Portuguese Honeys from Different Geographical and Botanical Origins: A 4-Year Stability Study Regarding Quality Parameters and Antioxidant Activity
Abstract
:1. Introduction
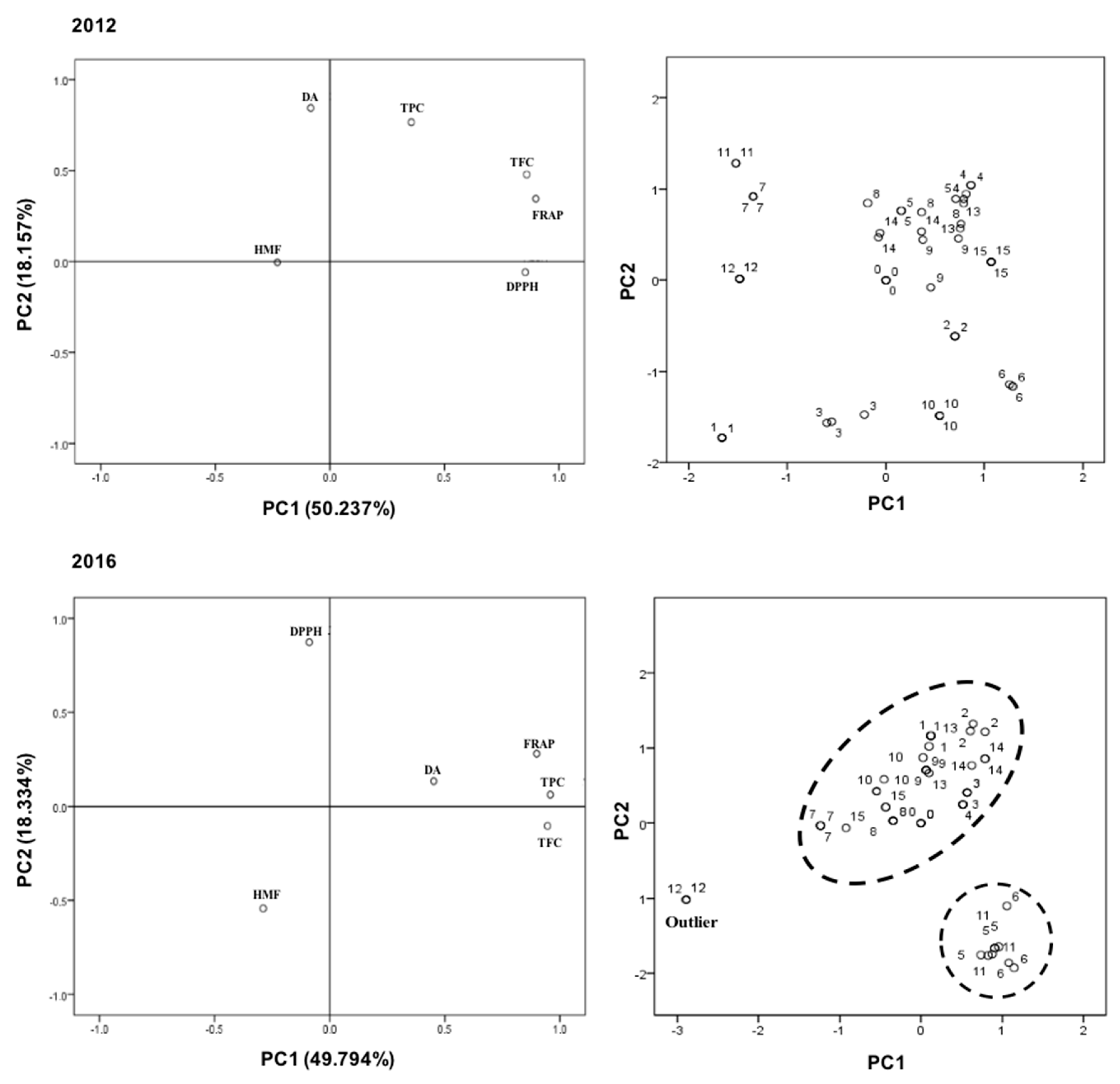
2. Results and Discussion
3. Materials and Methods
3.1. Standards and Reagents
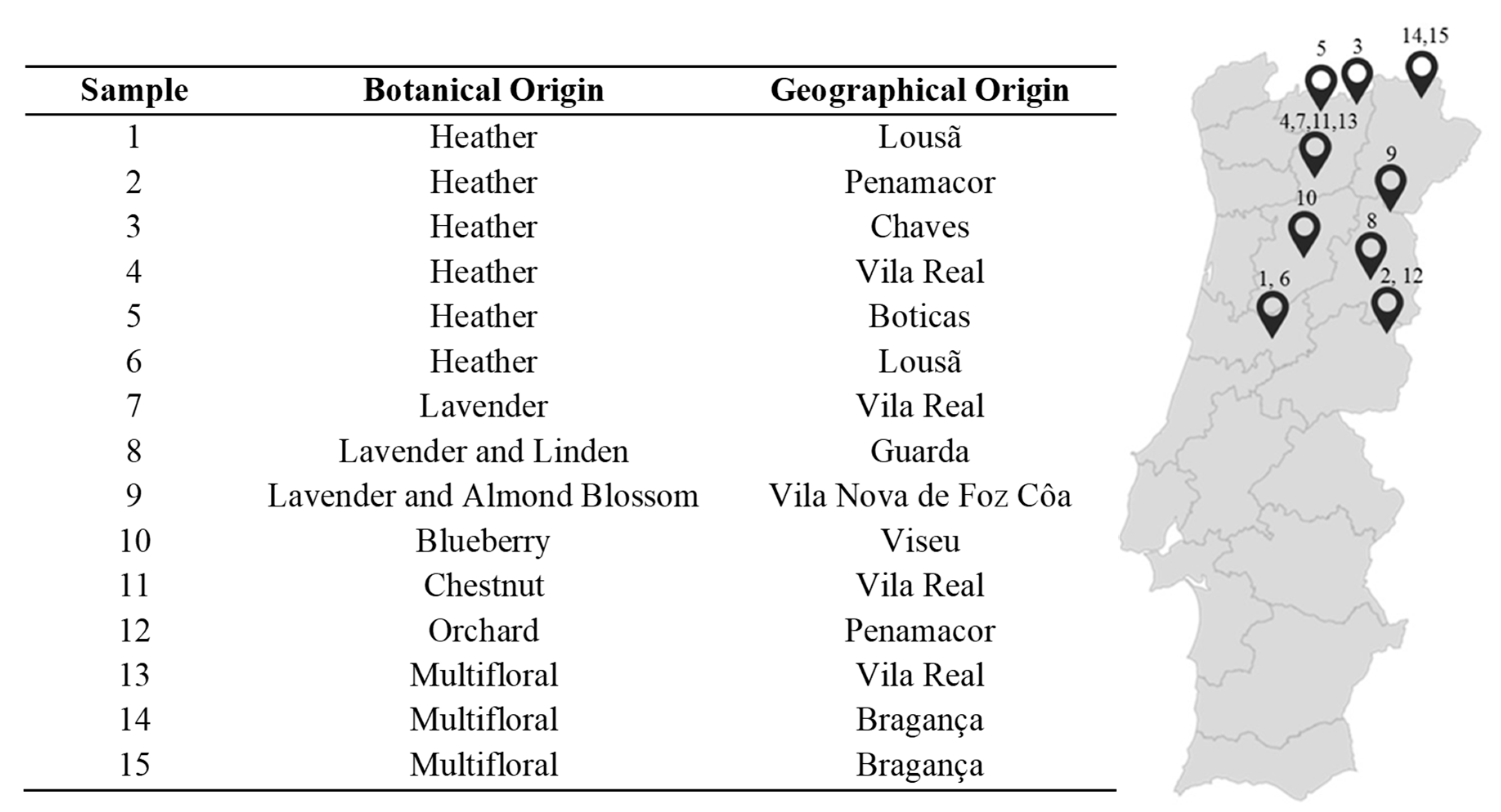
3.2. Samples
3.3. Colour Analysis
3.4. pH
3.5. Moisture
3.6. Diastase Activity (DA)
3.7. Hydroxymethylfurfural Quantification
3.8. Antioxidant Activity Analysis
3.8.1. DPPH• Scavenging Activity
3.8.2. Ferric Reducing Antioxidant Power Assay
3.9. Total Phenolics Content
3.10. Total Flavonoids Content
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Codex Alimentarius. Revised Codex Standard for Honey; Food and Agriculture Organization of the United Nations: Rome, Italy, (no. Codex stan 12–1981, rev.1 (1987), rev.2 (2001).
