BILP-19—An Ultramicroporous Organic Network with Exceptional Carbon Dioxide Uptake
Abstract
:1. Introduction
2. Results and Discussion
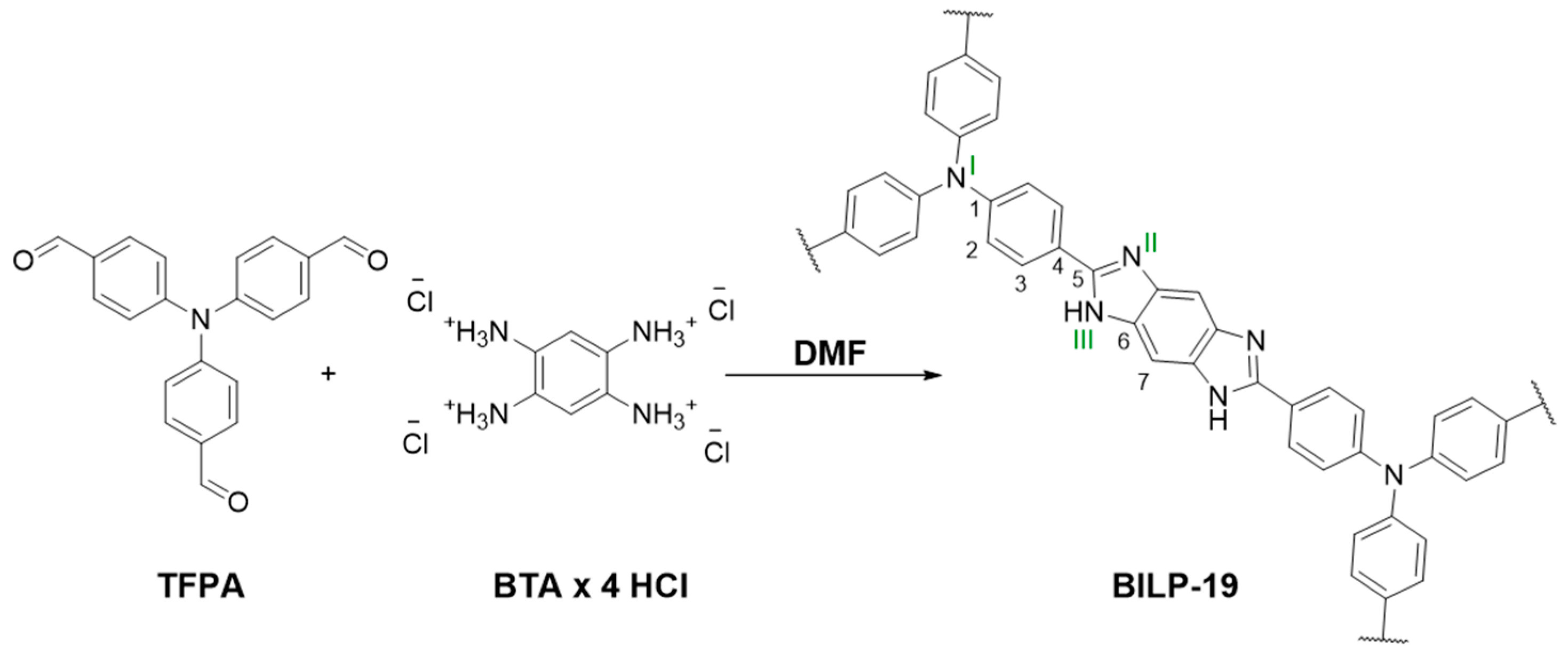
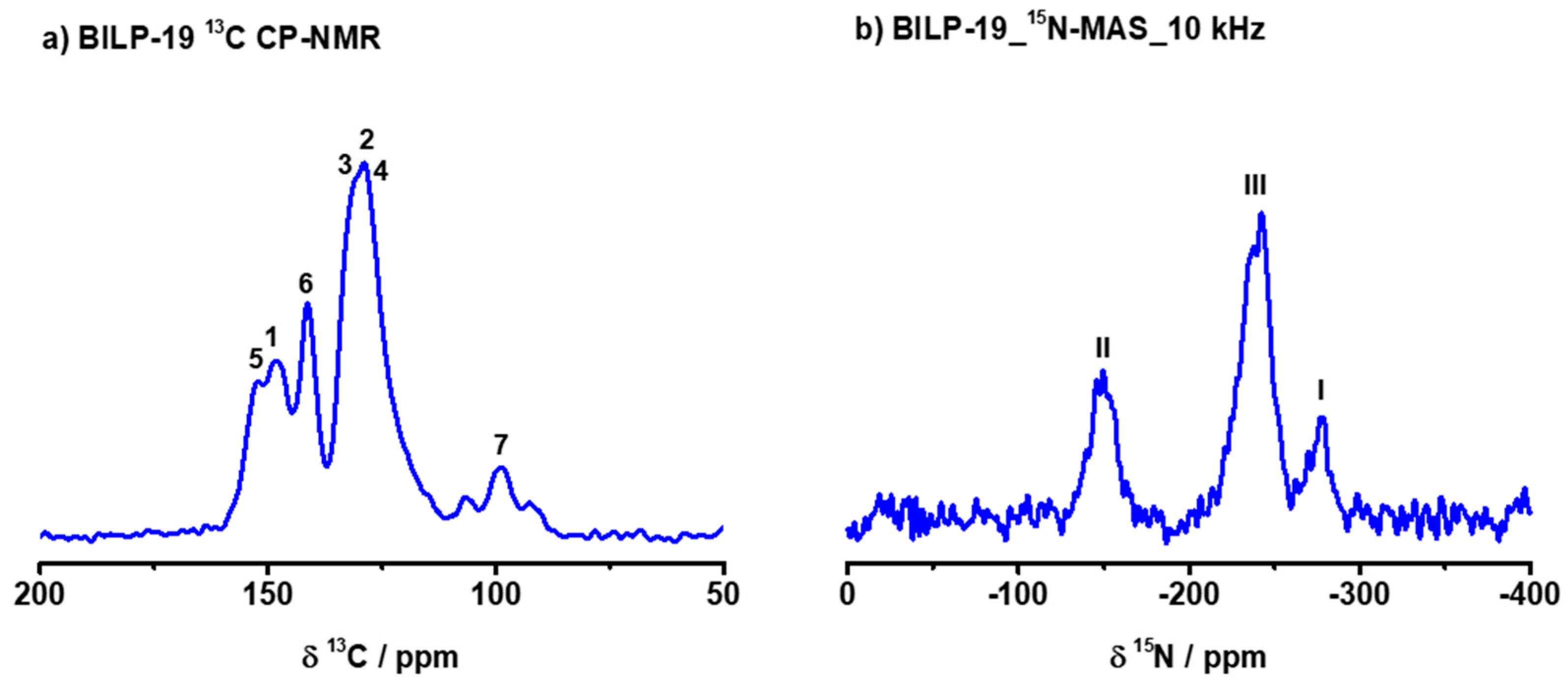
2.1. Synthesis and Characterization
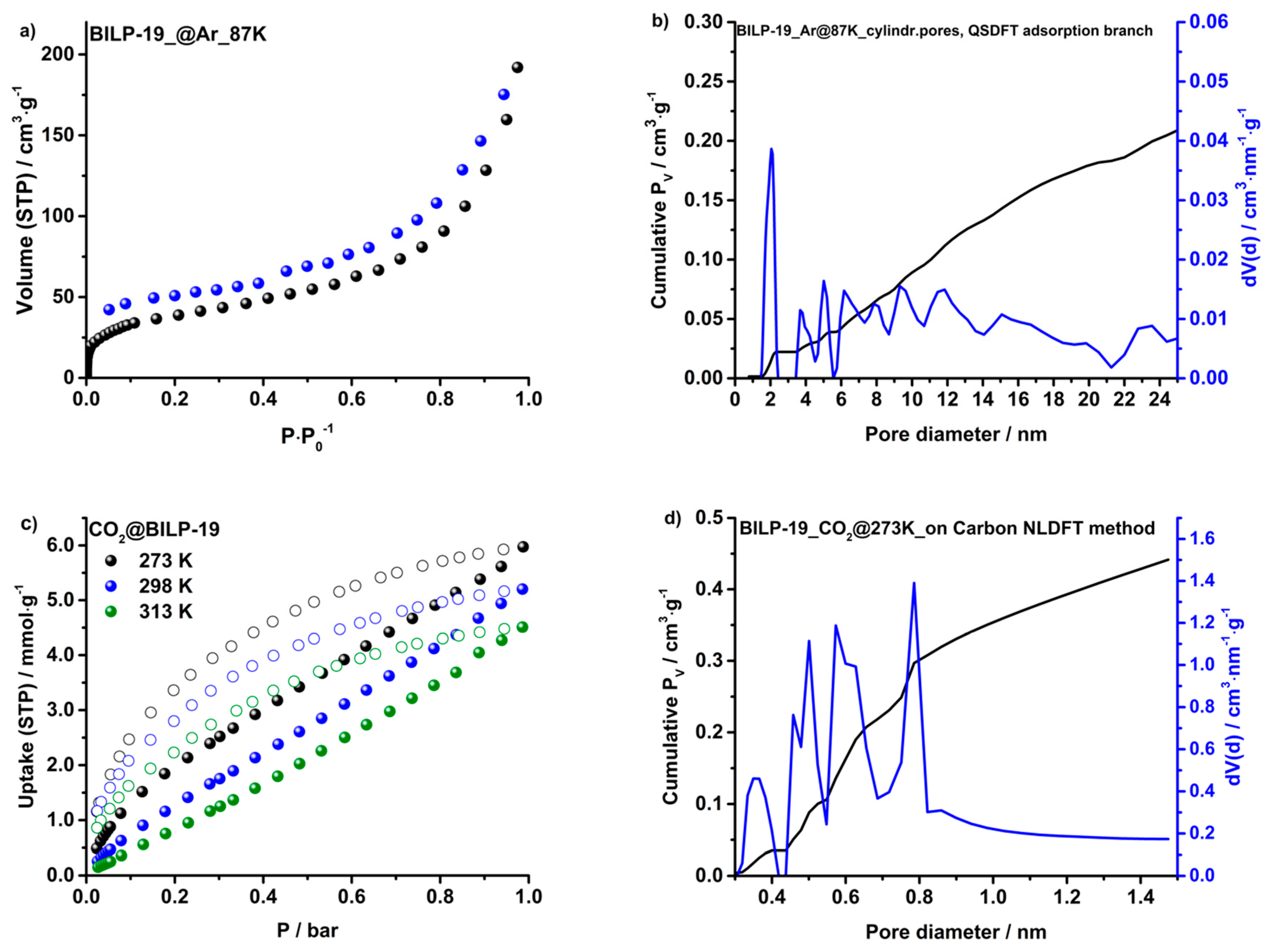
2.2. Surface Area and Porosity
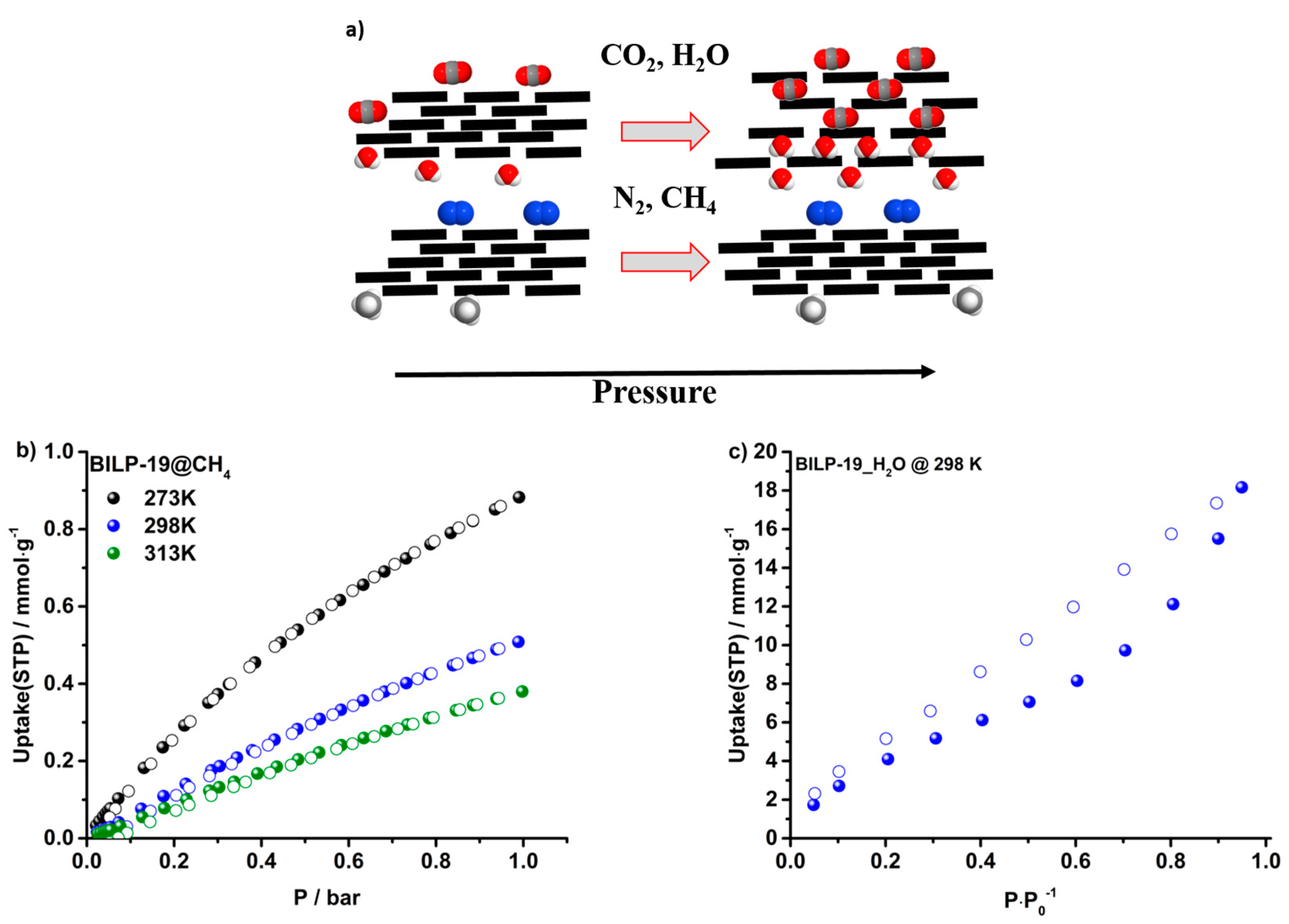
2.3. Gas Adsorption of CO2, N2, CH4 and H2O
2.4. Selectivities over N2 and CH4
3. Materials and Methods
3.1. General Information
3.2. Synthesis of BILP-19
3.3. Experimental Details
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- UNFCCC. Conference of the Parties (COP). In Adoption of the Paris Agreement, Proceedings of Paris Climate Change Conference; Paris, France, 2015, Available online: http://unfccc.int/meetings/paris_nov_2015/meeting/8926/php/view/decisions.php (accessed on 11 August 2017).
- The Global Status of CCS: 2016. Summary Report; “Time To Accelerate”; Global CCS Institute: Docklands, Australia, 2016.
- Dawson, R.; Cooper, A.I.; Adams, D.J. Chemical functionalization strategies for carbon dioxide capture in microporous organic polymers. Polym. Int. 2013, 62, 345–352. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- Sakaushi, K.; Antonietti, M. Carbon- and Nitrogen-Based Organic Frameworks. Acc. Chem. Res. 2015, 48, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Ren, H. Porous Organic Frameworks; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Klumpen, C.; Breunig, M.; Homburg, T.; Stock, N.; Senker, J. Microporous Organic Polyimides for CO2 and H2O Capture and Separation from CH4 and N2 Mixtures: Interplay between Porosity and Chemical Function. Chem. Mater. 2016, 28, 5461–5470. [Google Scholar] [CrossRef]
