Homoisoflavonoids and Chalcones Isolated from Haematoxylum campechianum L., with Spasmolytic Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of the Ethanolic Extract and Fractions
2.2. Evaluation of Compounds 1, 2 and 3a–5a
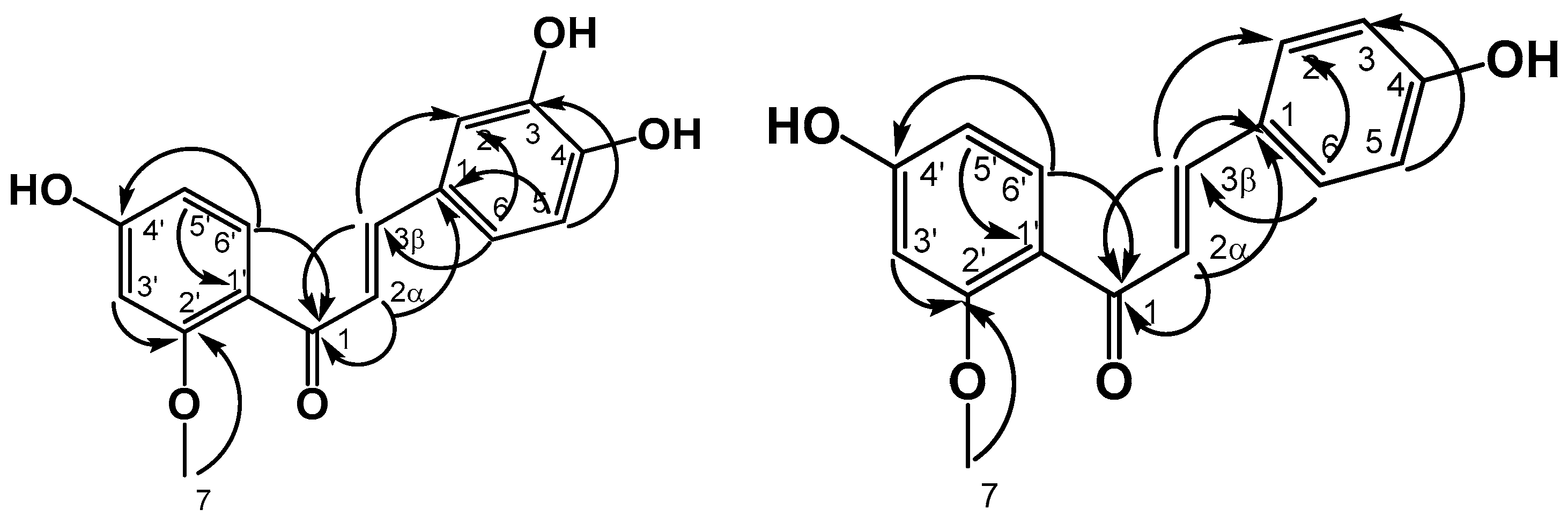
2.3. Structural Elucidation of Homoisoflavoids
2.3.1. Sappanchalcone (1)
2.3.2. 3-Deoxysappachalcone (2)

2.3.3. Hematoxylol A Tetraacetate (3a)

2.3.4. 4-O-Methylhematoxylol Tetracetate (4a)

2.3.5. Hematoxin Diacetate (5a)

3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Chromatographic Fractionation of Heartwood
3.4. Isolated of the Chalcones (1,2) and Homoisoflavonoids Peracetate (3a–5a)
3.5. Reaction of Acetylation
3.6. Model of Ileum Isolated Guinea Pigs
3.7. Preparation of Extracts, Fractions and Compounds for Evaluations
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yasunaka, K.; Abe, F.; Nagayama, A.; Okabe, H.; Lozada-Pérez, L.; López-Villafranco, E.; Estrada-Muñiz, E.; Aguilar, A.; Reyes Chilpa, R. Antibacterial activity of crude extracts from Mexican medicinal plants and purified coumarins and xanthones. J. Ethnopharmacol. 2005, 97, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Durán, R.; Méndez, M. Biodiversidad y Desarrollo Humano en Yucatán; Centro de Investigación Científica de Yucatán: Mérida, Mexico, 2010; Volume 7, pp. 370–372.