- Roshan, A.-R.A.; Gad, H.A.; El-Ahmady, S.H.; Abou-Shoer, M.I.; Khanbash, M.S.; Al-Azizi, M.M. Characterization and discrimination of the floral origin of sidr honey by physicochemical data combined with multivariate analysis. Food Anal. Methods 2017, 10, 137–146. [Google Scholar] [CrossRef]
- Kek, S.P.; Chin, N.L.; Tan, S.W.; Yusof, Y.A.; Chua, L.S. Classification of honey from its bee origin via chemical profiles and mineral content. Food Anal. Methods 2017, 10, 19–30. [Google Scholar] [CrossRef]
- Tucak, Z.; Periskic, M.; Beslo, D.; Tucak, I. Influence of the beehive type on the quality of honey. Coll. Antropol. 2004, 28, 463–467. [Google Scholar] [PubMed]
- Molan, P.C. Potential of honey in the treatment of wounds and burns. Am. J. Clin. Dermatol. 2001, 2, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, M.; Musa Özcan, M.; Çoklar, H. Evaluation of antioxidant activity, phenolic, mineral contents and some physicochemical properties of several pine honeys collected from western anatolia. Int. J. Food Sci. Nutr. 2009, 60, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Honey: A novel antioxidant. Molecules 2012, 17, 4400–4423. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, F.; Grobler, S.; Gedalia, I. The antifungal action of three south african honeys on candida albicans. Apidologie 2001, 32, 371–379. [Google Scholar] [CrossRef]
- Gomes, S.; Dias, L.G.; Moreira, L.L.; Rodrigues, P.; Estevinho, L. Physicochemical, microbiological and antimicrobial properties of commercial honeys from portugal. Food. Chem. Toxicol. 2010, 48, 544–5458. [Google Scholar] [CrossRef] [PubMed]
- Feás, X.; Estevinho, M.L. A survey of the in vitro antifungal activity of heather (Erica sp.) organic honey. J. Med. Food 2011, 14, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Bardy, J.; Slevin, N.J.; Mais, K.L.; Molassiotis, A. A systematic review of honey uses and its potential value within oncology care. J. Clin. Nurs. 2008, 17, 2604–2623. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Amaral, J.S.; Oliveira, M.B.P.P.; Mafra, I. Improving DNA isolation from honey for the botanical origin identification. Food Control. 2015, 48, 130–136. [Google Scholar] [CrossRef]
- Guler, A.; Bakan, A.; Nisbet, C.; Yavuz, O. Determination of important biochemical properties of honey to discriminate pure and adulterated honey with sucrose (Saccharum officinarum L.) syrup. Food Chem. 2007, 105, 1119–1125. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Gheldof, N.; Engeseth, N.J. Effect of processing and storage on antioxidant capacity of honey. J. Food Sci. 2004, 69. [Google Scholar] [CrossRef]
- Gheldof, N.; Xiao-Hong, W.; Engeseth, N. Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food Chem. 2002, 50, 5870–5877. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Estatistica (INE). Estatisticas Agricolas 2015; Instituto Nacional de Estatisticas—Statistics Portugal: Lisbon, Portugal, 2016. [Google Scholar]
- Portuguese Government. Programa Apícola Nacional 2017–2019; Gabinete de Planeamento, Politicas e Administração Geral: Lisbon, Portugal, 2016. [Google Scholar]
- Gomes, T.; Feas, X.; Iglesias, A.; Estevinho, L.M. Study of Organic Honey from the Northeast Portugal. Molecules 2011, 16, 5374–5386. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Costa, A.; Jerez, M.; Casal, S.; Sineiro, J.; Núñez, M.; Oliveira, M.B.P.P. Antiradical activity, phenolics profile, and hydroxymethylfurfural in espresso coffee: Influence of technological factors. J. Agric. Food Chem. 2010, 58, 12221–12229. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Barreira, S.V.P.; Nunes, M.A.; Cunha, L.M.; Oliveira, M.B.P.P. Optimization of antioxidants extraction from coffee silverskin, a roasting by-product, having in view a sustainable process. Ind. Crops Prod. 2014, 53, 350–357. [Google Scholar] [CrossRef]
- European Parliament. Directive 2014/63/EU of the European Parliament and of the Council of 15 May 2014 amending Council Directive 2001/110/EC relating to Honey. OJEU 2014, L164, 1–5. [Google Scholar]
- Estevinho, M.L.; Feás, X.; Seijas, J.A.; Vázquez-Tato, M.P. Physicochemical and pollen analysis of local honeys from the Lima Valley (Portugal). Electron. J. Environ. Agric. Food Chem. 2012, 11, 214–221. [Google Scholar]
- Estevinho, L.M.; Feás, X.; Seijas, J.A.; Pilar Vázquez-Tato, M. Organic honey from Trás-os-montes region (Portugal): Chemical, palynological, microbiological and bioactive compounds characterization. Food Chem. Toxicol. 2012, 50, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, A.; Feas, X.; Rodrigues, S.; Seijas, J.A.; Vazquez-Tato, M.P.; Dias, L.G.; Estevinho, L.M. Comprehensive study of honey with protected denomination of origin and contribution to the enhancement of legal specifications. Molecules 2012, 17, 8561–8577. [Google Scholar] [CrossRef] [PubMed]
