Bis(1-pyrenylmethyl)-2-benzyl-2-methyl-malonate as a Cu2+ Ion-Selective Fluoroionophore
Abstract
:1. Introduction
2. Results and Discussion
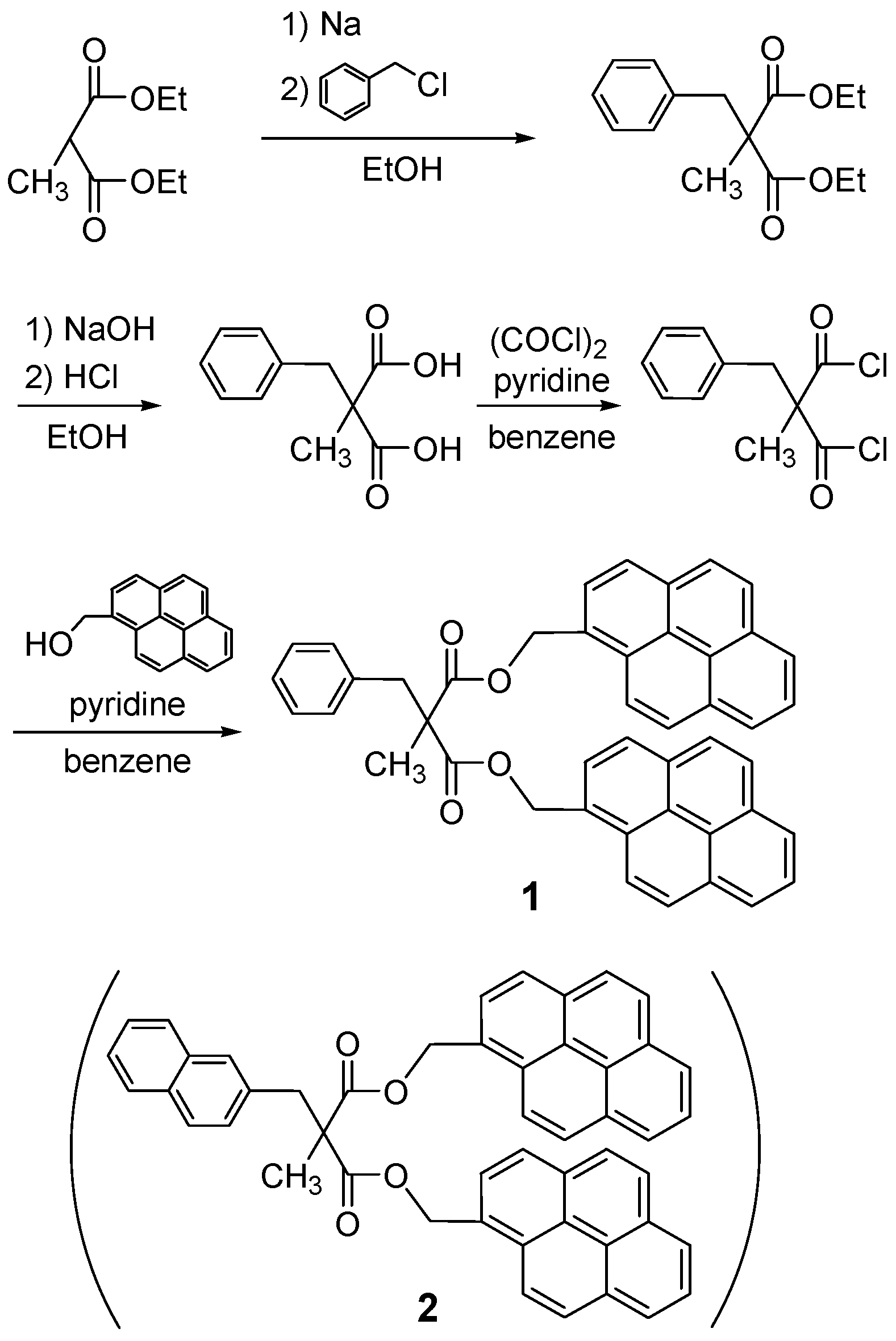
2.1. Synthesis
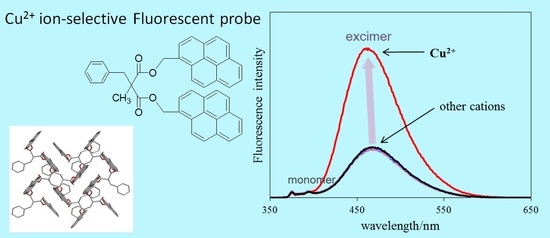
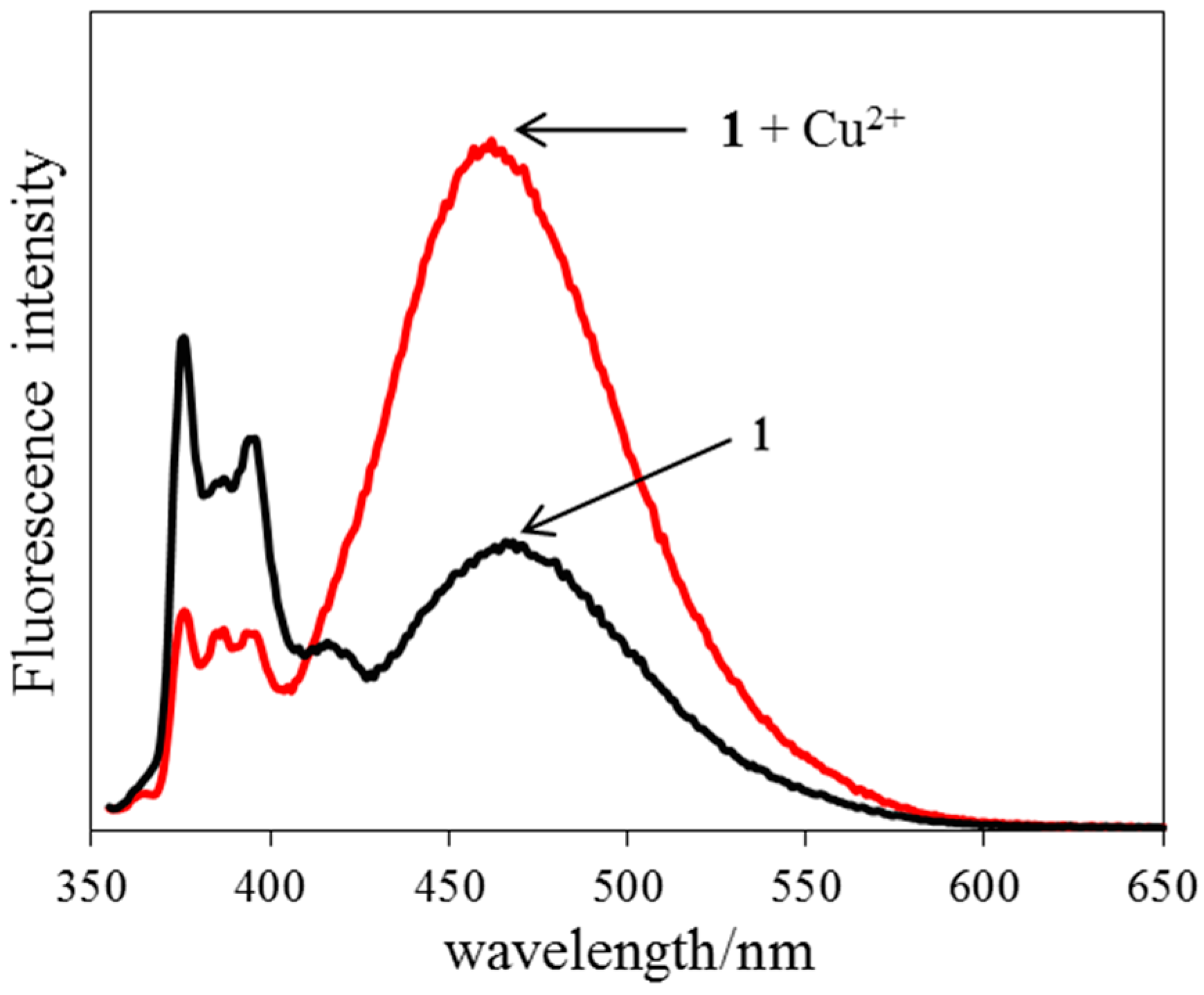
2.2. Absorption and Fluorescence Properties
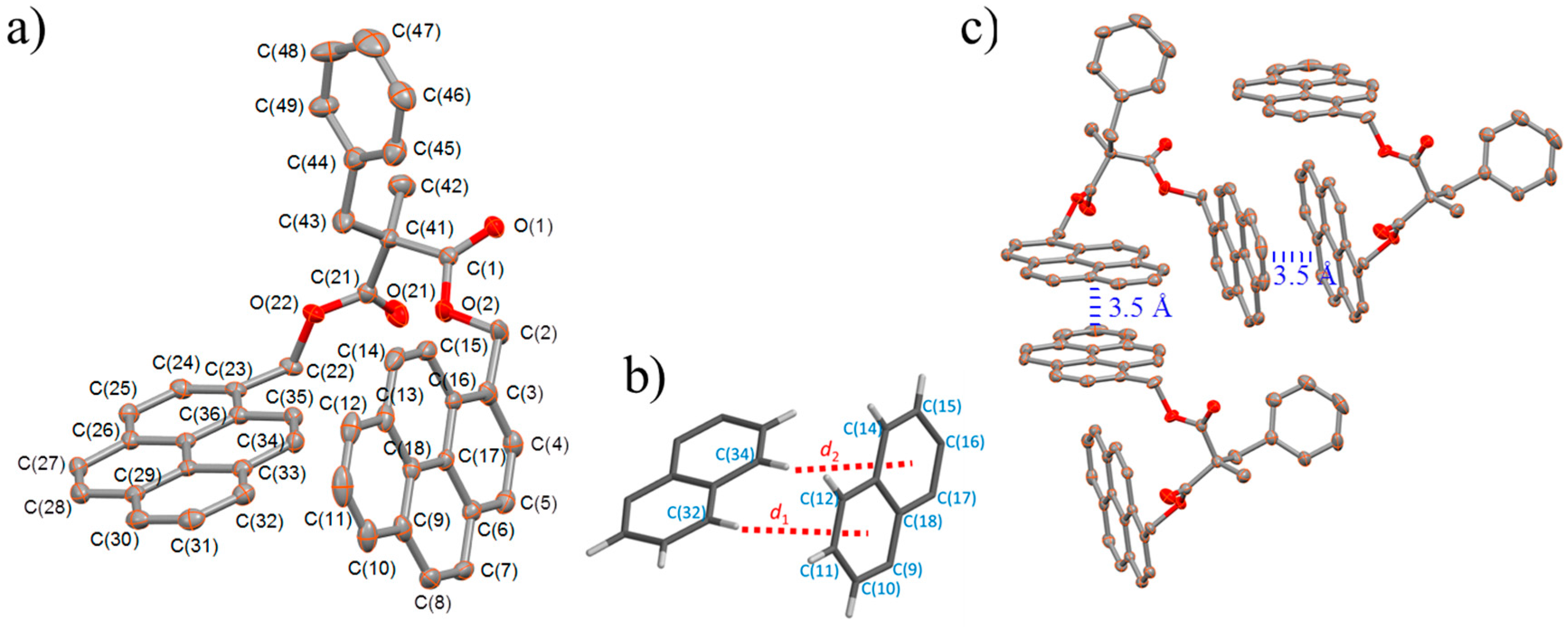
2.3. X-ray Structural Studies
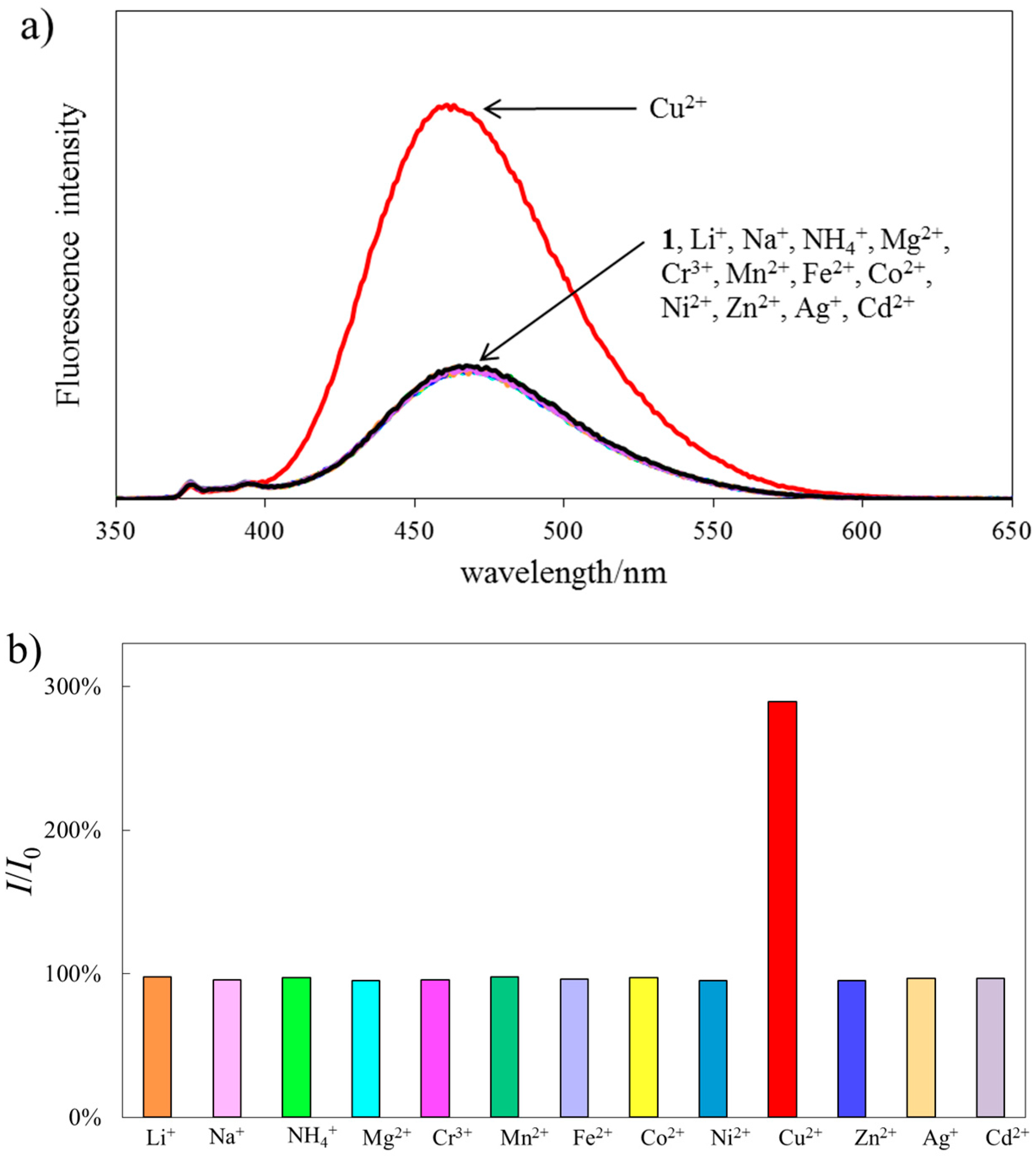
2.4. Fluorescence Response
3. Experimental Section
3.1. Reagents and Chemicals
3.2. Apparatus
3.3. Syntheses
3.4. X-ray Crystallographic Analysis
3.5. UV-Vis and Fluorescence Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Feng, X.; Hu, J.Y.; Redshaw, C.; Yamato, T. Functionalization of Pyrene To Prepare Luminescent Materials—Typical Examples of Synthetic Methodology. Chem. Eur. J. 2016, 22, 11898–11916. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Tang, Y.; Zhua, W.; Xie, Y. Fluorescent and colorimetric ion probes based onconjugated oligopyrroles. Chem. Soc. Rev. 2015, 44, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Basabe-Desmonts, L.; Reinhoudt, D.N.; Crego-Calama, M. Design of fluorescent materials for chemical sensing. Chem. Soc. Rev. 2007, 36, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Callan, J.F.; de Silva, A.P.; Magri, D.C. Luminescent sensors and switches in the early 21st century. Tetrahedron 2005, 61, 8551–8588. [Google Scholar] [CrossRef]
- Kimura, E. Macrocyclic polyamines with intelligent functions. Tetrahedron 1992, 48, 6175–6217. [Google Scholar] [CrossRef]
- Jia, H.; Yang, M.; Meng, Q.; He, G.; Wang, Y.; Hu, Z.; Zhang, R.; Zhang, Z. Synthesis and Application of an Aldazine-Based Fluorescence Chemosensor for the Sequential Detection of Cu2+ and Biological Thiols in Aqueous Solution and Living Cells. Sensors 