- Norton, S. The useful plants of dermatology. II. Haematoxylum and hematoxylin. J. Am. Acad. Dermatol. 1996, 34, 149–151. [Google Scholar] [CrossRef]
- Mazzuco, G.; Basolo, B.; Monga, G. The use of mallory’s phosphotungstic acid-hematoxilin (PTAH) stain in renal pathology. Pathol. Res. Pract. 1982, 175, 380–391. [Google Scholar] [CrossRef]
- Waldeyer, W. Untersuchungen über den Ursprung und den Verlauf des Axsencylinders bei Wirbellosen und Wirbelthieren sowie über dessen Endverhalten in der quergestreiften Muskelfaser. Henle Pfeifer’s Z. Rat. Med. 1863, 20, 193–256. [Google Scholar]
- Masuda, H.; Ohtani, K.; Mizutani, K.; Ogawa, S.; Kasai, R.; Tanaka, O. Chemical study on Haematoxylum campechianum: A sweet principle and new dibenz[b,d]oxocin derivates. Chem. Pharm. Bull. 1991, 39, 1382–1384. [Google Scholar] [CrossRef]
- Maldonado, F. Flora Medicinal del Estado de Tabasco: Uso, Manejo y Conservación; Universidad Juárez Autónoma de Tabasco (UJAT): Villahermosa, Mexico, 2005; p. 97. [Google Scholar]
- Martínez, M. Las Plantas Medicinales de México Tomo 1; Editorial Botas: Distrito Federal, Mexico, 2005; Volume 386, pp. 234–235. [Google Scholar]
- Gómez-Méndez, E.; López-Noverola, U.; López-Naranjo, J.; Salaya-Domínguez, J.; Díaz-González, J.; Hernández, M. Catálogo de Plantas Medicinales de Uso Actual del Estado de Tabasco; Fundación Produce Tabasco A.C., Universidad Juárez Autónoma del Estado de Tabasco Villahermosa: Villahermosa, Mexico, 2004; p. 55. [Google Scholar]
- Perkin, W.; Robinson, R. Brazilin and Haematoxylin. Part VII. Synthesis of derivatives of hydrindene closely allied to Brazilin and Haematoxylin. J. Chem. Soc. Trans. 1907, 91, 1073–1103. [Google Scholar] [CrossRef]
