Two New Tetravacant Organometallic Keggin-Type Heteropolyoxomolybdates-Supported Manganese Carbonyl Derivatives
Abstract
:1. Introduction
2. Results and Discussion
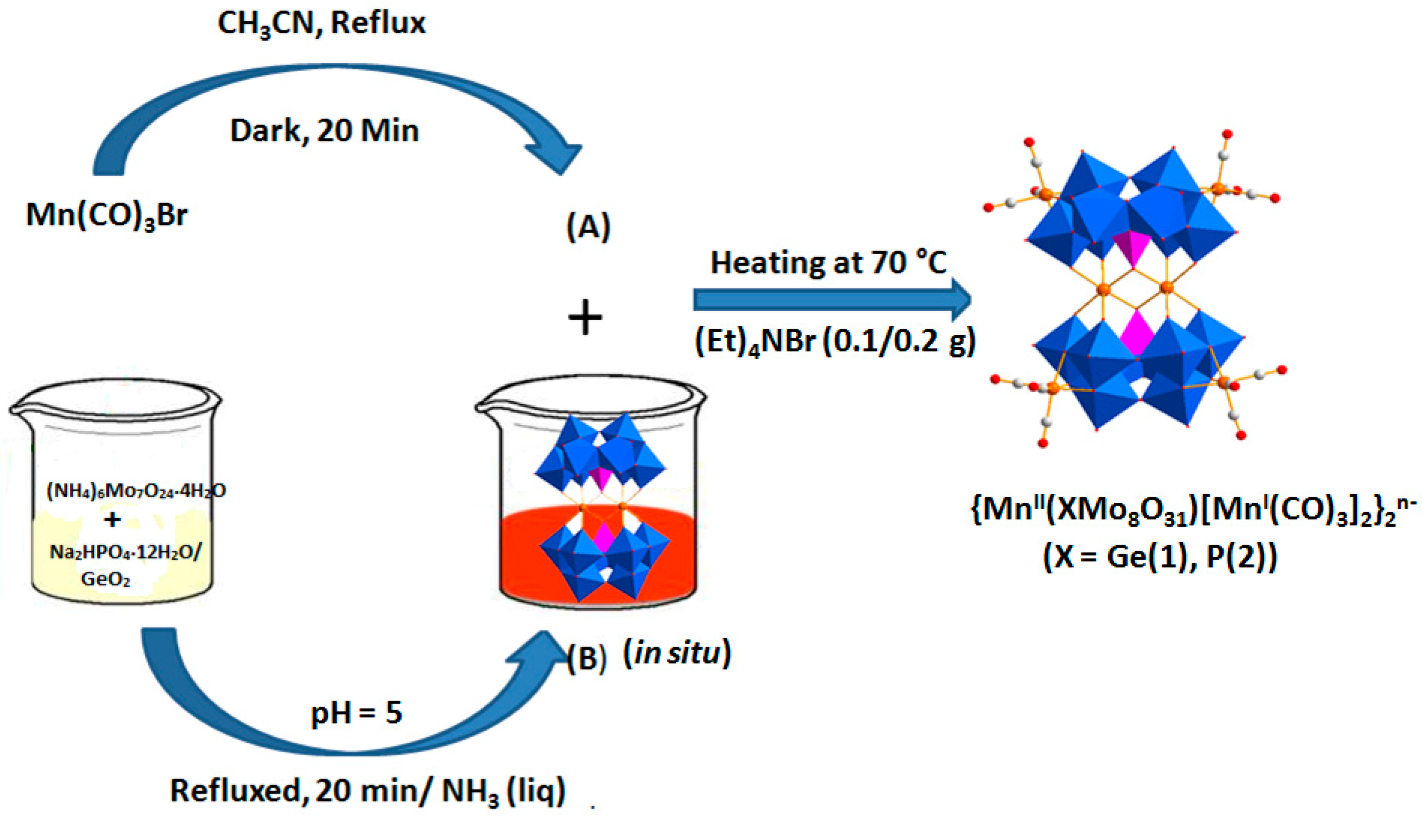
2.1. Synthesis
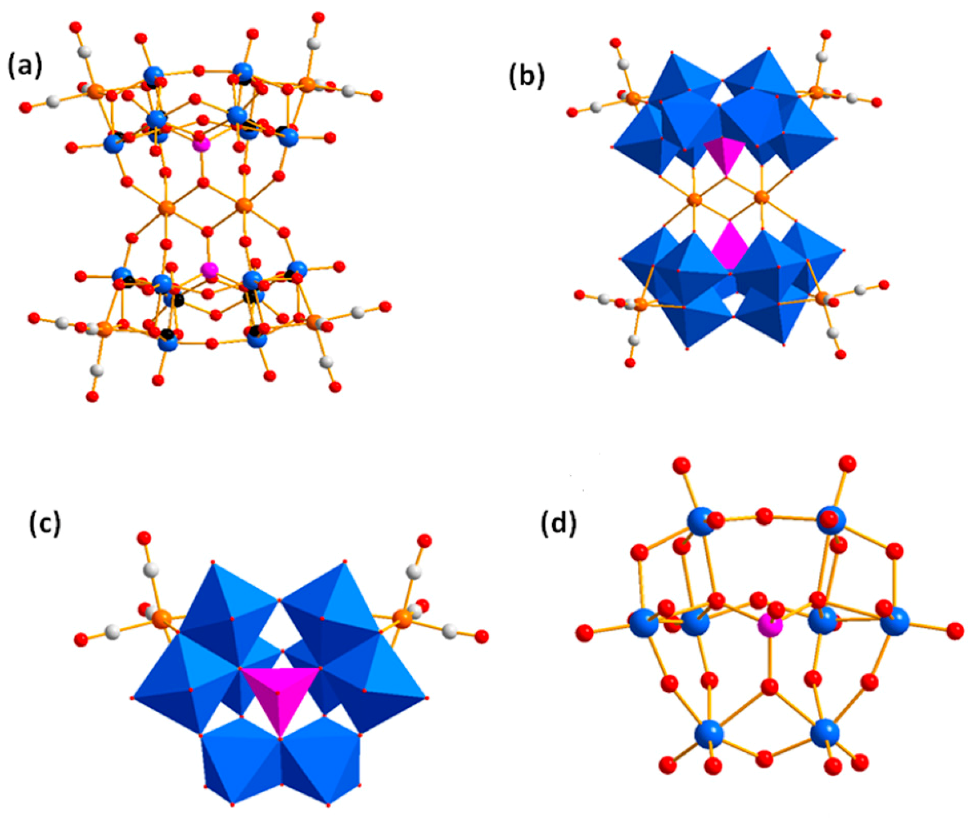
2.2. Crystal Structures
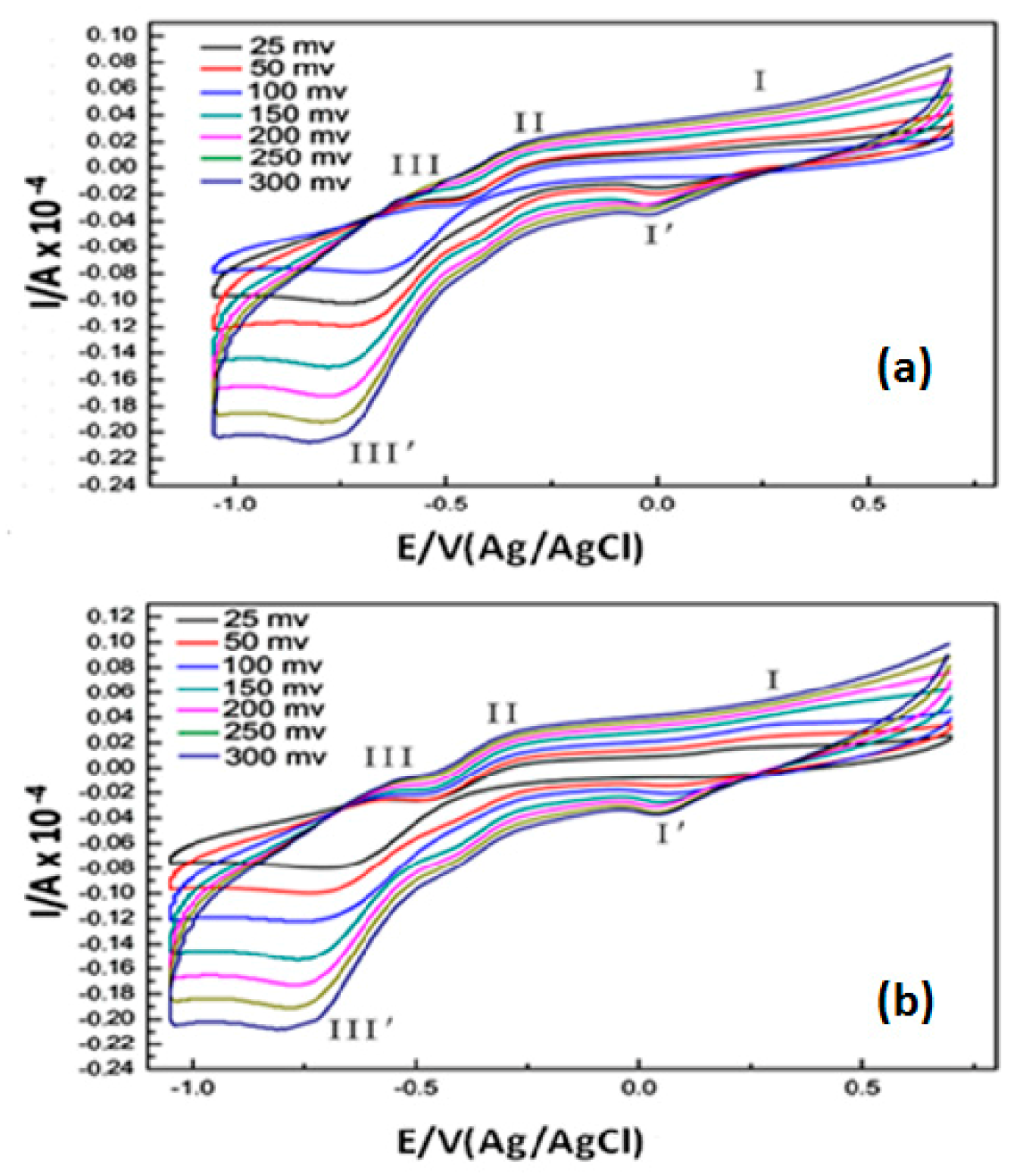
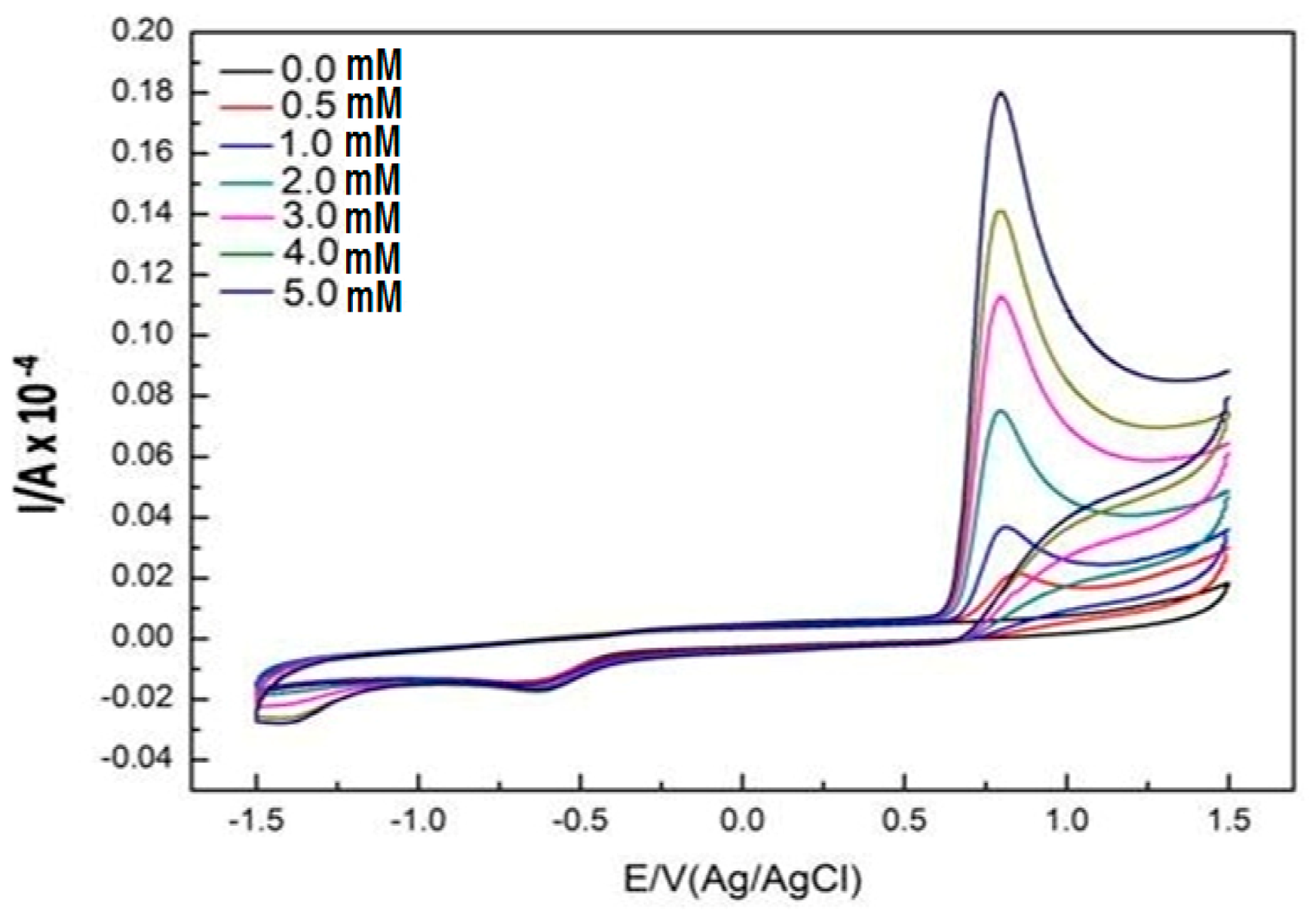
2.3. Electrochemistry
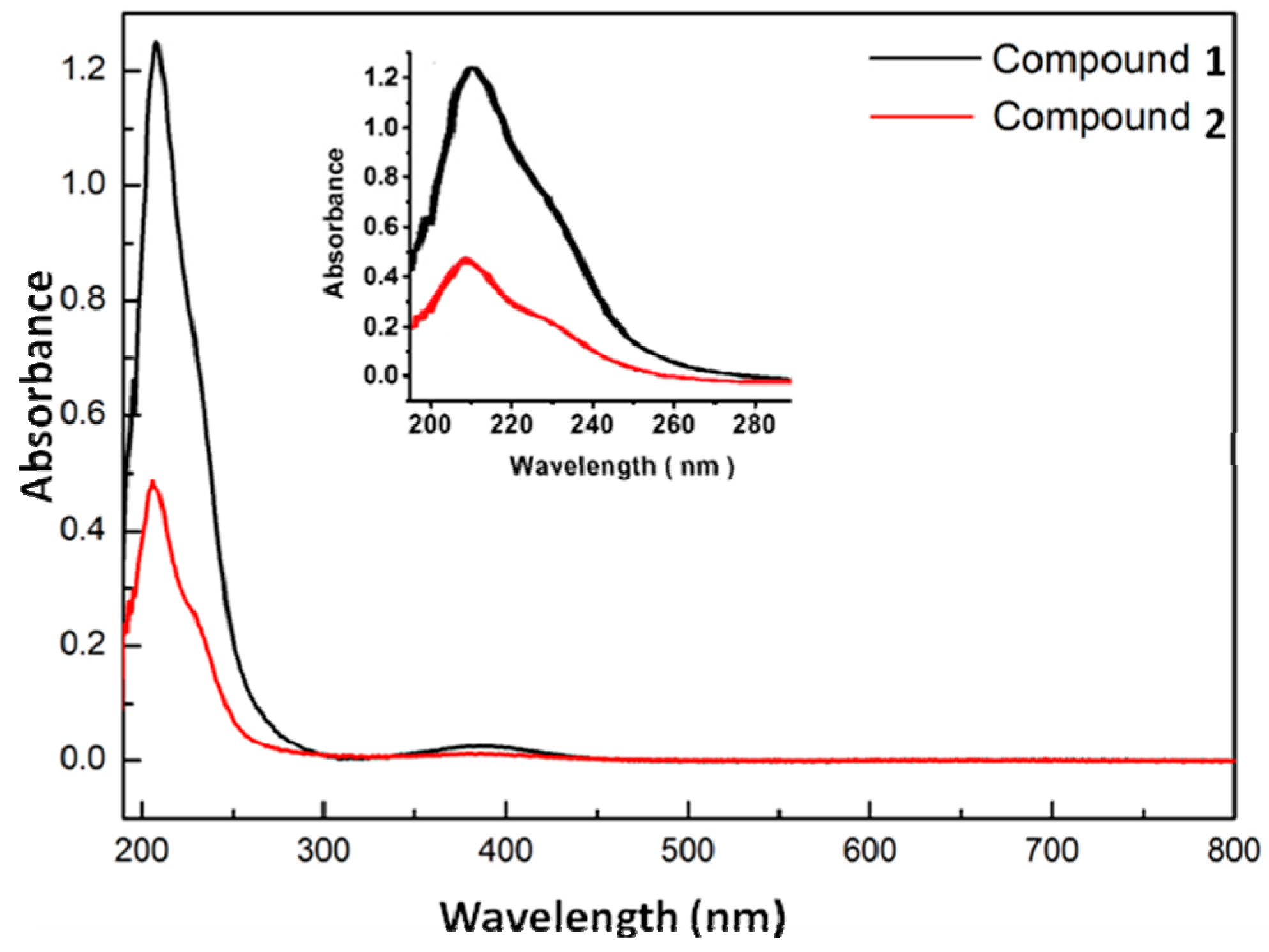
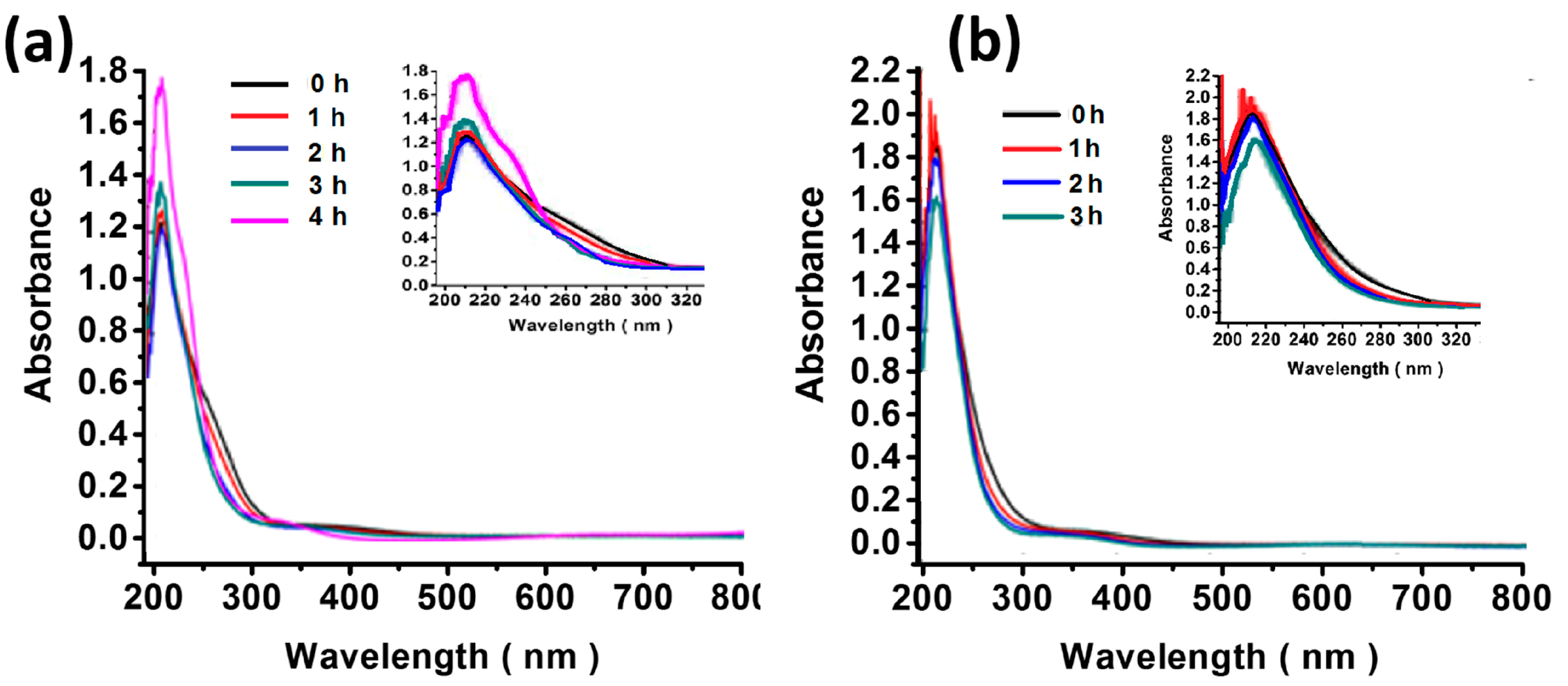
2.4. UV–Vis Spectroscopy
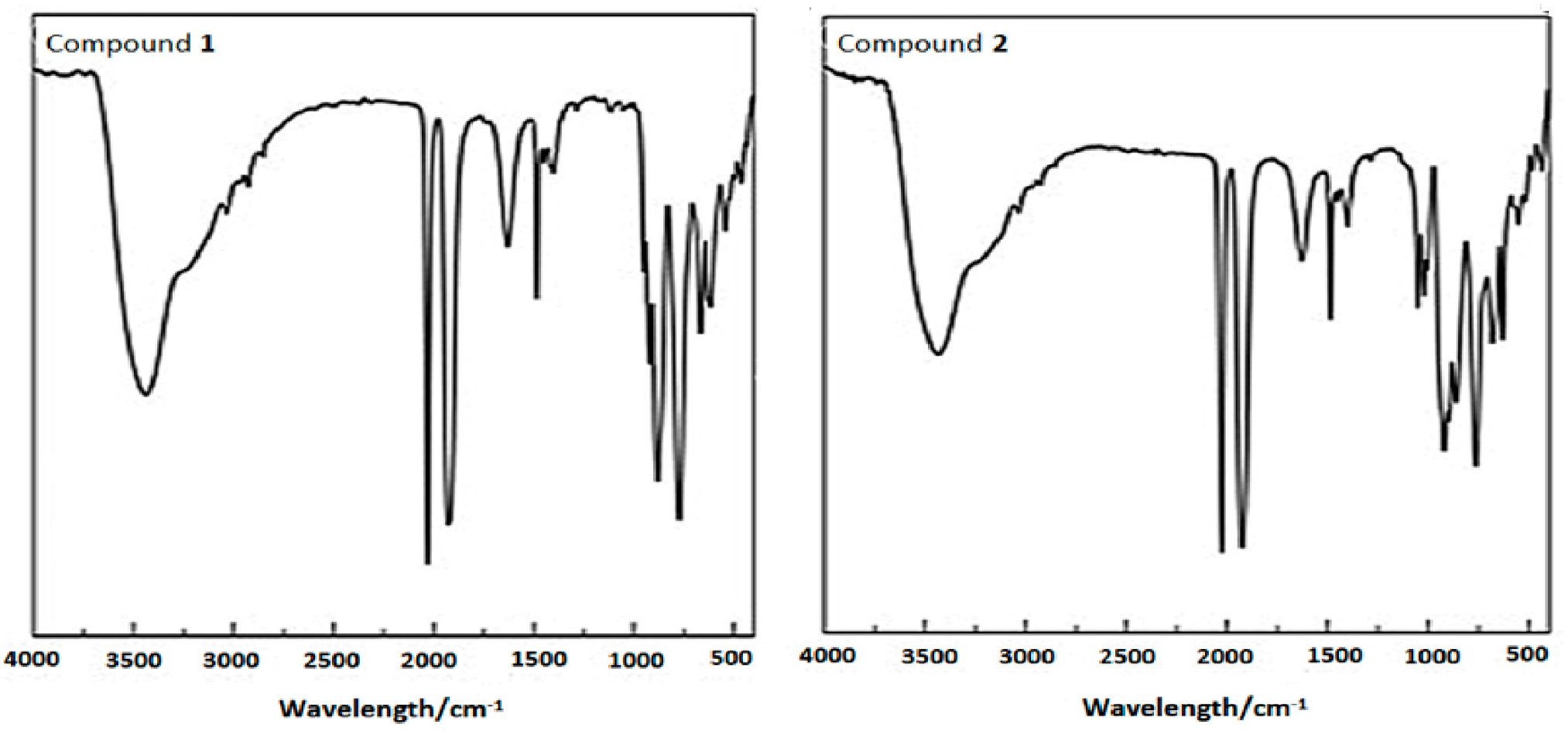
2.5. IR Spectroscopy
2.6. Thermogravimetric Analysis
3. Experimental Section
3.1. Materials and General Methods
3.2. Synthesis of [(CH3)4N]6H6{MnII(GeMo8O31)[MnI(CO)3]2}2·12H2O (1)
3.3. Synthesis of [(CH3)4N]4H6{MnII(PMo8O31) [MnI(CO)3]2}2·14H2O (2)
3.4. Crystallography
4. Conclusions
Supplementary Materials
Acknowledgments
Authors Contributions
Conflicts of Interest
References
- Gouzerh, P.; Proust, A. Main-group element, organic and organometallic derivatives of polyoxometalates. Chem. Rev. 1998, 98, 77–112. [Google Scholar] [CrossRef] [PubMed]
- Contant, R.; Herveb, G. The heteropolyoxotungstates: Relationships between routes of formation and structures. Inorg. Chem. 2002, 22, 63–112. [Google Scholar] [CrossRef]
- Long, D.L.; Burkholder, E.; Cronin, L. Polyoxometalate clusters, nanostructures and materials: From self assembly to designer materials and devices. Chem. Soc. Rev. 2007, 36, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.L.; Prosser-McCartha, C.M. Homogeneous catalysis by transition metal oxygen anion clusters. Coord. Chem. Rev. 1995, 143, 407–455. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Ammam, M. A molecular hybrid polyoxometalate organometallic moieties and its relevance to supercapacitors in physiological electrolytes. J. Power Sources 2015, 284, 524–535. [Google Scholar] [CrossRef]