- Hao, S.; Liu, Y.; Shang, C.; Liang, Z.; Yu, J. CO2 adsorption and catalytic application of imidazole ionic liquid functionalized porous organic polymers. Polym. Chem 2017, 8. [Google Scholar] [CrossRef]
- Wang, J.; Sng, W.; Yi, G.; Zhang, Y. Imidazolium salt-modified porous hypercrosslinked polymers for synergistic CO2 capture and conversion. Chem. Commun. 2015, 51, 12076–12079. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, M.G.; El-Kaderi, H.M. Template-Free Synthesis of a Highly Porous Benzimidazole-Linked Polymer for CO2 Capture and H2 Storage. Chem. Mater. 2011, 23, 1650–1653. [Google Scholar] [CrossRef]
- Rabbani, M.G.; El-Kaderi, H.M. Synthesis and Characterization of Porous Benzimidazole-Linked Polymers and Their Performance in Small Gas Storage and Selective Uptake. Chem. Mater. 2012, 24, 1511–1517. [Google Scholar] [CrossRef]
- Rabbani, M.G.; Reich, T.E.; Kassab, R.M.; Jackson, K.T.; El-Kaderi, H.M.; Zhang, W.; Lipkowski, J.; Guenther, J.; Blümel, J.; Krishna, R.; Li, Z.; Zhou, H.-C. High CO2 uptake and selectivity by triptycene-derived benzimidazole-linked polymers. Chem. Commun. 2012, 48, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Sekizkardes, A.K.; İslamoğlu, T.; Kahveci, Z.; El-Kaderi, H.M. Application of pyrene-derived benzimidazole-linked polymers to CO2 separation under pressure and vacuum swing adsorption settings. J. Mater. Chem. A 2014, 2, 12492. [Google Scholar] [CrossRef]