- Aazza, S.; Lyoussi, B.; Antunes, D.; Miguel, M.G. Physicochemical characterization and antioxidant activity of commercial portuguese honeys. J. Food Sci. 2013, 78, C1159–C1165. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.; Estevinho, M.L.; Feás, X.; Cantalapiedra, J.; Iglesias, A. Pollen spectrum and physico-chemical attributes of heather (Erica sp.) honeys of North Portugal. J. Sci. Food Agric. 2009, 89, 1862–1870. [Google Scholar] [CrossRef]
- Feás, X.; Pires, J.; Estevinho, M.L.; Iglesias, A.; De Araujo, J.P.P. Palynological and physicochemical data characterisation of honeys produced in the Entre-Douro e Minho region of Portugal. Int. J. Food Sci. Technol. 2010, 45, 1255–1262. [Google Scholar] [CrossRef]
- Fallico, B.; Zappala, M.; Arena, E.; Verzera, A. Effects of conditioning on hmf content in unifloral honeys. Food Chem. 2004, 85, 305–313. [Google Scholar] [CrossRef]
- Acquarone, C.; Buera, P.; Elizalde, B. Pattern of ph and electrical conductivity upon honey dilution as a complementary tool for discriminating geographical origin of honeys. Food Chem. 2007, 101, 695–703. [Google Scholar] [CrossRef]
- Terrab, A.; Recamales, A.F.; Hernanz, D.; Heredia, F.J. Characterisation of spanish thyme honeys by their physicochemical characteristics and mineral contents. Food Chem. 2004, 88, 537–542. [Google Scholar] [CrossRef]
- González-Miret, M.L.; Terrab, A.; Hernanz, D.; Fernández-Recamales, M.Á.; Heredia, F.J. Multivariate correlation between color and mineral composition of honeys and by their botanical origin. J. Agric. Food Chem. 2005, 53, 2574–2580. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Maffei Facino, R. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal. Chim. Acta 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Rosa, A.; Tuberoso, C.I.G.; Atzeri, A.; Melis, M.P.; Bifulco, E.; Dessì, M.A. Antioxidant profile of strawberry tree honey and its marker homogentisic acid in several models of oxidative stress. Food Chem. 2011, 129, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Wilczyńska, A. Effect of filtration on colour, antioxidant activity and total phenolics of honey. LWT Food Sci. Technol. 2014, 57, 767–774. [Google Scholar] [CrossRef]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Costa, E.; Costa, C.S.G.; Nunes, M.A.; Almeida, A.A.; Santos-Silva, A.; Oliveira, M.B.P.P. Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. In Food Chem.; 2017; in press. [Google Scholar]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Alves, A.; Ramos, A.; Gonçalves, M.M.; Bernardo, M.; Mendes, B. Antioxidant activity, quality parameters and mineral content of portuguese monofloral honeys. J. Food Compos. Anal. 2013, 30, 130–138. [Google Scholar] [CrossRef]
- Chen, C.; Campbell, L.T.; Blair, S.E.; Carter, D.A. The effect of standard heat and filtration processing procedures on antimicrobial activity and hydrogen peroxide levels in honey. Front. Microbiol. 2012, 3, 265. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Zheng, W. Effect of plant growth temperature on antioxidant capacity in strawberry. J. Agric. Food Chem. 2001, 49, 4977–4982. [Google Scholar] [CrossRef] [PubMed]
- Kuś, P.M.; Congiu, F.; Teper, D.; Sroka, Z.; Jerković, I.; Tuberoso, C.I.G. Antioxidant activity, color characteristics, total phenol content and general hplc fingerprints of six polish unifloral honey types. LWT Food Sci. Technol. 2014, 55, 124–130. [Google Scholar] [CrossRef]
- Ahmad, N.S.; Aziz, A.A.; Kong, K.W.; Hamid, M.S.A.; Cheong, J.P.G.; Hamzah, S.H. Dose–response effect of Tualang honey on postprandial antioxidant activity and oxidative stress in female athletes: A pilot study. In J. Altern. Complement. Med.; 2017; in press. [Google Scholar]
- Sairazi, N.S.M.; Sirajudeen, K.; Asari, M.A.; Mummedy, S.; Muzaimi, M.; Sulaiman, S.A. Effect of Tualang honey against KA-induced oxidative stress and neurodegeneration in the cortex of rats. BMC Complement. Altern. Med. 2017, 17, 31. [Google Scholar]
- Alvarez-Suarez, J.M.; Giampieri, F.; Cordero, M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Afrin, S.; Beltrán-Ayala, P.; González-Paramás, A.M.; Santos-Buelga, C.; et al. Activation of AMPK/Nrf2 signalling by Manuka honey protects human dermal fibroblasts against oxidative damage by improving antioxidant response and mitochondrial function promoting wound healing. J. Funct. Food 2016, 25, 38–49. [Google Scholar] [CrossRef]
- Gonzales, A.P.; Burin, L.; Buera, M.A.D.P. Color changes during storage of honeys in relation to their composition and initial color. Food Res. Int. 1999, 32, 185–191. [Google Scholar] [CrossRef]
- Gheldof, N.; Engeseth, N.J. Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J. Agric. Food Chem. 2002, 50, 3050–3055. [Google Scholar] [CrossRef] [PubMed]
- Chataway, H.D. Honey tables showing the relationship between various hydrometer scales and refractive index to moisture content and weight per gallon of honey. Can. Bee J. 1935, 43, 215–222. [Google Scholar]
- AOAC: Official Methods of Analysis of the Association of Official Analytical Chemists, 19th ed.AOAC International: Gaithersburg, MD, USA, 2012.