2016, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Shi, Y.; Wang, C.; Jia, H.; Gao, X.; Zhang, R.; Wanga, Y.; Zhang, Z. NBD-based fluorescent chemosensor for the selective quantification of copper and sulfide in an aqueous solution and living cells. Org. Biomol. Chem. 2015, 13, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Templeton, D.M. Mechanisms of immunosensitization to metals. Pure Appl. Chem. 2004, 76, 1255–1268. [Google Scholar] [CrossRef]
- Rurack, K. Flipping the light switch ‘on’—The design of sensor molecules that show cation-induced fluorescence enhancement with heavy and transition metal ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 2161–2195. [Google Scholar] [CrossRef]
- Varners, A.W.; Dodson, R.B.; Wehry, E.L. Interactions of transition-metal ions with photoexcited states of flavines. Fluorescence quenching studies. J. Am. Chem. Soc. 1972, 94, 946–950. [Google Scholar] [CrossRef]
- Cheng, D.; Liu, X.; Yang, H.; Zhang, T.; Han, A.; Zang, L. A Cu2+-Selective Probe Based on Phenanthro-Imidazole Derivative. Sensors 2017, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Xia, H.; Mu, Y.; Liu, X. A robust and luminescent covalent organic framework as a highly sensitive and selective sensor for the detection of Cu2+ ions. Chem. Commun. 2016, 52, 6613–6616. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Han, S.; Hu, Y.; Zhang, L. Enhanced Fluorescence in Tetraylnitrilomethylidyne−Hexaphenyl Derivative-Functionalized Periodic Mesoporous Organosilicas for Sensitive Detection of Copper(II). J. Phys. Chem. C 2016, 120, 9299–9307. [Google Scholar] [CrossRef]
