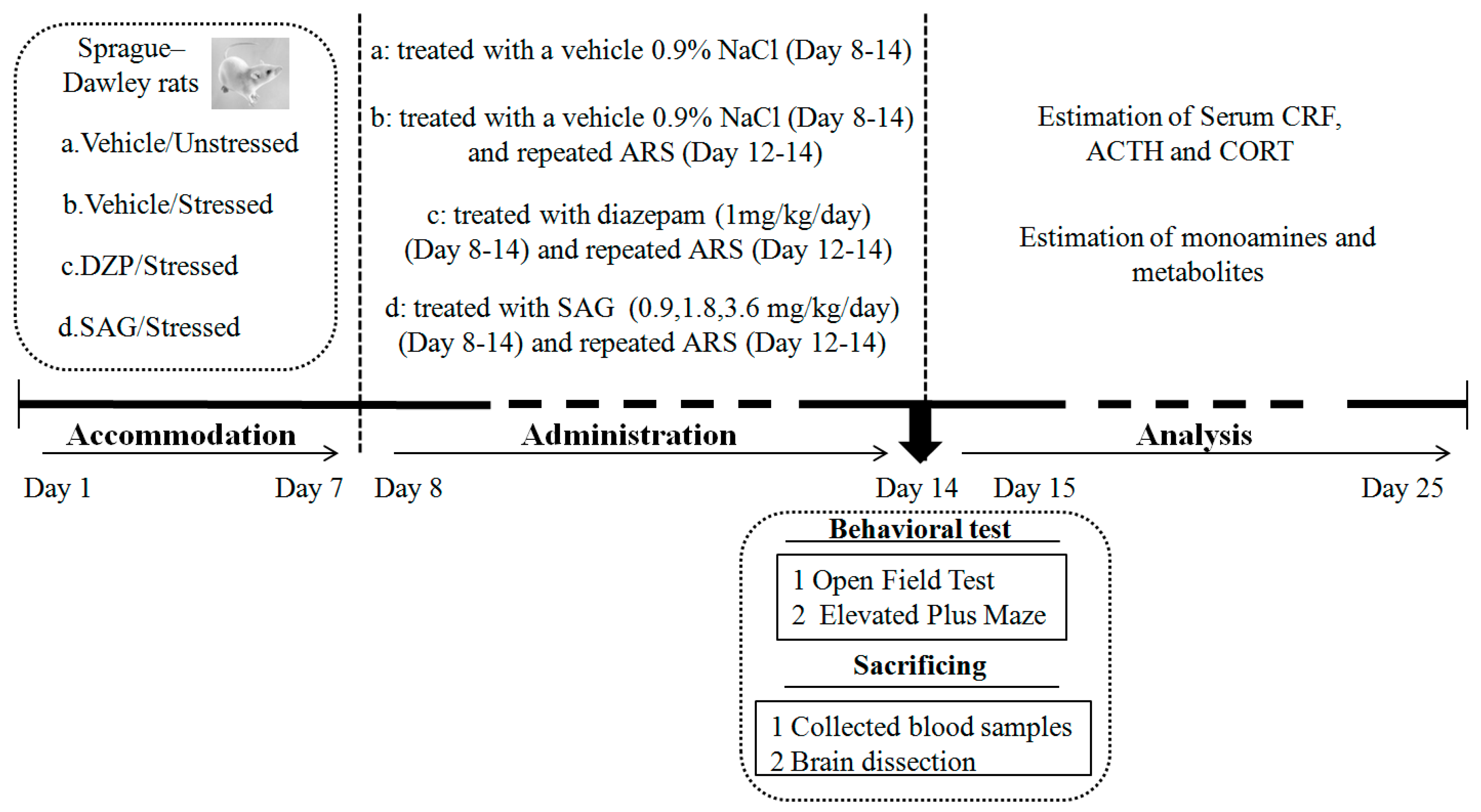
4. Materials and Methods
4.1. Animals
4.2. Drugs and Reagents
4.3. Treatment
4.4. AcuteRestraint Stress(ARS)
4.5. Behavioral Tests
4.5.1. Open Field Test (OFT)
4.5.2. Elevated Plus Maze (EPM)
4.6. Estimation of Serum CRF, ACTH and CORT
4.7. Estimation of Monoamines and Metabolites
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Naismith, S.L.; Norrie, L.M.; Mowszowski, L.; Hicki, I. The neurobiology of depression in later-life: Clinical, neuropsychological, neuroimaging and pathophysiological feateres. Prog. Neurobiol. 2012, 98, 99–143. [Google Scholar] [CrossRef] [PubMed]
- McLean, C.P.; Asnaani, A.; Litz, B.T.; Hofmann, S.G. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011, 45, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Youssef, N.A.; Rich, C.L. Does acute treatment with sedatives/hypnotics for anxiety in depressed patients affect suicide risk? A literature review. Ann. Clin. Psychiatry 2008, 20, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P. Chinese Materiamedica Chemistry, Pharmacology and Applications; Harwood Academic Publishers: Boca Raton, The Netherlands, 1999; pp. 519–520. [Google Scholar]
- Kim, W.K.; Jung, J.W.; Ahn, N.Y.; Oh, H.R.; Lee, B.K.; Oh, J.K.; Cheong, J.H.; Chun, H.S.; Ryu, J.H. Anxiolytic-like effects of extracts from Albizziajulibrissin bark in the elevated Plus-maze in rats. Life Sci. 2004, 75, 2787–2795. [Google Scholar] [CrossRef] [PubMed]
- Bo, F. Study on the Antianxiety Effects of Albiziajulibrissin on Mice. Master’s Thesis, Henan College of Traditional Chinese Medicine, Zhengzhou, China, 2014. [Google Scholar]
- Millan, M.J. The role of monoamines in the actions of established and “novel” antidepressant agents: A critical review. Eur. J. Pharmacol. 2004, 500, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, G.K.; Sprouse, J.S.; Sheldon, P.; Rasmussen, K. Electrophysiology of the central serotonin system: Receptor subtypes and transducer mechanisms. Ann. N. Y. Acad. Sci. 1990, 600, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Revelli, A.; Tesarik, J.; Massobrio, M. Nongenomic effects of neurosteroids. Gynecol. Endocrinol. 