- Namikoshi, M.; Nakata, H.; Nuno, M.; Ozawa, T.; Saitoh, T. Homoisoflavonoid and Related Compounds. Ill. Phenolic Constituents of Caesalpinia Japonica SIEB. et ZUCC. Chem. Pharm. Bull. 1987, 35, 3568–3575. [Google Scholar] [CrossRef]
- Masahiro, N.; Seiji, N.; Shu-Mei, L.; Ikuyo, E.; Ken-Ichi, K. Protosappanin A, a novel biphenyl compound from Sappan Lignum. Chem. Pharm. Bull. 1986, 34, 1–6. [Google Scholar]
- Lin, L.G.; Xie, H.; Li, H.L.; Tong, L.J.; Tang, C.P.; Ke, C.Q.; Liu, Q.F.; Lin, L.P.; Geng, M.Y.; Jiang, H.; et al. Naturally occurring homoisoflavonoids function as potent protein tyrosine kinase inhibitors by c-Src-based high-throughput screening. J. Med. Chem. 2008, 51, 4419–4429. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.G.; Liu, Q.Y.; Ye, Y. Naturally occurring homoisoflavonoids and their pharmacological activities. Planta Med. 2014, 80, 1053–1066. [Google Scholar]
- Zhao, M.B.; Li, J.; Shi, S.P.; Cai, C.Q.; Tu, P.F.; Tang, L.; Zeng, K.W.; Jiang, Y. Two new phenolic compounds from the Heartwood of Caesalpinia sappan L. Molecules 2014, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.G.; Han, K.I.; Kwon, H.J.; Patnaik, B.B.; Kim, W.J.; Hur, G.M.; Namm, K.W.; Han, M.D. Anti-inflammatory activity of sappanchalcone isolated from Caesalpinia sappan L. in a collagen-induced arthritis mouse model. Arch. Pharm. Res. 2015, 38, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, J.R.; Chebib, M.; Johnston, G.A. Flavonoid modulation of GABAA receptors. Br. J. Pharmacol. 