- Li, W.; Zhu, G.; Li, J.; Wang, Z.; Jin, Y. An Amidochlorin-Based Colorimetric Fluorescent Probe for Selective Cu2+ Detection. Molecules 2016, 21, 107. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Chatti, M.; Adusumalli, V.N.K.B.; Mahalingam, V. Highly Selective and Sensitive Detection of Cu2+ Ions Using Ce(III)/Tb(III)-Doped SrF2 Nanocrystals as Fluorescent Probe. ACS Appl. Mater. Interfaces 2015, 7, 25702–25708. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.L.; Choi, M.G.; Choe, J.I.; Chang, S.K. Cu2+- and Hg2+-Selective Chemosensing by Dioxocyclams Having Two Appended Pyrenylacetamides. Bull. Korean Chem. Soc. 2009, 30, 1093–1096. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Q.; Yu, B.; Yu, C. A Pyridine-Containing Cu2+-Selective Probe Based on Naphthalimide Derivative. Sensors 2014, 14, 24146–24155. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, Y. A DNAzyme Catalytic Beacon Sensor for Paramagnetic Cu2+ Ions in Aqueous Solution with High Sensitivity and Selectivity. J. Am. Chem. Soc. 2007, 129, 9838–9893. [Google Scholar] [CrossRef] [PubMed]
- Oter, O.; Ertekin, K.; Kirilmis, C.; Koca, M. Spectral characterization of a newly synthesized fluorescent semicarbazone derivative and its usage as a selective fiber optic sensor for copper(II). Anal. Chim. Acta 2007, 584, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Jun, E.J.; Xu, L.; Kim, S.J.; Hong, J.S.J.; Yoon, Y.J.; Yoon, J. New BODIPY Derivatives as OFF-ON Fluorescent Chemosensor and Fluorescent Chemodosimeter for Cu2+: Cooperative Selectivity Enhancement toward Cu2+. J. Org. Chem. 2006, 71, 2881–2884. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kumar, S. Photoactive chemosensors: A unique case of fluorescence enhancement with Cu(II). Chem. Commun. 2002, 23, 2840–2841. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: From mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef] [PubMed]