1998, 12, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, W.; Zhang, J. Effects of novel anxiolytic 4-butyl-alpha-agarofuran on levels of monoamine neurotransmitters in rats. Eur. J. Pharmacol. 2004, 504, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Gold, P.W. The organization of the stress system and its dysregulation in depressive illness. Mol. Psychiatry 2015, 20, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Vreeburg, S.A.; Hoogendijk, W.J.G.; Van Pelt, J.; DeRijk, R.H.; Verhagen, J.C.M.; Van Dyck, R.; Smit, J.H.; Zitman, F.G.; Penninx, B.W. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: Results from a large cohort study. Arch. Gen. Psychiatry 2009, 66, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Adinoff, B.; Junghanns, K.; Keifer, F.; Krishnan-Sarin, S. Suppression of the HPA axis stress-response: Implications for relapse. Alcohol. Clin. Exp. Res. 2010, 29, 1351–1355. [Google Scholar] [CrossRef]
- Kent, J.M.; Mathew, S.J.; Gorman, J.M. Molecular targets in the treatment of anxiety. Biol. Psychiatry 2002, 52, 1008–1030. [Google Scholar] [CrossRef]
- Luo, Y.W.; Xu, Y.; Cao, W.Y.; Zhong, X.L.; Duan, J.; Wang, X.Q.; Hu, Z.L.; Li, F.; Zhang, J.Y.; Zhou, M.; et al. Insulin-like growth factor 2 mitigates depressive behavior in a rat model of chronic stress. Neuropharmacology 2015, 89, 318–324. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Stress, adaptation, and disease. Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Jereme, G.S.; Hsiao-Jou, C.C.; James, S.M.C.; Conrad, S.; Nickolas, A.L. Acute restraint stress induces rapid changes in central redox status and protective antioxidant genes in rats. Psychoneuroendocrinology 2016, 67, 104–112. [Google Scholar]
- Padovan, C.M.; Del Bel, E.A.; Guimaraes, F.S. Behavioral effects in the elevated plus maze of an NMDA antagonist injected into the dorsal hippocampus: Influence of restraint stress. PharmacolBiochem. Behav. 2000, 67, 325–330. [Google Scholar] [CrossRef]
- Busnardo, C.; Alves, F.H.; Crestani, C.C.; Scopinho, A.A.; Resstel, L.B.; Correa, F.M. Paraventricular nucleus of the hypothalamus glutamate neurotransmission modulates autonomic, neuroendocrine and behavioral responses to acute restraint stress in rats. Eur. Neuropsychopharmacol. 2013, 23, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, N.T.; Valmik, D.D.; Bhoomika, M.P. Potential antidepressant-like activity of silymarin in the acute restraint stress in mice: Modulation of corticosterone and oxidative stress response in cerebral cortex and hippocampus. Pharmacol. Rep. 2016, 68, 1020–1027. [Google Scholar]