2011, 163, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Grosso, C.; Valentao, P.; Ferreres, F.; Andrade, P.B. The use of flavonoids in Central Nervous System Disorders. In Current Medicinal Chemistry; Atta-ur-Rahman, Ed.; Bentham Science Publishers: Cambridge, UK, 2013; Volume 20, pp. 4694–4719. [Google Scholar]
- Cheng, S.Y.; Wang, C.M.; Cheng, H.L.; Chen, H.J.; Hsu, Y.M.; Lin, Y.C.; Chou, C.H. Biological activity of oleanane triterpene derivatives obtained by chemical derivatization. Molecules 2013, 18, 13003–13019. [Google Scholar] [CrossRef] [PubMed]
- Lozoya, X.; Becerril, G.; Martínez, M. Modelo de perfusión intraluminal del íleon del cobayo in vitro en el estudio de propiedades antidiarréicas de la guayaba (Psidium guajava). Arch. Investig. Méd. 1990, 21, 155–162. [Google Scholar]
Sample Availability: Samples of the compounds 1, 2 and 3a–5a are available from the authors. |
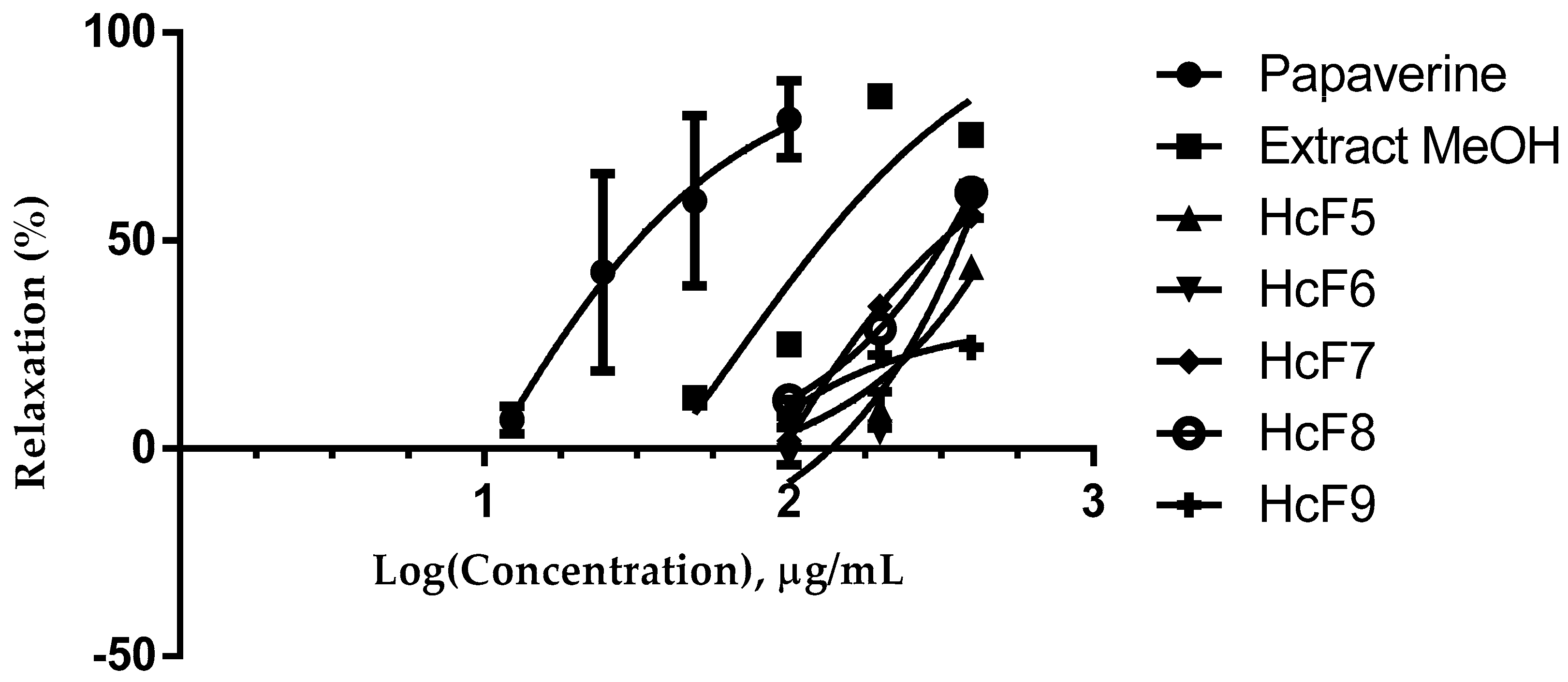
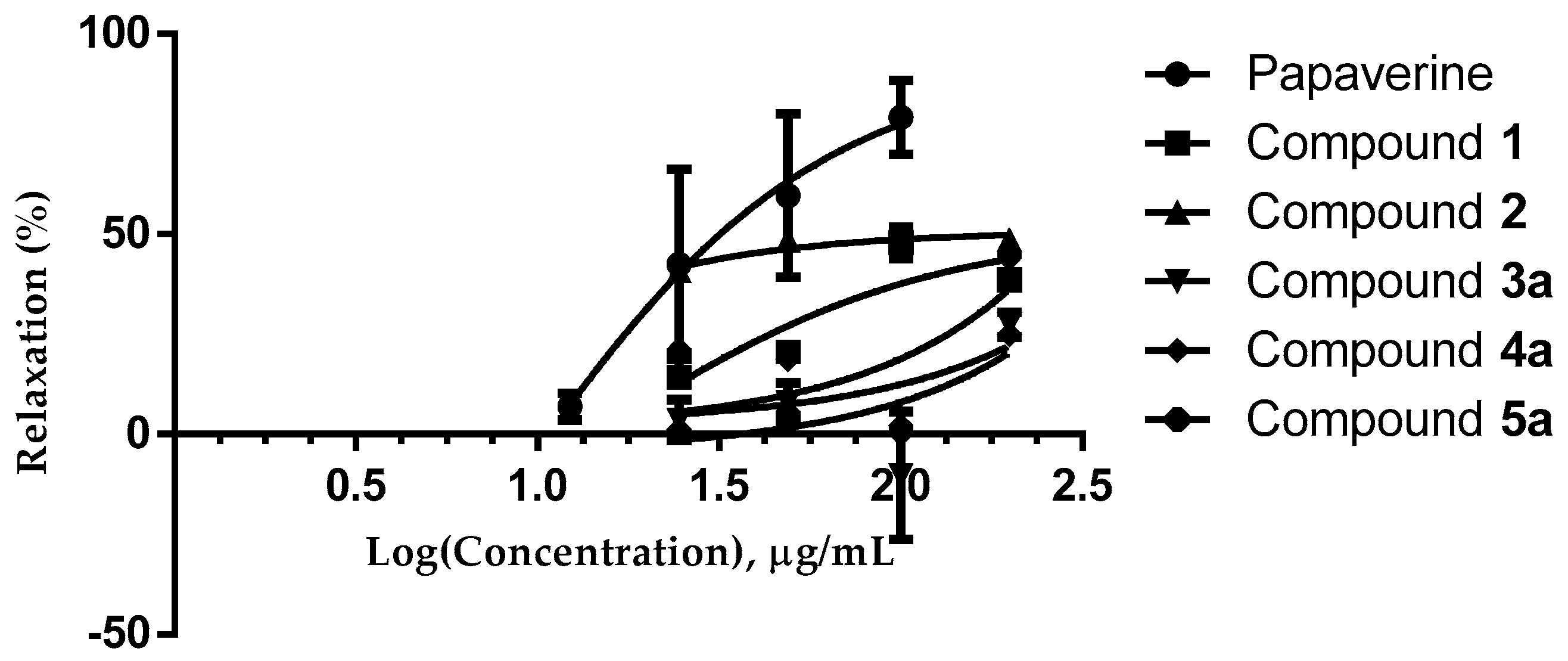
| Extract, Fraction and Compounds | Effective Concentration Fifty (CE50 = µg/mL) | Relaxation (%) Emax |
|---|---|---|
| Extract MeOH | 62.11 ± 3.23 b | 85.16 ± 6.34 d |
| Fraction | ||
| HcF5 | 346.7± 19.32 f | 56.7 ± 1.8 c |
| HcF6 | 324.9 ± 10.15 f | 80.9 ± 2.5 d |
| HcF7 | 61.75 ± 3.55 b | 84.6 ± 1.7 d |
| HcF8 | 343.7 ± 10.04 f | 95.1 ± 1.2 e |
| HcF9 | 125.5 ± 10.65 c | 24.45 ±1.6 a |
| Compound | ||
| 1 | 16.06 ± 2.15 a | 50.9 ± 2.2 b,c |
| 2 | 25.37 ± 3.47 a | 48.5 ± 2.0 b |
| 3a | 204.5 ± 2.75 d | 85.78 ± 2.7 d |
| 4a | 285 ± 11.0 e | ND |
| 5a | 203.1 ±7.2 d | 100 ±0.3 e |
| Papaverine (+) | 20.08 ± 2.0 a | 85.72 ± 2.8 d |