Sample Availability: Not available. |
| Sample | HMF (mg/kg) | DA (Gothe Degrees) | pH | Moisture (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 2012 | 2016 | 2012 | 2016 | 2012 | 2016 | 2012 | 2016 | |
| 1 | 16.99 ± 0.52 a | 55.11 ± 0.87 d,* | 19.90 ± 0.58 g,h,i | 15.83 ± 0.29 c,d,e,f,* | 4.52 ± 0.04 f | 3.92 ± 0.01 h,* | 14.04 ± 0.14 c,d,e,f,g | 14.00 ± 0.00 f,g |
| 2 | 2.38 ± 0.25 c | 12.89 ± 0.12 g,h,* | 27.78 ± 0.76 c | 19.23 ± 0.11 a,* | 4.88 ± 0.00 b | 4.50 ± 0.01 a,* | 13.14 ± 0.01 g | 13.47 ± 0.09 h,i,* |
| 3 | 1.13 ± 0.06 c,d,e,f | 20.99 ± 0.07 e,f,* | 32.64 ± 0.85 b | 17.83 ± 0.22 a,b,c,* | 4.80 ± 0.00 c,d | 4.26 ± 0.01 d,* | 13.77 ± 0.02 c,e,f,g | 14.40 ± 0.00 d,e,* |
| 4 | 2.22 ± 0.03 c,d | 4.15 ± 0.06 i,* | 24.80 ± 0.45 d,e | 15.68 ± 0.18 d,e,f,g,* | 5.05 ± 0.01 a | 4.21 ± 0.00 e,* | 16.85 ± 0.09 d,e,f,g | 15.67 ± 0.12 b,* |
| 5 | 1.73 ± 0.08 c,d | 23.31 ± 0.00 e,* | 21.74 ± 1.06 f,g | 15.57 ± 0.38 e,f,g,* | 4.74 ± 0.01 d | 4.37 ± 0.01 b,* | 13.97 ± 0.21 b,c,d,e | 15.00 ± 0.00 i,* |
| 6 | 7.70 ± 0.18 b | 105.05 ± 2.14 c,* | 37.77 ± 1.41 a | 18.10 ± 0.19 a,b,* | 4.82 ± 0.00 b,c | 4.30 ± 0.01 c,* | 15.90 ± 0.41 e,f,g | 14.40 ± 0.00 e,f,* |
| 7 | 0.71 ± 0.06 e,f | 15.21 ± 0.31 f,g,* | 20.85 ± 1.22 g,h | 8.88 ± 0.58 h,* | 3.98 ± 0.05 i | 3.80 ± 0.01 i,* | 15.40 ± 0.20 a,b | 14.80 ± 0.00 d,e,* |
| 8 | 0.79 ± 0.03 e,f | 21.00 ± 0.22 e,f,* | 21.68 ± 0.93 f,g | 17.69 ± 0.27 a,b,c,d,* | 4.60 ± 0.01 e | 3.91 ± 0.01 h,* | 15.16 ± 0.94 c,d,e,f,g | 13.27 ± 0.12 g,h |
| 9 | 0.33 ± 0.01 f | 22.49 ± 0.13 e,* | 18.93 ± 1.18 i | 17.69 ± 0.27 a,b,c,d | 4.64 ± 0.02 e | 4.11 ± 0.01 f,* | 15.37 ± 0.79 f,g | 14.20 ± 0.20 h,i |
| 10 | 7.11 ± 0.25 b | 115.95 ± 0.01 b,* | 26.48 ± 0.80 c,d | 17.39 ± 0.21 a,b,c,d,e,* | 4.34 ± 001 g | 3.96 ± 0.01 g,* | 14.73 ± 0.06 b,c,d | 14.67 ± 0.23 e,f |
| 11 | 1.06 ± 0.17 d,e,f | 4.06 ± 0.04 i,* | 19.79 ± 0.47 h,i | 16.93 ± 0.31 b,c,d,e,f,* | 5.01 ± 0.01 a | 4.26 ± 0.01 d,* | 14.29 ± 0.40 b,c,d,e,f | 13.73 ± 0.12 c,d |
| 12 | 17.38 ± 1.05 a | 148.97 ± 6.14 a,* | 19.62 ± 0.33 h,i | 13.60 ± 0.43 g,* | 4.01 ± 0.03 i | 3.58 ± 0.01 j,* | 13.65 ± 0.89 a | 13.47 ± 0.12 a |
| 13 | 1.01 ± 0.00 d,e,f | 14.14 ± 0.11 g,h,* | 25.32 ± 0.89 d | 17.48 ± 1.70 a,b,c,d,e,* | 4.49 ± 0.00 f | 4.36 ± 0.02 b,* | 13.91 ± 0.76 b | 14.20 ± 0.00 b,c |
| 14 | 0.74 ± 0.03 e,f | 15.31 ± 0.03 f,g,* | 16.15 ± 0.13 j | 15.68 ± 0.37 d,e,f,g | 4.09 ± 0.01 h | 4.12 ± 0.01 f,* | 14.68 ± 0.12 b,c,d,e,f | 14.40 ± 0.00 d,e |