- Vila-verde, C.; Marinho, A.L.Z.; Lisboa, S.F.; Guimaraes, F.S. Nitric oxide in the prelimbic medial prefrontal cortex is involved in the anxiogenic-like effect induced by acute restraint stress in rats. Neuroscience 2016, 320, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Busnardo, C.; Tavares, R.F.; Correa, F.M. Angiotensinergic neurotransmission in the paraventricular nucleus of the hypothalamus modulates the pressor response to acute restraint stress in rats. Neuroscience 2014, 270, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.; Fitzgerald, L.W.; Lane, S.; Terwilliger, R.; Eric, J.N. Biochemical Adaptations in the Mesolimbic Dopamine System in Response to Repeated Stress. Neuropsychopharmacology 1996, 14, 443–452. [Google Scholar] [CrossRef]
- Lv, Y.W.; Liu, J.; Shi, S.N.; Guo, J.Y.; Liu, Y.; Shi, J.L. Anxiolytic effect of antianxietic compound prescription capsule on acute stress in rats and influence upon expression of ERK/CREB signaling pathway and BDNF in the brain of rats. Chin. Pharmacol. Bull. 2015, 31, 1614–1619. [Google Scholar]
- Kinjo, J.; Fukui, K.; Higuchi, H.; Nohara, T. The first isolation of lignan tri- and tetra- glycosides. Chem. Pharm. Bull. 1991, 39, 1623–1625. [Google Scholar] [CrossRef]
- Kang, T.H.; Jeong, S.J.; Kim, N.Y.; Higuchi, R.; Kim, Y.C. Sedative activity of two flavonol glycosides isolated from the flowers of Albizziajulibrissin Durazz. J. Ethnopharmacol. 2000, 71, 321–323. [Google Scholar] [CrossRef]
- Choleris, E.; Thomas, A.W.; Kavaliers, M.; Prato, F.S. A detailed ethological analysis of the mouse open field test: Effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci. Biobehav. Rev. 2001, 25, 235–260. [Google Scholar] [CrossRef]
- Belzung, C.; Griebel, G. Measuring normal and pathological anxiety-like behaviour in mice: A review. Behav. Brain Res. 2001, 125, 141–149. [Google Scholar] [CrossRef]