- Bains, G.K.; Kim, S.H.; Sorin, E.J.; Narayanaswami, V. The Extent of Pyrene Excimer Fluorescence Emission Is a Reflector of Distance and Flexibility: Analysis of the Segment Linking the LDL Receptor-Binding and Tetramerization Domains of Apolipoprotein E3. Biochemistry 2012, 51, 6207–6219. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, J. New Insights in the Study of Pyrene Excimer Fluorescence to Characterize Macromolecules and their Supramolecular Assemblies in Solution. Langmuir 2012, 28, 6527–6538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, R.; Liu, F.; Li, K.A. Fluorescent Sensor for Imidazole Derivatives Based on Monomer-Dimer Equilibrium of a Zinc Porphyrin Complex in a Polymeric Film. Anal. Chem. 2004, 76, 7336–7345. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, A.; Hayashita, T.; Nishizawa, S.; Watanabe, M.; Teramae, N. Benzo-15-crown-5 Fluoroionophore/γ-Cyclodextrin Complex with Remarkably High Potassium Ion Sensitivity and Selectivity in Water. J. Am. Chem. Soc. 1999, 121, 2319–2320. [Google Scholar] [CrossRef]
- Sahoo, D.; Narayanaswami, V.; Kay, M.C.; Ryan, O.R. Pyrene Excimer Fluorescence: A Spatially Sensitive Probe to Monitor Lipid-Induced Helical Rearrangement of Apolipophorin III. Biochemistry 2000, 39, 6594–6601. [Google Scholar] [CrossRef] [PubMed]
- Bodenant, B.; Fages, F.; Delville, M.H. Metal-Induced Self-Assembly of a Pyrene-Tethered Hydroxamate Ligand for the Generation of Multichromophoric Supramolecular Systems. The Pyrene Excimer as Switch for Iron(III)-Driven Intramolecular Fluorescence Quenching. J. Am. Chem. Soc. 1998, 120, 7511–7519. [Google Scholar] [CrossRef]
- Sandhu, S.; Kumar, R.; Singh, P.; Walia, A.; Vanita, V.; Kumar, S. Ratiometric fluorophore for quantification of iodide under physiological conditions: Applications in urine analysis and live cell imaging. Org. Biomol. Chem. 2016, 14, 3536–3543. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhu, H.; Zhang, M.; Tan, T.; Chen, J.; Qiu, H. A new highly Zn2+-selective and “off–on” fluorescent chemosensor based on the pyrene group. Anal. Methods 2015, 7, 8172–8176. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, M.G.; Eor, S.; Chang, S.K. Fluorescence Signaling of Zr4+ by Hydrogen Peroxide Assisted Selective Desulfurization of Thioamide. Inorg. Chem. 2012, 51, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Yan, Y.; Chen, L.; Cao, W.; Li, H.; Yang, L.; Wu, Y. A fluorescence reagent for the highly selective recognition and separation of lead ion(II) from aqueous solutions. Anal. Chim. Acta 2012, 751, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Gong, S.; Gong, S.; Yang, C.; Qin, J. Novel Pyrene-armed Calix[4]arenes through Triazole Connection: Ratiometric Fluorescent Chemosensor for Zn2+ and Promising Structure for Integrated Logic Gates. Chin. J. Chem. 2008, 26, 1424–1430. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Tokitoh, Y.; Hirai, T. pH- and H2O-Driven Triple-Mode Pyrene Fluorescence. Org. Lett. 2006, 8, 3841–3844. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Lee, A.; Kim, S.; Ham, S.; No, K.; Kim, J.S. Fluorescent Ratiometry of Tetrahomodioxacalix[4]arene Pyrenylamides upon Cation Complexation. Org. Lett. 2006, 8, 1601–1604. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, S.H.; Kim, H.J.; Lee, S.H.; Lee, S.W.; Ko, J.; Bartsch, R.A.; Kim, J.S. Indium(III)-Induced Fluorescent Excimer Formation and Extinction in Calix[4]arene-Fluoroionophores. Inorg. Chem. 2005, 44, 7866–7875. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Lee, S.H.; Lee, J.Y.; Lee, J.Y.; Bartsch, R.A.; Kim, J.S. An Excimer-Based, Binuclear, On-Off Switchable Calix[4]crown Chemosensor. J. Am. Chem. Soc. 2004, 126, 16499–16506. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ganguly, A.; Uddin, M.R.; Mandal, S.; Alam, M.A.; Guchhait, N. Dual mode selective chemosensor for copper and fluoride ions: A fluorometric, colorimetric and theoretical investigation. Dalton Trans. 