| 15 | 0.81 ± 0.04 e,f | 7.65 ± 0.04 h,i,* | 23.40 ± 0.24 e,f | 15.00 ± 0.41 f,g,* | 4.60 ± 0.01 e | 3.61 ± 0.02 j,* | 14.57 ± 0.0 b,c,d,e,f | 15.80 ± 0.00 a,* |
| Sample | 2012 | 2016 | ||||
|---|---|---|---|---|---|---|
| L* (Lightness) | a* (Redness) | b* (Yellowness) | L* (Lightness) | a* (Redness) | b* (Yellowness) | |
| 1 | 37.71 ± 0.03 e,f | 3.52 ± 0.01 a | 10.06 ± 0.02 g | 17.74 ± 0.35 d,* | 2.77 ± 0.11 d | 1.85 ± 0.07 g,* |
| 2 | 39.51 ± 0.00 d | 2.73 ± 0.03 b | 12.52 ± 0.01 e | 18.68 ± 0.35 c,d,* | 3.45 ± 0.08 c,* | 2.84 ± 0.06 f,* |
| 3 | 38.67 ± 0.00 d,e | −2.06 ± 0.02 i | 8.42 ± 0.01 h | 17.90 ± 0.16 d,* | 3.28 ± 0.02 c,* | 2.04 ± 0.03 g,* |
| 4 | 37.91 ± 0.19 e,f | −0.28 ± 0.07 f | 8.16 ± 0.14 h | 21.26 ± 0.40 b,* | 4.32 ± 0.13 b,* | 4.25 ± 0.06 d,* |
| 5 | 37.09 ± 0.51 f | 2.24 ± 0.14 c | 7.36 ± 0.08 i | 17.09 ± 0.25* | 0.98 ± 0.01 f | 0.73 ± 0.04 h,* |
| 6 | 41.68 ± 0.04 c | 0.88 ± 0.01 e | 14.96 ± 0.04 c | 20.02 ± 0.45 b,c,* | 4.68 ± 0.20 a,b,* | 3.52 ± 0.11 e,* |
| 7 | 44.58 ± 0.03 b | −1.92 ± 0.02 i | 17.81 ± 0.01 a | 21.53 ± 0.43 a,b,* | 1.80 ± 0.09 e,* | 5.57 ± 0.16 b,* |
| 8 | 41.47 ± 0.04 c | 1.11 ± 0.03 e | 14.98 ± 0.00 c | 23.02 ± 0.25 a,* | 3.61 ± 0.16 c,* | 6.60 ± 0.22 a* |
| 9 | 47.24 ± 0.13 a | −4.26 ± 0.03 k | 18.42 ± 0.11 a | 20.63 ± 0.13 b,* | 4.44 ± 0.04 b* | 4.77 ± 0.03 c,* |
| 10 | 41.16 ± 0.01 c | 1.67 ± 0.00 d | 13.56 ± 0.05 d | 21.42 ± 0.25 a,b,* | 4.95 ± 0.20 a,* | 5.62 ± 0.18 b,* |
| 11 | 36.70 ± 0.14 | −1.38 ± 0.00 h | 6.91 ± 0.03 i | 17.74 ± 0.71 d,* | 0.93 ± 0.07 f,* | 0.95 ± 0.04 h,* |
| 12 | 33.93 ± 0.21 g | −0.73 ± 0.02 g | 4.09 ± 0.05 j | 20.25 ± 0.30 b,c,* | 1.95 ± 0.13 e,* | 2.80 ± 0.18 f,* |
| 13 | 39.67 ± 0.71 d | −0.25 ± 0.13 f | 10.82 ± 0.37 f | 21.34 ± 0.95 b,* | 3.46 ± 0.05 c,* | 5.81 ± 0.19 b |
| 14 | 45.08 ± 0.15 b | −3.60 ± 0.01 j | 15.95 ± 0.32 b | 18.25 ± 0.05 d,* | 1.07 ± 0.05 f,* | 1.64 ± 0.05 g,* |
| 15 | 38.21 ± 0.26 e | 3.50 ± 0.23 a | 10.69 ± 0.50 f,g | 18.64 ± 0.31 c,d,* | 1.06 ± 0.07 f,* | 2.03 ± 0.03 g,* |
| Sample | TPC (mg GAE/kg) | TFC (mg ECE/kg) | DPPH• SA (%) | FRAP (mg FSE/kg) | ||||
|---|---|---|---|---|---|---|---|---|
| 2012 | 2016 | 2012 | 2016 | 2012 | 2016 | 2012 | 2016 | |
| 1 | 269.03 ± 2.01 f | 301.70 ± 1.95 f,* | 21.77 ± 0.92 d,e | 48.58 ± 2.52 c,d,* | 24.6 ± 0.2 d | 21.2 ± 0.4 c,d | 1948.0 ± 24.2 a,b,c | 2170.8 ± 4.2 b,c* |