- Sekizkardes, A.K.; Culp, J.T.; Islamoglu, T.; Marti, A.; Hopkinson, D.; Myers, C.; El-Kaderi, H.M.; Nulwala, H.B. An ultra-microporous organic polymer for high performance carbon dioxide capture and separation. Chem. Commun. Chem. Commun 2015, 51, 13393–13396. [Google Scholar] [CrossRef] [PubMed]
- Altarawneh, S.; İslamoğlu, T.; Sekizkardes, A.K.; El-Kaderi, H.M. Effect of Acid-Catalyzed Formation Rates of Benzimidazole-Linked Polymers on Porosity and Selective CO2 Capture from Gas Mixtures. Environ. Sci. Technol. 2015, 49, 4715–4723. [Google Scholar] [CrossRef] [PubMed]
- Ashourirad, B.; Sekizkardes, A.K.; Altarawneh, S.; El-Kaderi, H.M. Exceptional Gas Adsorption Properties by Nitrogen-Doped Porous Carbons Derived from Benzimidazole-Linked Polymers. Chem. Mater. 2015, 27, 1349–1358. [Google Scholar] [CrossRef]
- Sekizkardes, A.K.; Altarawneh, S.; Kahveci, Z.; Islamoʇlu, T.; El-Kaderi, H.M. Highly selective CO2 capture by triazine-based benzimidazole-linked polymers. Macromolecules 2014, 47, 8328–8334. [Google Scholar] [CrossRef]
- Lu, W.; Sculley, J.P.; Yuan, D.; Krishna, R.; Wei, Z.; Zhou, H.-C. Polyamine-Tethered Porous Polymer Networks for Carbon Dioxide Capture from Flue Gas. Angew. Chem. Int. Ed. 2012, 51, 7480–7484. [Google Scholar] [CrossRef] [PubMed]
- Arab, P.; Rabbani, M.G.; Sekizkardes, A.K.; İslamoğlu, T.; El-Kaderi, H.M. Copper(I)-Catalyzed Synthesis of Nanoporous Azo-Linked Polymers: Impact of Textural Properties on Gas Storage and Selective Carbon Dioxide Capture. Chem. Mater. 2014, 26, 1385–1392. [Google Scholar] [CrossRef]