- Han, X.-B.; Li, Y.-G.; Zhang, Z.-M.; Tan, H.-Q.; Lu, Y.; Wang, E.-B. Polyoxometalate-based nickel clusters as visible light-driven water oxidation catalysts. J. Am. Chem. Soc. 2015, 137, 5486–5493. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhao, J.; Mi, J.; Ding, Y.; Zhou, P.; Ma, B.; Zhao, J.; Song, J. Efficient photocatalytic H2 evolution catalyzed by an unprecedented robust molecular semiconductor {Fe11} nanocluster without co-catalysts at neutral conditions. Nano Energy 2015, 16, 247–255. [Google Scholar] [CrossRef]
- Wang, J.; Lu, X.; Fan, S.; Zhao, W.; Li, W. In situ growth of gold nanoparticles on SiO2/lanthanide–polyoxometalates composite spheres: An efficient catalytic and luminescent system. J. Alloys Comp. 2015, 632, 87–93. [Google Scholar] [CrossRef]
- Fang, X.; Speldrich, M.; Schilder, H.; Cao, R.; O’Halloran, K.P.; Hill, C.L.; Kögerler, P. Switching slow relaxation in a MnIII3MnIV cluster: An example of grafting single-molecule magnets onto polyoxometalates. Chem. Commun. 2010, 46, 2760–2762. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, Y.-G.; Wang, Y.-H.; Wang, E.-B.; Zhang, Z.-M.; Clérac, R. Mixed-valent {Mn14} aggregate encapsulated by the inorganic polyoxometalate shell: [MnIII13MnIIO12(PO4)4(PW9O34)4]31−. Inorg. Chem. 2009, 48, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Erikson, H.; Sarapuu, A.; Gullón, J.S.; Tammevesk, K. Recent progress in oxygen reduction electrocatalysis on Pd-based catalysts. J. Electroanal. Chem. 2016, 780, 327–336. [Google Scholar] [CrossRef]
