Inositol Derivatives and Phenolic Compounds from the Roots of Taraxacum coreanum
Abstract
:1. Introduction
2. Results
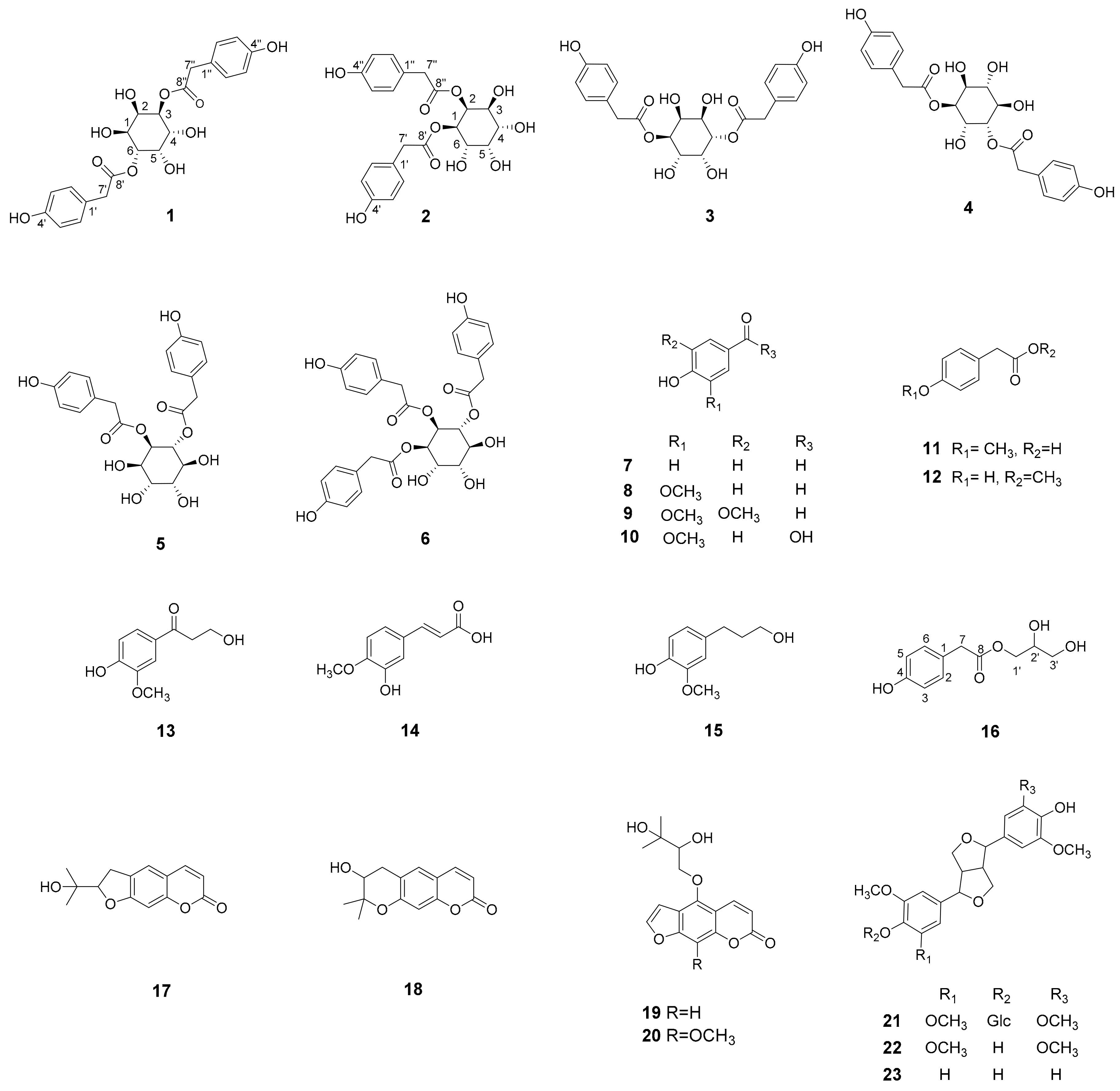
2.1. Structure Elucidation of the New Compounds
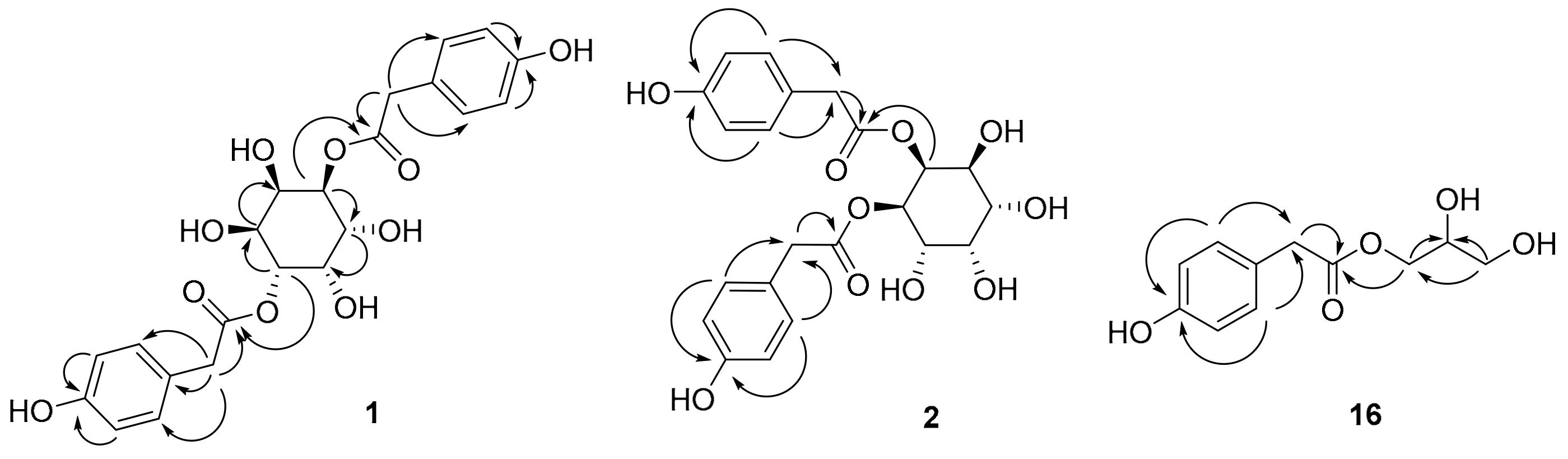
2.1.1. Taraxinositol A (1)
2.1.2. Taraxinositol B (2)
2.1.3. Taraxinol (16)
2.2. Identification of Known Compounds
2.3. Antioxidative Activity of Isolated Compounds
3. Materials and Methods
3.1. General Information
3.2. Isolation of Compounds
3.3. Evaluation of Antioxidant Activity
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- González-Castejón, M.; Visioli, F.; Rodriguez-Casado, A. Diverse biological activities of dandelion. Nutr. Rev. 2012, 70, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H. The Medicinal Plants of Korea; Kyo-Hak Publishing Co.: Seoul, Korea, 1999; pp. 655–656. [Google Scholar]
- Mingarro, D.M.; Plaza, A.; Galán, A.; Vicente, J.A.; Martínez, M.P.; Acero, N. The effect of five Taraxacum species on in vitro and in vivo antioxidant and antiproliferative activity. Food Funct. 2015, 6, 2787–2793. [Google Scholar] [CrossRef] [PubMed]
- Kenny, O.; Brunton, N.P.; Walsh, D.; Hewage, C.M.; McLoughlin, P.; Smyth, T.J. Characterisation of antimicrobial extracts from dandelion root (Taraxacum officinale) using LC-SPE-NMR. Phytother. Res. 2015, 29, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhu, L.; Wang, J.; He, H.; Chang, X.; Gao, J.; Shumin, W.; Yan, T. Anti-inflammatory effects of water extract of Taraxacum mongolicum hand.-Mazz on lipopolysaccharide-induced inflammation in acute lung injury by suppressing PI3K/Akt/mTOR signaling pathway. J. Ethnopharmacol. 2015, 168, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.S.; Ko, W.; Lee, D.S.; Kim, D.C.; Kim, J.; Choi, M.; Beom, J.S.; An, R.B.; Oh, H.; Kim, Y.C. Taraxacum coreanum protects against glutamate-induced neurotoxicity through heme oxygenase-1 expression in mouse hippocampal HT22 cells. Mol. Med. Rep. 2017, 15, 2347–2352. [Google Scholar] [PubMed]