- Artur, H.S.; Adrian, J.D. Effects of interleukin-1β and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol. Biochem. Behav. 2007, 86, 651–659. [Google Scholar]
- Dawson, G.R.; Tricklebank, M.D. Use of the elevated plus maze in the search for novel anxiolytic agents. Trends Pharmacol. Sci. 1995, 16, 33–36. [Google Scholar] [CrossRef]
- Nestler, E.J.; Barrot, M.; DiLeone, R.J.; Eisch, A.J.; Gold, S.J.; Monteggia, L.M. Neurobiology of depression. Neuron 2002, 34, 13–25. [Google Scholar] [CrossRef]
- Pitts, A.F.; Samuelson, S.D.; Meller, W.H.; Bissette, G.; Nemeroff, C.B.; Kathol, R.G. Cerebrospinal fluid corticotropin-releasing hormone, vasopressin, and oxytocin concentrations in treated patients with major depression and controls. Biol. Psychiatry 1995, 38, 330–335. [Google Scholar] [CrossRef]
- Van Bockstaele, E.J.; Colago, E.E.; Valentino, R.J. Corticotropin-releasing factorcontaining axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J. Comp. Neurol. 1996, 364, 523–534. [Google Scholar] [CrossRef]
- Kioukia-Fougia, N.; Antoniou, K.; Bekris, S.; Liapi, C.; Christofidis, I.; PapadopoulouDaiafoti, Z. The effects of stress exposure on the hypothalamic-pituitary -adrenal axis, thymus, thyroid hormones, and glucose levels. Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 823–830. [Google Scholar] [CrossRef]
- Christiansen, S.; Bouzinova, E.V.; Palme, R.; Wiborg, O. Circadian activity of the hypothalamic-pituitary-adrenal axis is differentially affected in the rat chronic mild stress model of depression. Stress 2012, 15, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.M.; Coplan, J.D.; Kent, J.M.; Gorman, J.M. The noradrenergic system in pathological anxiety: A focus on panic with relevance to generalized anxiety and phobias. Biol. Psychiatry 1999, 46, 1205–1218. [Google Scholar] [CrossRef]
- Bondi, C.O.; Jett, J.D.; Morilak, D.A. Beneficial effects of desipramine on cognitive function of chronically stressed rats are mediated by alpha1-adrenergic receptors in medial prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.; Li, B.M. Neurobiology of executive functions: Catecholamine influences on prefrontal cortical functions. Biol. Psychiatry 2005, 57, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L. The Study on Pharmacodynamics and Mechanism of Compound Ma Ti Xiang (CMTX). Master’s Thesis, Beijing University of Chinese Medicine, Beijing, China, 2011. [Google Scholar]