- Kandasamy, B.; Bassil, B.S.; Haider, A.; Beckmann, J.; Chen, B.; Dalal, N.S.; Kortz, U. Incorporation of organotellurium(IV) in polyoxometalates. J. Organomet. Chem. 2015, 796, 33–38. [Google Scholar] [CrossRef]
- Guajardo, N.; Fuentealba, M.; Manzur, C.; Carrillo, D. Organometallic inorganic charge transfer salts containing a cationic iron mixed sandwich and polyoxomolybdate anions: Syntheses, X-ray molecular structures and spectroscopic properties. J. Organomet. Chem. 2011, 696, 2306–2312. [Google Scholar] [CrossRef]
- Zhao, C.; Rodríguez-Córdoba, W.; Kaledin, A.L.; Yang, Y.; Geletii, Y.V.; Lian, T.; Musaev, D.G.; Hill, C.L. An Inorganic chromophore based on a molecular oxide supported metal carbonyl cluster: [P2W17O61{Re(CO)3}3{ORb(H2O)}(μ3-OH)]9−. Inorg. Chem. 2013, 52, 13490–13495. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, J.A.; García-Álvarez, P. The N-heterocyclic carbene chemistry of transition-metal carbonyl clusters. Chem. Soc. Rev. 2011, 40, 5389–5405. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dubois, K.D.; Louis, M.E. Photocatalytic CO2 reduction and surface immobilization of a tricarbonyl Re(I) compound modified with amide groups. ACS Catal. 2013, 3, 655–662. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F. Organometallic polymorphism and phase transitions. Chem. Soc. Rev. 2000, 29, 229–238. [Google Scholar] [CrossRef]
- Knoth, W.H. Derivatives of heteropolyanions. 2. Metal-metal-bonded derivatives. J. Am. Chem. Soc. 1979, 101, 2211–2213. [Google Scholar] [CrossRef]