| Position | 1 | 2 | 3a | 4a | 5a |
|---|---|---|---|---|---|
| 2α | 124.8 | 125.2 | |||
| 3β | 144.2 | 144.2 | |||
| 1 C=O | 192.5 | 193.2 | |||
| 1′ | 121.8 | 121.8 | 134.3 | ||
| 2′ | 162.5 | 162.6 | 125.7 | ||
| 3′ | 99.8 | 100.3 | 141.6 | ||
| 4′ | 164.4 | 164.6 | 140.8 | ||
| 5′ | 108.7 | 109.0 | 122.9 | ||
| 6′ | 133.4 | 134.1 | 129.0 | ||
| 1 | 128.7 | 128.2 | 126.8 | 144.1 | |
| 2 | 114.9 | 131.5 | 120.6 | 69.4 | 130.1 |
| 3 | 146.8 | 117.0 | 136.5 | 69.9 | 180.4 |
| 4 | 149.6 | 161.3 | 132.7 | 76.2 | 136.6 |
| 4a | 149.2 | 118.2 | 160.7 | ||
| 5 | 116.2 | 117.0 | 128.5 | ||
| 6 | 123.0 | 131.5 | 76.9 | 113.9 | 77.5 |
| 7 | 55.87 | 56.28 | 203.6 | 143.6 | 70.0 |
| 8 | 49.0 | 130.9 | 38.67 | ||
| 8a | 142.0 | 146.6 | 133.9 | ||
| 9 | 125.2 | 38.2 | 123.3 | ||
| 10 | 142.1 | 56.2 | 141.8 | ||
| 11 | 144.3 | 167.7 | 140.6 | ||
| 12 | 125.0 | 168.2 | 121.7 | ||
| 12a | 136.3 | 130.0 | |||
| 12b | 131.0 | 52.7 | |||
| 13 | 167.9 | 168.4 | 83.75 | ||
| 14 | 168.5 | 168.4 | 62.51 | ||
| 15 | 168.6 | 20.64 | 168.0 | ||
| 16 | 168.7 | 20.65 | 168.0 | ||
| 17 | 20.80 | 20.3 | 20.52 | ||
| 18 | 21.23 | 20.3 | 20.68 | ||
| 19 | 21.16 | ||||
| 20 | 21.16 |
| Position | 1 | 2 | 3a | 4a | 5a |
|---|---|---|---|---|---|
| 2α | 7.37, d, 15.7 | 7.40, d, 15.7 | |||
| 3β | 7.49, d, 15.7 | 7.55, d, 16.5 | |||
| 2′ | 7.18, s | ||||
| 3′ | 6.52, d, 2.2 | 6.51,d, 2.2 | |||
| 5′ | 6.46, dd, 2.2, 8.4 | 6.45, dd, 2.2, 8.4 | 7.14, d, 8.07 | ||
| 6′ | 7.57, d, 8.4 | 7.57, d, 8.4 | 7.21, d, 8.07 | ||
| 1 | 7.28, d, 8.4 | 7.08, d, 9.9 | |||
| 2α | 7.11, d, 2.2 | 7.49, dd, 1.8, 8.4 | 7.17, d, 8.8 | 3.94, d, 11.3 | 6.63, d, 10.2 |