2016, 45, 11042–11051. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.S.; Li, C.Y.; Li, Y.F.; Li, D.; Li, Z. Development of a simple pyrene-based ratiometric fluorescentchemosensor for copper ion in living cells. Sens. Actuators B 2016, 222, 1226–1232. [Google Scholar] [CrossRef]
- Moriuchi-Kawakami, T.; Hisada, Y.; Shibutani, Y. A Cu2+ ion-selective fluoroionophore with dual off/on switches. Chem. Cent. J. 2010, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhang, Y.; Li, K.; Liu, F.; Chan, W. Fluorescent ratioable recognition of Cu2+ in water using a pyrene-attached macrocycle/γ-cyclodextrin complex. Anal. Chim. Acta 2004, 525, 97–103. [Google Scholar] [CrossRef]
- Sasaki, D.Y.; Shnek, D.R.; Pack, D.W.; Arnold, F.H. Metal-Induced Dispersion of Lipid Aggregates: A Simple, Selective, and Sensitive Fluorescent Metal Ion Sensor. Angew. Chem. Int. Ed. Engl. 1995, 34, 905–907. [Google Scholar] [CrossRef]
- Sarkar, S.; Roy, S.; Sikdar, A.; Saha, R.N.; Panja, S.S. A pyrene-based simple but highly selective fluorescence sensor for Cu2+ ions via a static excimer mechanism. Analyst 2013, 138, 7119–7126. [Google Scholar] [CrossRef] [PubMed]
- Jun, E.J.; Won, H.N.; Kim, J.S.; Lee, K.H.; Yoon, J. Unique blue shift due to the formation of static pyrene excimer: Highly selective fluorescent chemosensor for Cu2+. Tetrahedron Lett. 2006, 47, 4577–4580. [Google Scholar] [CrossRef]
- Martínez, R.; Zapata, F.; Caballero, A.; Espinosa, A.; Tárraga, A.; Molina, P. 2-Aza-1,3-butadiene Derivatives Featuring an Anthracene or Pyrene Unit: Highly Selective Colorimetric and Fluorescent Signaling of Cu2+ Cation. Org. Lett. 2006, 8, 3235–3238. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.; Espinosa, A.; Tárraga, A.; Molina, P. New Hg2+ and Cu2+ Selective Chromoand Fluoroionophore Based on a Bichromophoric Azine. Org. Lett. 2005, 7, 5869–5872. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Lin, C.S.; Hwang, C.Y. Cu2+-Induced Blue Shift of the Pyrene Excimer Emission: A New Signal Transduction Mode of Pyrene Probes. Org. Lett. 2001, 3, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Moriuchi-Kawakami, T.; Kawata, K.; Nakamura, S.; Koyama, Y.; Shibutani, Y. Design of bisquinolinyl malonamides as Zn2+ ion-selective fluoroionophores based on the substituent effect. Tetrahedron 2014, 70, 9805–9813. [Google Scholar] [CrossRef]
- Moriuchi-Kawakami, T.; Aoki, R.; Morita, K.; Tsujioka, H.; Fujimori, K.; Shibutani, Y.; Shono, T. Conformational analysis of 12-crown-3 and sodium ion selectivity of electrodes based on bis(12-crown-3) derivatives with malonate spacers. Anal. Chim. Acta 2003, 480, 291–298. [Google Scholar] [CrossRef]
- Shibutani, Y.; Mino, S.; Shen, S.; Moriuchi-Kawakami, T.; Yakabe, K.; Shono, T. Chiral Bis(12-crown-4)-based Electrodes for Sodium Ion. Chem. Lett. 1997, 26, 49–50. [Google Scholar] [CrossRef]
- Shrestha, B.B.; Higashibayashi, S.; Sakurai, H. Columnar/herringbone dual crystal packing of pyrenylsumanene and its photophysical properties. Beilstein J. Org. Chem. 2014, 10, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Yeşilot, S.; Çoşut, B.; Alidağı, H.A.; Hacıvelioğlu, F.; Özpınar, G.A.; Kılıç, A. Intramolecular excimer formation in hexakis-(pyrenyloxy)cyclotriphosphazene: Photophysical properties, crystal structure, and theoretical investigation. Dalton Trans. 2014, 43, 3428–3433. [Google Scholar] [CrossRef] [PubMed]