- Sadakane, M.; Iimuro, Y.; Tsukuma, D.; Bassil, B.S.; Dickman, M.H.; Kortz, U.; Zhang, Y.; Ye, S.; Ueda, W. Carbonyl–ruthenium substituted α-Keggin-tungstosilicate, [α-SiW11O39RuII(CO)]6−: Synthesis, structure, redox studies and reactivity. Dalton Trans. 2008, 47, 6692–6698. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, J.; Zhao, J.; Ma, P.; Wang, J.; Niu, J. Two novel trivacant Keggin-type polytungstates supported manganese carbonyl derivatives synthesized by degradation of metastable [γ-XW10O36]8− (X = GeIV, SiIV). Dalton Trans. 2012, 41, 5832–5837. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Kambara, C.S.; Yang, Y.; Kaledin, A.L.; Musaev, D.G.; Lian, T.; Hill, C.L. Synthesis, structures, and photochemistry of tricarbonyl metal polyoxoanion complexes, [X2W20O70{M(CO)3}2]12− (X = Sb, Bi and M = Re, Mn). Inorg. Chem. 2013, 52, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Huang, Z.; Rodríguez-Cόrdoba, W.; Kambara, C.S.; O’Halloran, K.P.; Hardcastle, K.I.; Musaev, D.G.; Lian, T.; Hill, C.L. Synthesis and characterization of a metal-to-polyoxometalate charge transfer molecular chromophore. J. Am. Chem. Soc. 2011, 133, 20134–20137. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Yang, L.; Zhao, J.; Ma, P.; Wang, J. Novel octatungstate-supported tricarbonyl metal derivatives: {[H2W8O30][M(CO)3]2}8− (M = MnI and ReI). Dalton Trans. 2011, 40, 8298–8300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhao, J.; Zhang, Y.; Hu, X.; Li, L.; Ma, P.; Wang, J.; Niu, J. Octamolybdate-supported tricarbonyl metal derivatives: [{H2Mo8O30}{M(CO)3}2]8− (M = MnI and ReI). Dalton Trans. 2013, 42, 2696–2699. [Google Scholar] [CrossRef] [PubMed]