- McDonald, T.M.; Lee, W.R.; Mason, J.A.; Wiers, B.M.; Hong, C.S.; Long, J.R. Capture of carbon dioxide from air and flue gas in the alkylamine-appended metal-organic framework mmen-Mg2(dobpdc). J. Am. Chem. Soc. 2012, 134, 7056–7065. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, Z.; Xing, W.; Shen, H.; Bi, X.; Zhu, T.; Qiu, Z.; Zhuo, S. A New Approach to Tuning Carbon Ultramicropore Size at Sub-Angstrom Level for Maximizing Specific Capacitance and CO2 Uptake. Adv. Funct. Mater. 2016, 26, 7955–7964. [Google Scholar] [CrossRef]
- Liebl, M.R.; Senker, J. Microporous Functionalized Triazine-Based Polyimides with High CO2 Capture Capacity. Chem. Mater. 2013, 25, 970–980. [Google Scholar] [CrossRef]
- Seema, H.; Kemp, K.C.; Le, N.H.; Park, S.-W.; Chandra, V.; Lee, J.W.; Kim, K.S. Highly selective CO2 capture by S-doped microporous carbon materials. Carbon N. Y. 2014, 66, 320–326. [Google Scholar] [CrossRef]
- Hesse, M.; Meier, H.; Zeeh, B. Spektroskopische Methoden in der Organischen Chemie; Georg Thieme Verlag: Stuttgart, Germany, 2012. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Galarneau, A.; Villemot, F.; Rodriguez, J.; Fajula, F.; Coasne, B. Validity of the t-plot method to assess microporosity in hierarchical micro/mesoporous materials. Langmuir 2014, 30, 13266–13274. [Google Scholar] [CrossRef] [PubMed]
- Islamoglu, T.; Behera, S.; Kahveci, Z.; Tessema, T.-D.; Jena, P.; El-Kaderi, H.M. Enhanced Carbon Dioxide Capture from Landfill Gas Using Bifunctionalized Benzimidazole-Linked Polymers. ACS Appl. Mater. Interfaces 2016, 8, 14648–14655. [Google Scholar] [CrossRef] [PubMed]
- Mohr, R.; Rao, M.B. Isosteric Heat of Adsorption: Theory and Experiment. J. Phys. Chem. B 1999, 103, 6539–6546. [Google Scholar]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Particle Technology Series; Springer: Dordrecht, The Netherlands, 2004; Volume 16. [Google Scholar]