- Li, J.; Liu, Q.T.; Chen, Y.; Liu, J.; Shi, J.L.; Liu, Y.; Guo, J.Y. Involvement of 5-HT1A Receptors in the Anxiolytic-Like Effects of Quercitrin and Evidence of the Involvement of the Monoaminergic System. Evid.-Based Compl. Alt. Med. 2016. [Google Scholar] [CrossRef]
- Liu, J.; Zhai, W.M.; Yang, Y.X.; Shi, J.L.; Liu, Q.T.; Liu, G.L.; Fang, N.; Li, J.; Guo, J.Y. GABA and 5-HT systems are implicated in the anxiolytic-like effect of spinosin in mice. Pharmacol. Biochem. Behav. 2015, 128, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; He, R.R.; Li, Y.F.; Li, S.B.; Tsoi, B.; Kurihara, H. Pharmacological studies on the anxiolytic effect of standardized Schisandralignans extract on restraint-stressed mice. Phytomedicine 2011, 18, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Roche, M.; Kerr, D.M.; Hunt, S.P.; Kelly, J.P. Neurokinin-1 receptor deletion modulatesbehavioural and neurochemical alterations in an animal model of depression. Behav. Brain Res. 2012, 228, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Shi, J.L.; Yong, L.; Zhao, R.; Zhai, Y.J.; Guo, J.Y. Anxiolytic-like effects of compound Zhi Zhu Xiang in rats. Evid.-Based Compl. Alt. Med. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Speisky, M.B.; Kharouba, S.N. Rapid and sensitive method for measuring norepinephrin, dopamine, 5-hydroxytryptamine and their major metabolites in rat brain by high performance liquid chromatography. Differential effect of probenecid, haloperidol and yohimbine on the concentrations of biogenic amines and metabolites in various regions of rat brain. J. Chromatogr. A 1987, 386, 25–35. [Google Scholar]
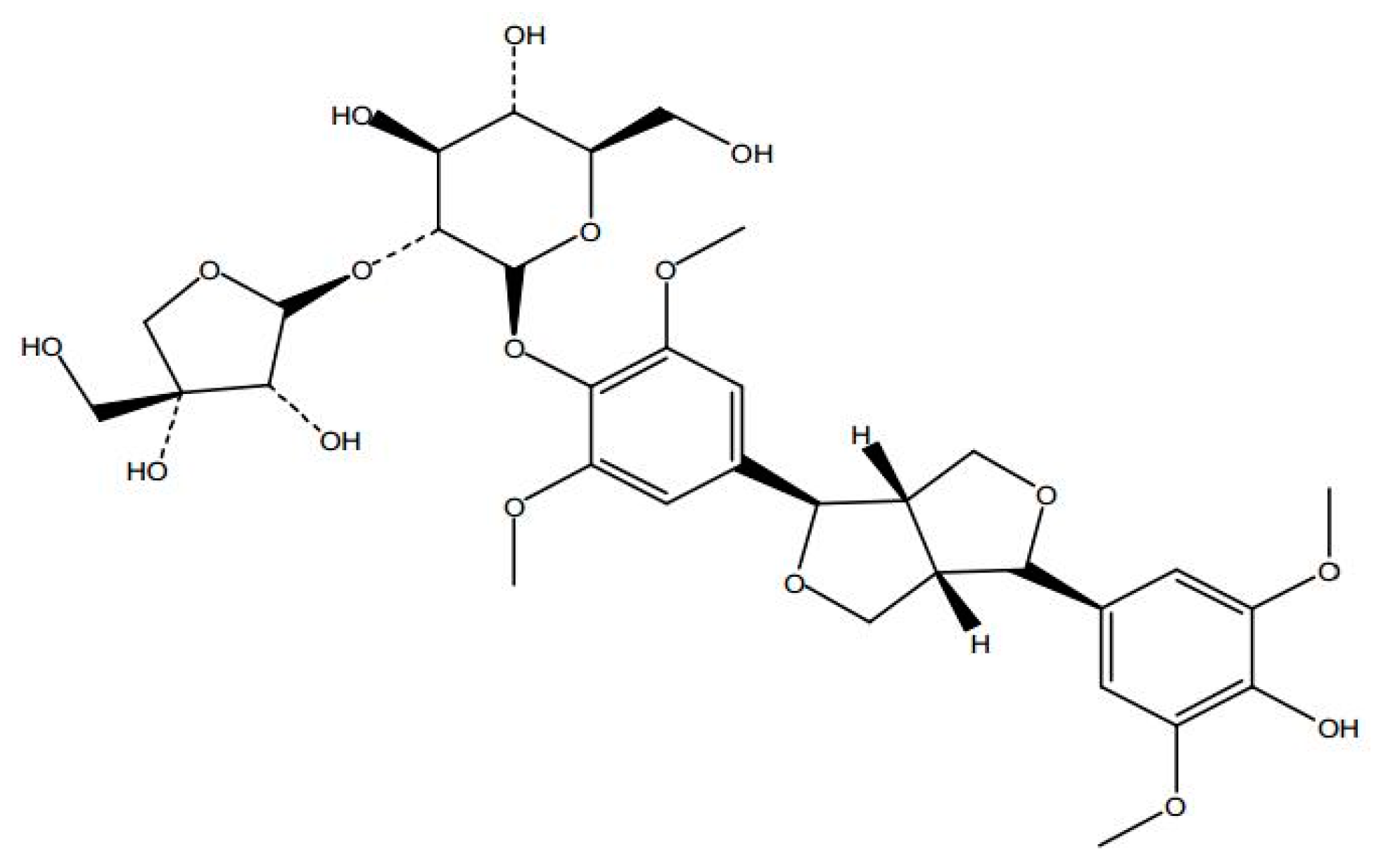
Sample Availability: Samples of the SAG ((−)-syringaresnol-4-O-β-d-apiofuranosyl-(1→2)-β-d-glucopyranoside)are available from the authors. |

| Groups | NE (μg/g) | DA (μg/g) | DOPAC (μg/g) | HVA (μg/g) | 5-HT (μg/g) | 5-HIAA (μg/g) |
|---|---|---|---|---|---|---|
| V/US | 121.35 ± 8.32 | 39.33 ± 5.38 | 35.99 ± 4.61 | 34.03 ± 3.58 | 20.31 ± 1.89 | 48.11 ± 3.27 |
| V/S | 188.87 ± 9.45 ## | 65.24 ± 6.69 # | 49.51 ± 5.08 | 47.76 ± 5.15 # | 28.03 ± 2.43 # | 58.15 ± 3.92 # |