- Huo, Z.; Guo, J.; Lu, J.; Xu, Q.; Ma, P.; Zhao, J.; Zhang, D.; Niu, J. A nona-vacant Keggin-type tricarbonyl rhenium derivative {[PMo3O16][Re(CO)3]4}5− and its catalytic performance for CO2 cycloaddition reactions. RSC Adv. 2015, 5, 69006–69009. [Google Scholar] [CrossRef]
- Li, J.; Guo, J.; Jia, J.; Ma, P.; Zhang, D.; Wang, J.; Niu, J. Isopentatungstate-supported metal carbonyl derivative: Synthesis, characterization, and catalytic properties for alkene epoxidation. Dalton Trans. 2016, 45, 6726–6731. [Google Scholar] [CrossRef] [PubMed]
- Huo, Z.; Zhao, J.; Bu, Z.; Ma, P.; Liu, Q.; Niu, J.; Wang, J. Synthesis of cyclic carbonates from carbon dioxide and epoxides catalyzed by a Keggin-type polyoxometalate-supported rhenium carbonyl derivate in ionic. Liquid. ChemCatChem 2014, 6, 3096–3100. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, Y.; Zhang, P.; Ma, P.; Zhang, D.; Wang, J.; Niu, J. Synthesis and characterization of a series of novel polyoxometalate-supported carbonyl manganese derivatives. RSC Adv. 2016, 6, 108335–108342. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Ma, P.; Dong, Y.; Niu, J.; Wang, J. Synthesis, crystal structure and characterization of trivacant-Keggin-polyoxometalate-based carbonyl manganese derivative. Inorg. Chem. Commun. 2015, 56, 45–47. [Google Scholar] [CrossRef]
- Choudhary, A.; Gandla, D.; Krow, G.R.; Raines, R.T. Nature of Amide Carbonyl-Carbonyl Interactions in Proteins. J. Am. Chem. Soc. 2009, 131, 7244–7246. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Ghosh, B.N.; Bauza, A.; Rissanen, K.; Frontera, A.; Chattopadhyay, S. Observation of novel oxygen···oxygen interaction in supramolecular assembly of cobalt(III) Schiff base complexes: A combined experimental and computational study. RSC Adv. 2015, 5, 73028–73039. [Google Scholar] [CrossRef]