| 2 | 327.34 ± 4.02 d | 380.40 ± 8.92 d,* | 30.08 ± 0.89 c | 54.83 ± 9.53 c,d | 18.5 ± 0.4 e | 16.2 ± 0.9 f,g | 2956.0 ± 20.7 a,b,c | 3282.7 ± 7.3 a,b,c* |
| 3 | 300.32 ± 12.56 e | 382.79 ± 8.49 d,* | 23.15 ± 0.02 d | 49.47 ± 2.19 c,d,* | 18.2 ± 0.3 e | 16.4 ± 0.3 f,g | 2225.7 ± 9.8 a,b,c | 2957.7 ± 4.2 a,b,c,* |
| 4 | 251.97 ± 2.01 g | 335.09 ± 1.95 e,* | 17.61 ± 1.96 f | 36.98 ± 1.26 d,e,* | 27.9 ± 0.5 c | 20.5 ± 0.6 c,d,e | 1728.3 ± 16.5 a,c | 3062.7 ± 7.3 b,c* |
| 5 | 500.83 ± 3.48 a | 591.87 ± 11.74 a | 44.64 ± 0.95 a | 111.04 ± 2.19 a,b,* | 33.2 ± 0.1 b | 31.0 ± 1.3 b | 4086.7 ± 74.3 b,c | 4863.8 ± 16.9 a,b,c,* |
| 6 | 378.53 ± 4.02 c | 461.49 ± 1.95 c,* | 31.47 ± 0.03 c | 94.09 ± 12.81 b,* | 33.3 ± 0.2 b | 30.1 ± 0.9 b | 2286.7 ± 36.4 a,b,c | 3067.6 ± 8.5 b,c,* |
| 7 | 149.58 ± 2.01 l | 267.51 ± 10.73 g,* | 8.60 ± 0.02 i | 17.35 ± 5.78 f | 5.3 ± 0.1 g | 7.0 ± 1.2 h | 497.7 ± 21.1 c | 1598.9 ± 16.9 c,* |
| 8 | 220.68 ± 2.01 h,i | 232.53 ± 8.49 h | 14.15 ± 0.95 g | 26.27 ± 1.26 e,f,* | 32.5 ± 0.2 b | 22.6 ± 0.7 c,* | 989.3 ± 9.8 a,b,c | 1708.9 ± 18.5 a,b* |
| 9 | 378.53 ± 4.02 c | 297.72 ± 7.37 f,* | 19.69 ± 0.88 e,f | 53.93 ± 2.52 c,d,* | 13.0 ± 0.9 f | 15.0 ± 0.3 g | 1706.3 ± 9.8 a | 2109.7 ± 19.4 a,b* |
| 10 | 395.60 ± 2.01 b | 280.23 ± 3.89 f,g,* | 23.85 ± 1.01 d | 43.23 ± 2.52 d,e,* | 18.9 ± 0.2 e | 19.0 ± 0.7 d,e,f | 1631.3 ± 17.2 c | 2139.0 ± 14.7 c,* |
| 11 | 183.71 ± 2.01 k | 509.19 ± 11.85 b,* | 37.01 ± 0.99 b | 112.83 ± 5.50 a,* | 48.8 ± 0.6 a | 47.5 ± 0.3 a | 3944.7 ± 38.2 a,b,c | 5130.2 ± 12.7 a,b,c,* |
| 12 | 200.77 ± 2.01 j | 139.52 ± 10.84 i,* | 10.68 ± 0.01 h | 16.46 ± 2.52 f | 18.7 ± 0.5 e | 17.3 ± 0.4 e,f,g,* | 764.7 ± 4.6 a,b,c | 1391.2 ± 7.3 a,b,c* |
| 13 | 224.95 ± 4.02 h | 293.75 ± 4.90 f,g,* | 17.61 ± 0.87 f | 28.06 ± 0.00 e,f,* | 11.9 ± 0.0 f | 9.7 ± 1.7 h | 1506.0 ± 5.2 a,b,c | 3827.6 ± 18.5 a,b,c,* |
| 14 | 207.88 ± 4.02 i,j | 389.94 ± 3.89 d,* | 14.15 ± 0.92 g | 63.75 ± 1.26 c,* | 18.6 ± 0.8 e | 16.9 ± 0.4 f,g | 914.3 ± 9.8 a,b,c | 3162.9 ± 23.6 a,b,c,* |
| 15 | 378.53 ± 2.01 c | 288.98 ± 5.95 f,g,* | 31.47 ± 0.03 c | 25.38 ± 4.37 e,f | 27.0 ± 0.6 c | 31.0 ± 1.3 b | 2656.3 ± 9.8 a,b | 1540.3 ± 15.3 a,* |
| 2012 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TPC | TFC | DPPH• SA | FRAP | L* | a* | b* | HMF | DA | |
| TPC | - | 0.683 | 0.143 | 0.546 | 0.383 | 0.395 | 0.322 | −0.027 | 0.149 |