- Kisiel, W.; Michalska, K. Sesquiterpenoids and phenolics from Taraxacum hondoense. Fitoterapia 2005, 76, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Marciniuk, J.; Kisiel, W. Sesquiterpenoids and phenolics from roots of Taraxacum udum. Fitoterapia 2010, 81, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Tanaka, A.; Uriuda, M.; Yamada, T.; Tanaka, R. Three novel triterpenoids from Taraxacum officinale roots. Molecules 2016, 21, 1121. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, N.; Liu, M. A new inositol trimester from Taraxacum mongolicum. Nat. Prod. Res. 2014, 28, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Kenny, O.; Smyth, T.J.; Hewage, C.M.; Brunton, N.P.; McLoughlin, P. 4-Hydroxyphenylacetic acid derivatives of inositol from dandelion (Taraxacum officinale) root characterised using LC–SPE–NMR and LC–MS techniques. Phytochemistry 2014, 98, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Bupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation and cancer: How are they linked? Free Radic. Biol. Med. 2010, 47, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Alzheimer’s disease and oxidative stress: A review. Curr. Med. Chem. 2014, 21, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidant: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Viapiana, A.; Wesolowski, M. The phenolic content and antioxidant activities of infusions of Sambucus nigra L. Plant Foods Hum. Nutr. 2017, 72, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Bolling, B.W. Review of nut phytochemicals, fat-soluble bioactives, antioxiant components and health effects. Br. J. Nutr. 2015, 113, S68–S78. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ralph, J.; Lu, F.; Ralph, S.A.; Boudet, A.M.; MacKay, J.J.; Sederoff, R.R.; Ito, T.; Kawai, S.; Ohashi, H.; et al. NMR analysis of lignins in CAD-deficient plants. Part 1. Incorporation of hydroxycinnamaldehydes and hydroxybenzaldehydes into lignins. Org. Biomol. Chem. 2003, 1, 268–281. [Google Scholar] [CrossRef] [PubMed]
- González-Baró, A.C.; Parajón-Costa, B.S.; Franca, C.A.; Pis-Diez, R. Theoretical and spectroscopic study of vanillic acid. J. Mol. Struct. 2008, 889, 204–210. [Google Scholar] [CrossRef]
- James, M.C.; Priyabrata, D. Development of a one-pot method for the homologation of aldehydes to carboxylic acids. Tetrahedron 2009, 65, 7794–7800. [Google Scholar]