- Sharma, C.; Singh, A.K.; Joy, J.; Jemmis, E.D.; Awasthi, S.K. Experimental and theoretical study of intermolecular O···O interaction in structurally rigid β-keto carboxylic esters. RSC Adv. 2016, 6, 91689. [Google Scholar] [CrossRef]
- Li, Y.-W.; Li, Y.-G.; Wang, Y.-H.; Feng, X.-J.; Lu, Y.; Wang, E.-B. A New Supramolecular Assembly Based on Triple-Dawson-Type Polyoxometalate and 3d-4f Heterometallic Cluster. Inorg. Chem. 2009, 48, 6452–6458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qi, Y.; Qin, C.; Li, Y.; Wang, E.-B.; Wang, X.; Su, Z.; Lin, X. Two Multi-Copper-Containing Heteropolyoxotungstates Constructed from the Lacunary Keggin Polyoxoanion and the High-Nuclear Spin Cluster. Inorg. Chem. 2007, 46, 8162–8169. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhang, Z.; Li, Y.; Wang, E.-B. Two hexa-TM-containing (TM = Co2+ and Ni2+){P2W12}-based trimeric tungstophosphates. Dalton Trans. 2010, 39, 3884–3889. [Google Scholar] [CrossRef] [PubMed]
- Mal, S.S.; Bassil, B.S.; Ibrahim, M.; Nellutla, S.; Tol, J.; Dalal, N.S.; Fernandez, J.A.; Lopez, X.; Poblet, J.M.; Ngo, B.R.; et al. Wheel-Shaped Cu20-Tungstophosphate [Cu20X(OH)24(H2O)12(P8W48O184)]25− Ion (X = Cl, Br, I) and the Role of the Halide Guest. Inorg. Chem. 2009, 48, 11636–11645. [Google Scholar] [CrossRef] [PubMed]
- Bassil, B.S.; Nellutla, S.; Kortz, U. The satellite-shaped Co-15 polyoxotungstate [Co6(H2O)30{Co9Cl2(OH)3(H2O)9(β-SiW8O31)3}]5−. Inorg. Chem. 2005, 44, 2659–2665. [Google Scholar] [CrossRef] [PubMed]