| 2β | 4.19, d, 11.0 | ||||
| 3 | 6.81, dd, 1.8, 8.4 | ||||
| 4 | 3.7, s | ||||
| 5 | 6.79, d, 8.0 | 6.81, dd, 1.8, 8.4 | 7.02, d, 8.07 | ||
| 6α | 6.99, dd, 2.2, 8.0 | 7.49, dd, 1.8, 8.4 | 4.5, s | 6.72, d, 8.07 | 3.86, dd, 2.2, 11 |
| 6β | 4.21, d, 11 | ||||
| 7 | 3.9, s | 3.88, s | |||
| 8α | 3.6, s | 3.10, d, 16.5 | |||
| 8β | 3.40, d, 16.1 | ||||
| 9α | 7.2, s | 2.84, d, 13.5 | 7.03, s | ||
| 9β | 3.02, d, 14.3 | ||||
| 10 | 3.34 | ||||
| 12 | 7.18, s | 6.78, s | |||
| 13 | 3.6, s | ||||
| 14 | 3.6, s | ||||
| 15 | 2.3, s | ||||
| 16 | 2.3, s | ||||
| 17 | 2.35, s | 2.3, s | 2.24, s | ||
| 18 | 2.33, s | 2.3, s | 2.26, s | ||
| 19 | 2.3, s | ||||
| 20 | 2.3, s |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar-Ramos, A.; Lobato-García, C.E.; Zamilpa, A.; Gómez-Rivera, A.; Tortoriello, J.; González-Cortazar, M. Homoisoflavonoids and Chalcones Isolated from Haematoxylum campechianum L., with Spasmolytic Activity. Molecules 2017, 22, 1405. https://doi.org/10.3390/molecules22091405
Escobar-Ramos A, Lobato-García CE, Zamilpa A, Gómez-Rivera A, Tortoriello J, González-Cortazar M. Homoisoflavonoids and Chalcones Isolated from Haematoxylum campechianum L., with Spasmolytic Activity. Molecules. 2017; 22(9):1405. https://doi.org/10.3390/molecules22091405
Chicago/Turabian StyleEscobar-Ramos, Armando, Carlos Ernesto Lobato-García, Alejandro Zamilpa, Abraham Gómez-Rivera, Jaime Tortoriello, and Manasés González-Cortazar. 2017. "Homoisoflavonoids and Chalcones Isolated from Haematoxylum campechianum L., with Spasmolytic Activity" Molecules 22, no. 9: 1405. https://doi.org/10.3390/molecules22091405