- Subhas, B.D.; Venkat, N.A. An efficient asymmetric synthesis of (S)-atenolol: Using hydrolytic kinetic resolution. Bioorg. Med. Chem. Lett. 2005, 13, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Supaluk, P.; Saowapa, S.; Apilak, W.; Ratana, L.; Somsak, R.; Virapong, P. Bioactive metabolites from Spilanthes acmella Murr. Molecules 2009, 14, 850–867. [Google Scholar]
- Kuo, Y.J.; Hwang, S.Y.; Wu, M.D.; Liao, C.C.; Liang, Y.H.; Kuo, Y.H.; Ho, H.O. Cytotoxic constituents from Podocarpus fasciculus. Chem. Pharm. Bull. 2008, 56, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Uddin, G.; Ullah, W.; Siddiqui, B.S.; Shah, S.Q. Gresialin and optivanin new constituents from the stem bark of Grewia optiva Drummond ex Burret (Tiliaceae). Nat. Prod. Res. 2013, 27, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Sun, Q.; Shi, Y.; Kong, L. Isolation and purification of coumarin compounds from the root of Peucedanum decursivum (Miq.) Maxim by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1076, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Lee, S.H.; Cha, C.J. Biotransformation of plant secondary metabolite decursin by Mycobacterium sp. PYR1001. J. Agric. Food Chem. 2010, 58, 2931–2934. [Google Scholar] [CrossRef] [PubMed]
- Row, E.C.; Brown, S.A.; Stachulski, A.V.; Lennard, M.S. Design, synthesis and evaluation of furanocoumarin monomers as inhibitors of CYP3A4. Org. Biomol. Chem. 2006, 4, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Ngunde, N.J.; Bedir, E.; Efange, S.M.N.; Okunji, C.O.; Iwu, M.M.; Schuster, B.G.; Khan, I.A. Constituents of Peucedanum zenkeri seeds and their antimicrobial effects. Pharmazie 2003, 58, 587–589. [Google Scholar]
- Jung, M.J.; Kang, S.S.; Jung, H.A.; Kim, G.J.; Choi, J.S. Isolation of flavonoids and a cerebroside from the stem bark of Albizzia julibrissin. Arch. Pharm. Res. 2004, 27, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.D.A.; Katayama, T.; Suzuki, T.; Nakagawa, T. Stereochemistry and biosynthesis of (+)-lyoniresinol, a syringyl tetrahydronaphthalene lignan in Lyonia ovalifolia var. elliptica I: Isolation and stereochemistry of syringyl lignans and predicted precursors to (+)-lyoniresinol from wood. J. Wood Sci. 2007, 53, 161–167. [Google Scholar] [CrossRef]