- Lisnard, L.; Dolbecq, A.; Mialane, P. Molecular and multidimensional polyoxotungstates functionalized by {Cu(bpy)}2+ groups. Dalton Trans. 2005, 24, 3913–3920. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Hua, J.; Ma, X.; Wang, J. Temperature-controlled assembly of a series of inorganic-organic hybrid arsenomolybdates. Crystengcomm 2012, 14, 4060–4067. [Google Scholar] [CrossRef]
- Burdinski, D.; Bothe, E.; Wieghardt, K. Synthesis and characterization of tris(bipyridyl) ruthenium(II)-modified mono-, di-, and trinuclear manganese complexes as electron-transfer models for photosystem II. Inorg. Chem. 2000, 39, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Reece, S.Y.; Nocera, D.G. Direct tyrosine oxidation using the MLCT excited states of rhenium polypyridyl complexes. J. Am. Chem. Soc. 2005, 127, 9448–9458. [Google Scholar] [CrossRef] [PubMed]
- Besecker, C.J.; Klemperer, W.G. Polyoxoanion-supported metal carbonyls: Syntheses of the [(CO)3M(Nb2W4O19)]3− anions (M = rhenium and manganese). J. Am. Chem. Soc. 1980, 102, 7598–7600. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1 and 2 are available from the authors. |




| Year | Formula | Reference |
|---|---|---|
| 2008 | Cs6[SiW11O39RuII(CO)]·8H2O | [19] |
| 2011 | (NH4)4[H4{[H2Mo8O30][Mn(CO)3]2}]·12H2O | [24] |
| (NH4)4[{H6Mo8O30}−{Re(CO)3}2]·14H2O | ||
| 2013 | H6[Na(H2O)5]2{[H2W8O30][Mn(CO)3]2}·13H2O | [23] |
| H2[Na(H2O)5]2[Na(H2O)4]2[Na(H2O)2]2{[H2W8O30][Re(CO)3]2} | ||
| [Na(H2O)5]2[Na2(µ2-H2O)2(H2O)4]2[Mn(H2O)2]{[H2W8O30][Mn(CO)3]2} | ||
| 2016 | KH[(CH3)4N]3{[Re(CO)3]4[(μ2-OH)(μ3-O)(W5O18)]}·6H2O | [26] |