| DZP/S | 129.78 ± 4.60 ** | 44.88 ± 6.00 * | 38.40 ± 4.26 | 31.32 ± 4.23 * | 15.57 ± 0.97 ** | 31.32 ± 4.23 ** |
| SAG/S (0.9 mg/kg) | 167.45 ± 13.17 | 55.44 ± 6.57 | 45.87 ± 3.22 | 37.89 ± 3.41 | 21.14 ± 1.01 * | 55.21 ± 1.84 |
| SAG/S (1.8 mg/kg) | 164.30 ± 6.12 * | 52.93 ± 4.60 | 41.59 ± 4.01 | 36.47 ± 3.34 | 17.89 ± 1.01 ** | 50.92 ± 2.91 |
| SAG/S (3.6 mg/kg) | 136.10 ± 2.96 ** | 46.76 ± 4.44 * | 40.64 ± 4.52 | 34.00 ± 2.97 * | 17.69 ± 1.13 ** | 34.00 ± 2.97 ** |
| Groups | NE (μg/g) | DA (μg/g) | DOPAC (μg/g) | HVA (μg/g) | 5-HT (μg/g) | 5-HIAA (μg/g) |
|---|---|---|---|---|---|---|
| V/US | 148.71 ± 7.94 | 28.36 ± 2.58 | 18.71 ± 1.30 | 19.35 ± 2.88 | 23.44 ± 3.24 | 74.29 ± 2.69 |
| V/S | 188.87 ± 9.45 ## | 65.24 ± 6.69 # | 49.51 ± 5.08 | 47.76 ± 5.15 # | 28.03 ± 2.43 # | 58.15 ± 3.92 # |
| DZP/S | 124.13 ± 4.47 ** | 23.77 ± 1.20 | 14.78 ± 1.09 | 11.95±1.24 | 19.57 ± 1.76 ** | 72.54 ± 2.53 ** |
| SAG/S (0.9 mg/kg) | 153.39 ± 9.53 | 28.06 ± 1.96 | 19.93 ± 2.39 | 17.37 ± 1.44 | 25.76 ± 4.95 | 86.17 ± 3.88 |
| SAG/S (1.8 mg/kg) | 139.09 ± 4.89 ** | 28.49 ± 1.67 | 21.48 ± 4.66 | 13.28 ± 2.12 | 20.18 ± 1.40 * | 81.13 ± 10.86 |
| SAG/S (3.6 mg/kg) | 135.37 ± 14.78 ** | 26.45 ± 1.24 | 15.93 ± 1.43 | 14.71 ± 1.59 | 19.50 ± 1.56 ** | 76.99 ± 4.56 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Lv, Y.-W.; Shi, J.-L.; Ma, X.-J.; Chen, Y.; Zheng, Z.-Q.; Wang, S.-N.; Guo, J.-Y. Anti-Anxiety Effect of (−)-Syringaresnol-4-O-β-d-apiofuranosyl-(1→2)-β-d-glucopyranoside from Albizzia julibrissin Durazz (Leguminosae). Molecules 2017, 22, 1331. https://doi.org/10.3390/molecules22081331
Liu J, Lv Y-W, Shi J-L, Ma X-J, Chen Y, Zheng Z-Q, Wang S-N, Guo J-Y. Anti-Anxiety Effect of (−)-Syringaresnol-4-O-β-d-apiofuranosyl-(1→2)-β-d-glucopyranoside from Albizzia julibrissin Durazz (Leguminosae). Molecules. 2017; 22(8):1331. https://doi.org/10.3390/molecules22081331
Chicago/Turabian StyleLiu, Jie, Yue-Wei Lv, Jin-Li Shi, Xiao-Jie Ma, Yi Chen, Zhi-Quan Zheng, Sheng-Nan Wang, and Jian-You Guo. 2017. "Anti-Anxiety Effect of (−)-Syringaresnol-4-O-β-d-apiofuranosyl-(1→2)-β-d-glucopyranoside from Albizzia julibrissin Durazz (Leguminosae)" Molecules 22, no. 8: 1331. https://doi.org/10.3390/molecules22081331