- Jung, H.W.; Mahesh, R.; Lee, J.G.; Lee, S.H.; Kim, Y.S.; Park, Y.K. Pinoresinol from the fruits of Forsythia koreana inhibits inflammatory responses in LPS-activated microglia. Neurosci. Lett. 2010, 480, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Colle, D.; Arantes, L.P.; Rauber, R.; de Mattos, S.E.; Rocha, J.B.; Noqueira, C.W.; Soares, F.A. Antioxidant properties of Taraxacum officinale fruit extract are involved in the protective effect against cellular death induced by sodium nitroprusside in brain of rats. Pharm. Biol. 2012, 50, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, S. Dandelion extracts protect human skin fibroblasts from UVB damage and cellular senescence. Oxid. Med. Cell. Longev. 2015, 2015, 619560. [Google Scholar] [CrossRef] [PubMed]
- Jędrejek, D.; Kontek, B.; Lis, B.; Stochmal, A.; Olas, B. Evaluation of antioxidant activity of phenolic fractions from the leaves and petals of dandelion in human plasma treated with H2O2 and H2O2/Fe. Chem. Biol. Interact. 2017, 262, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choo, S.J.; Ryoo, I.J.; Ahn, J.S.; Yoo, I.D. Eudesmanolides from Taraxacum mongolicum and their inhibitory effects on the production of nitric oxide. Arch. Pharm. Res. 2011, 34, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiong, H.; Liu, L. Effects of taraxasterol on inflammatory responses in lipopolysaccharide-induced RAW 264.7 macrophages. J. Ethnopharmacol. 2012, 141, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Zidorn, C.; Ellmerer-Müller, E.P.; Stuppner, H. Eudesmanolides and inositol derivatives from Taraxacum linearisquameum. Phytochemistry 1999, 51, 991–994. [Google Scholar] [CrossRef]
- Michalska, K.; Zylewski, M.; Marciniuk, J.; Kisiel, W. Structural analysis of 1l-chiro-inositol diester from Taraxacum udum. Carbohydr. Res. 2010, 345, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Garayev, E.; Herbette, G.; Di Giorgio, C.; Chiffolleau, P.; Roux, D.; Sallanon, H.; Ollivier, E.; Elias, R.; Baghdikian, B. New sesquiterpene acid and inositol derivatives from Inula montana L. Fitoterapia 2017, 345, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Worawalai, W.; Wacharasindhu, S.; Phuwapraisirisan, P. N-Arylmethylaminoquercitols, a new series of effective antidiabetic agents having α-glucosidase inhibition and antioxidant activity. Bioorg. Med. Chem. Lett. 2015, 25, 2570–2573. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Some samples of the compounds are available from the authors. |