| 2013 | Na11H[Sb2W20O70{Re(CO)3}2]·34H2O | [21] |
| Na11H[Bi2W20O70{Re(CO)3}2]·33H2O | ||
| K9Na3[Sb2W20O70{Mn(CO)3}2]·32H2O | ||
| K9Na3[Bi2W20O70{Mn(CO)3}2]·32H2O | ||
| 2013 | [P2W17O61{Re(CO)3}3{ORb(H2O)}(μ3-OH)]9− | [14] |
| 2014 | [P4W35O124{Re(CO)3}2]16− | [22] |
| 2012 | K8[(OC)3Mn(A-α-H2GeW9O34)]·10H2O | [20] |
| K8[(OC)3Mn(A-α-H2SiW9O34)]·11H2O | ||
| 2014 | [(CH3)4N]5H23{(PW11O39){Re(CO)3}3(µ3-O)(µ2-OH)]4·24H2O | [27] |
| 2015 | {[PMo3O16][Re(CO)3]4}5− | [25] |
| 2016 | [(M4(H2O)10(XW9O33)2{Mn(CO)3}2]n− (X = Sb/Bi; Mn = Mn/Mn3.5W0.5) | [28] |
| 2015 | (NH4)3H3[{Mn(CO)3}(Mn(H2O)2(Mn(H2O)3)(TeW9O33)]2·31H2O | [29] |
| Atoms | 1 | 2 |
|---|---|---|
| Mo | 5.99–6.09 | 6.17–6.62 |
| Mn | 2.30 | 2.42 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, V.; Zhang, Y.; Yang, L.; Ma, P.; Zhang, D.; Zhang, C.; Yu, L.; Niu, J.; Wang, J. Two New Tetravacant Organometallic Keggin-Type Heteropolyoxomolybdates-Supported Manganese Carbonyl Derivatives. Molecules 2017, 22, 1351. https://doi.org/10.3390/molecules22081351
Singh V, Zhang Y, Yang L, Ma P, Zhang D, Zhang C, Yu L, Niu J, Wang J. Two New Tetravacant Organometallic Keggin-Type Heteropolyoxomolybdates-Supported Manganese Carbonyl Derivatives. Molecules. 2017; 22(8):1351. https://doi.org/10.3390/molecules22081351
Chicago/Turabian StyleSingh, Vikram, Yujiao Zhang, Linping Yang, Pengtao Ma, Dongdi Zhang, Chao Zhang, Li Yu, Jingyang Niu, and Jingping Wang. 2017. "Two New Tetravacant Organometallic Keggin-Type Heteropolyoxomolybdates-Supported Manganese Carbonyl Derivatives" Molecules 22, no. 8: 1351. https://doi.org/10.3390/molecules22081351