- Popp, N.; Homburg, T.; Stock, N.; Senker, J. Porous imine-based networks with protonated imine linkages for carbon dioxide separation from mixtures with nitrogen and methane. J. Mater. Chem. A 2015, 3, 18492–18504. [Google Scholar] [CrossRef]
- Stegbauer, L.; Hahn, M.W.; Jentys, A.; Savasci, G.; Ochsenfeld, C.; Lercher, J.A.; Lotsch, B.V. Tunable Water and CO2 Sorption Properties in Isostructural Azine-Based Covalent Organic Frameworks through Polarity Engineering. Chem. Mater. 2015, 27, 7874–7881. [Google Scholar] [CrossRef]
- Biswal, B.P.; Kandambeth, S.; Chandra, S.; Shinde, D.B.; Bera, S.; Karak, S.; Garai, B.; Kharul, U.K.; Banerjee, R. Pore surface engineering in porous, chemically stable covalent organic frameworks for water adsorption. J. Mater. Chem. A 2015, 3, 23664–23669. [Google Scholar] [CrossRef]
- Shimekit, B.; Mukhtar, H. Natural Gas Purification Technologies–Major Advances for CO2 Separation and Future Directions. Adv. Nat. Gas Technol. 2012, 235–270. [Google Scholar]
- Landa, H.O.R.; Flockerzi, D.; Seidel-Morgenstern, A. A method for efficiently solving the IAST equations with an application to adsorber dynamics. AIChE J. 2013, 59, 1263–1277. [Google Scholar] [CrossRef]
- Carroll, J.J. Henry’s law revisited. Chem. Eng. Prog. 1999, 95, 49–56. [Google Scholar]
- Xiang, Z.; Zhou, X.; Zhou, C.; Zhong, S.; He, X.; Qin, C.; Cao, D. Covalent-organic polymers for carbon dioxide capture. J. Mater. Chem. 2012, 22, 22663–22669. [Google Scholar] [CrossRef]