| TFC | 0.683 | - | 0.646 | 0.962 | 0.282 | −0.222 | 0.244 | −0.194 | −0.167 |
| DPPH• SA | 0.143 | 0.646 | - | 0.657 | 0.370 | −0.095 | 0.372 | −0.191 | −0.400 |
| FRAP | 0.546 | 0.962 | 0.657 | - | 0.185 | −0.163 | 0.150 | −0.180 | −0.241 |
| L | 0.383 | 0.282 | 0.370 | 0.185 | - | −0.517 | 0.955 | −0.463 | 0.062 |
| a | 0.395 | −0.222 | −0.095 | −0.163 | −0.517 | - | −0.293 | 0.330 | 0.084 |
| b | 0.322 | 0.244 | 0.372 | 0.150 | 0.955 | −0.293 | - | −0.379 | 0.104 |
| HMF | −0.027 | −0.194 | −0.191 | −0.180 | −0.463 | 0.330 | −0.379 | - | −0.285 |
| DA | 0.149 | −0.167 | −0.400 | −0.241 | 0.062 | 0.084 | 0.104 | −0.285 | - |
| 2016 | |||||||||
| TPC | TFC | DPPH• SA | FRAP | L* | a* | b* | HMF | DA | |
| TPC | - | 0.911 | 0.596 | 0.857 | −0.043 | 0.022 | −0.039 | 0.371 | −0.096 |
| TFC | 0.911 | - | 0.685 | 0.796 | −0.095 | −0.081 | −0.093 | −0.206 | 0.194 |
| DPPH• SA | 0.596 | 0.685 | - | 0.496 | 0.061 | −0.134 | 0.039 | −0.255 | −0.042 |
| FRAP | 0.857 | 0.796 | 0.496 | - | −0.095 | −0.216 | −0.116 | −0.335 | 0.275 |
| L | −0.043 | −0.095 | 0.061 | −0.095 | - | 0.569 | 0.947 | 0.174 | −0.123 |
| a | 0.022 | −0.081 | −0.134 | −0.216 | 0.569 | - | 0.643 | 0.492 | 0.291 |
| b | −0.039 | −0.093 | 0.039 | −0.116 | 0.947 | 0.643 | - | −0.036 | 0.095 |
| HMF | 0.371 | −0.206 | −0.255 | −0.335 | 0.174 | 0.492 | −0.036 | - | −0.026 |
| DA | −0.096 | 0.194 | −0.042 | 0.275 | −0.123 | 0.291 | 0.095 | −0.026 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, S.; Pinto, D.; Rodrigues, F.; Alves, R.C.; Oliveira, M.B.P.P. Portuguese Honeys from Different Geographical and Botanical Origins: A 4-Year Stability Study Regarding Quality Parameters and Antioxidant Activity. Molecules 2017, 22, 1338. https://doi.org/10.3390/molecules22081338
Soares S, Pinto D, Rodrigues F, Alves RC, Oliveira MBPP. Portuguese Honeys from Different Geographical and Botanical Origins: A 4-Year Stability Study Regarding Quality Parameters and Antioxidant Activity. Molecules. 2017; 22(8):1338. https://doi.org/10.3390/molecules22081338
Chicago/Turabian StyleSoares, Sonia, Diana Pinto, Francisca Rodrigues, Rita C. Alves, and M. Beatriz P.P. Oliveira. 2017. "Portuguese Honeys from Different Geographical and Botanical Origins: A 4-Year Stability Study Regarding Quality Parameters and Antioxidant Activity" Molecules 22, no. 8: 1338. https://doi.org/10.3390/molecules22081338