| Carbon No. | 1 | Carbon No. | 2 | ||
|---|---|---|---|---|---|
| 1H | 13C | 1H | 13C | ||
| 1, 4 | 3.93 (2H, dt, J = 7.0, 2.5 Hz) | 69.1 | 1 | 5.09 (1H, dt, J = 6.5, 3.5 Hz) | 72.2 |
| 2, 5 | 3.95 (2H, d, J = 2.0 Hz) | 72.1 | 2 | 5.29 (1H, t, J = 3.5 Hz) | 70.9 |
| 3, 6 | 5.22 (2H, dd, J = 7.0, 2.5 Hz) | 73.3 | 3 | 3.55 (1H, dd, J = 9.5, 3.0 Hz) | 71.0 |
| 4 | 3.66 (1H, dd, J = 9.5, 2.5 Hz) | 71.1 | |||
| 5 | 3.84 (1H, t, J = 3.5 Hz) | 69.5 | |||
| 6 | 3.66 (1H, d, J = 6.5, 2.5 Hz) | 72.9 | |||
| 1′, 1″ | - | 124.9 | 1′ | - | 124.7 |
| 2′, 6′, 2″, 6″ | 7.03 (4H, d, J = 8.5 Hz) | 130.1 | 2′, 6′ | 7.02 (1H, d, J = 8.5 Hz) | 130.1 |
| 3′, 5′, 3″, 5″ | 6.73 (4H, d, J = 8.5 Hz) | 114.8 | 3′, 5′ | 6.72 (1H, d, J = 8.5 Hz) | 115.0 |
| 4′, 4″ | - | 156.1 | 4′ | - | 156.0 |
| 7′, 7″ | 3.29 (2H, d, J = 15.5 Hz)3.41 (2H, d, J = 15.5 Hz) | 39.6 | 7′ | 3.55 (1H, s) 3.41 (1H, s) | 39.4 |
| 8′, 8″ | - | 172.3 | 8′ | - | 172.1 |
| 1″ | - | 124.7 | |||
| 2″, 6″ | 7.12 (1H, d, J = 8.5 Hz) | 130.1 | |||
| 3″, 5″ | 6.77 (1H, d, J = 8.5 Hz) | 115.0 | |||
| 4″ | - | 156.3 | |||
| 7″ | 3.50 (2H, d, J = 2.5 Hz) | 39.9 | |||
| 8″ | - | 171.1 | |||
| Carbon No. | 16 | |
|---|---|---|
| 1H | 13C | |
| 1 | - | 124.9 |
| 2, 6 | 7.12 (2H, d, J = 8.8 Hz) | 130.0 |
| 3, 5 | 6.74 (2H, d, J = 8.4 Hz) | 114.9 |
| 4 | - | 156.2 |
| 7 | 3.58 (2H, s) | 39.6 |
| 8 | - | 172.6 |
| 1′ | 4.09 (1H, dd, J = 11.2, 6.0 Hz) 4.17 (1H, dd, J = 11.6, 4.4 Hz) | 65.4 |
| 2′ | 3.83 (1H, m) | 69.7 |
| 3′ | 3.54 (2H, dd, J = 5.2, 2.4 Hz) | 62.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, E.J.; Ahn, J.H.; Jo, Y.H.; Kim, S.B.; Hwang, B.Y.; Lee, M.K. Inositol Derivatives and Phenolic Compounds from the Roots of Taraxacum coreanum. Molecules 2017, 22, 1349. https://doi.org/10.3390/molecules22081349
Mo EJ, Ahn JH, Jo YH, Kim SB, Hwang BY, Lee MK. Inositol Derivatives and Phenolic Compounds from the Roots of Taraxacum coreanum. Molecules. 2017; 22(8):1349. https://doi.org/10.3390/molecules22081349
Chicago/Turabian StyleMo, Eun Jin, Jong Hoon Ahn, Yang Hee Jo, Seon Beom Kim, Bang Yeon Hwang, and Mi Kyeong Lee. 2017. "Inositol Derivatives and Phenolic Compounds from the Roots of Taraxacum coreanum" Molecules 22, no. 8: 1349. https://doi.org/10.3390/molecules22081349