- Dawson, R.; Stevens, L.A.; Drage, T.C.; Snape, C.E.; Smith, M.W.; Adams, D.J.; Cooper, A.I. Impact of water coadsorption for carbon dioxide capture in microporous polymer sorbents. J. Am. Chem. Soc. 2012, 134, 10741–10744. [Google Scholar] [CrossRef] [PubMed]
- Fung, B.M.; Khitrin, A.K.; Ermolaev, K. An improved broadband decoupling sequence for liquid crystals and solids. J. Magn. Reson. 2000, 142, 97–101. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound BILP-19 are not available from the authors. |
| T (K) | CO2 (mmol·g−1) | CH4 (mmol·g−1) | N2 (mmol·g−1) | H2O (mmol·g−1) |
|---|---|---|---|---|
| 273 | 5.97 | 0.88 | - | - |
| 298 | 5.20 | 0.51 | 0.11 | 18.17 |
| 313 | 4.51 | 0.38 | - | - |
| T/K | CO2/CH4 | CO2/N2 | ||
|---|---|---|---|---|
| Henry | IAST (15:85) | Henry | IAST (15:85) | |
| 273 | 12.0 | 11.9 | - | - |
| 298 | 14.0 | 12.3 | 123 | 59.1 |
| 313 | 10.3 | 11.3 | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klumpen, C.; Radakovitsch, F.; Jess, A.; Senker, J. BILP-19—An Ultramicroporous Organic Network with Exceptional Carbon Dioxide Uptake. Molecules 2017, 22, 1343. https://doi.org/10.3390/molecules22081343
Klumpen C, Radakovitsch F, Jess A, Senker J. BILP-19—An Ultramicroporous Organic Network with Exceptional Carbon Dioxide Uptake. Molecules. 2017; 22(8):1343. https://doi.org/10.3390/molecules22081343
Chicago/Turabian StyleKlumpen, Christoph, Florian Radakovitsch, Andreas Jess, and Jürgen Senker. 2017. "BILP-19—An Ultramicroporous Organic Network with Exceptional Carbon Dioxide Uptake" Molecules 22, no. 8: 1343. https://doi.org/10.3390/molecules22081343