- Swift, J.A.; Pal, R.; McBride, J.M. Using Hydrogen-Bonds and Herringbone Packing to Design Interfaces of 4,4’-Disubstituted meso-Hydrobenzoin Crystals. The Importance of Recognizing Unfavorable Packing Motifs. J. Am. Chem. Soc. 1998, 120, 96–104. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. IL MILIONE: A suite of computer programs for crystal structure solution of proteins. J. Appl. Cryst. 2007, 40, 609–613. [Google Scholar] [CrossRef]
- CrystalStructure 4.2.2: Crystal Structure Analysis Package; Rigaku Corporation: Tokyo, Japan, 2000–2016.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound 1 are available from the authors. |


| Formula | C45H32O4 |
|---|---|
| Formula weight | 636.75 |
| Crystal system | Orthorhombic |
| Space group | P212121 (no. 19) |
| a, Å | 9.50566(17) |
| b, Å | 12.5344(2) |
| c, Å | 27.0375(5) |
| α, deg | 90 |
| β, deg | 90 |
| γ, deg | 90 |
| V, Å3 | 3221.46(10) |
| Z | 4 |
| Dcalc, g cm-3 | 1.313 |
| μ(Cu Kα), cm-1 | 6.569 |
| T, °C | −1.0 |
| λ(Cu Ka), Å | 1.54187 |
| R1 a | 0.0620 |
| wR2 b | 0.1780 |
| Bond Lengths | |||
| O(1)–C(1) | 1.212(5) | O(2)–C(1) | 1.348(5) |
| O(2)–C(2) | 1.453(6) | O(21)–C(21) | 1.208(5) |
| O(22)–C(21) | 1.331(5) | O(22)–C(22) | 1.463(5) |
| C(1)–C(41) | 1.512(6) | C(2)–C(3) | 1.502(6) |
| C(22)–C(23) | 1.500(6) | C(21)–C(41) | 1.542(6) |
| C(41)–C(42) | 1.573(7) | C(41)–C(43) | 1.512(7) |
| C(43)–C(44) | 1.520(7) | ||
| Bond Angles | |||
| C(1)–O(2)–C(2) | 115.8(3) | C(2)–C(3)–C(16) | 121.5(4) |
| O(1)–C(1)–O(2) | 122.3(4) | O(21)–C(21)–C(41) | 122.0(4) |
| O(2)–C(1)–C(41) | 112.0(3) | O(22)–C(22)–C(23) | 108.9(3) |
| C(2)–C(3)–C(4) | 118.4(4) | C(22)–C(23)–C(36) | 121.1(4) |
| O(21)–C(21)–O(22) | 124.9(4) | C(1)–C(41)–C(42) | 108.4(4) |
| O(22)–C(21)–C(41) | 113.0(4) | C(21)–C(41)–C(42) | 105.6(4) |
| C(22)–C(23)–C(24) | 118.7(4) | C(21)–C(41)–C(43) | 111.7(4) |
| C(1)–C(41)–C(21) | 107.0(4) | C(41)–C(43)–C(44) | 113.4(4) |
| C(1)–C(41)–C(43) | 110.7(4) | C(43)–C(44)–C(49) | 119.6(4) |
| C(21)–O(22)–C(22) | 115.7(3) | C(42)–C(41)–C(43) | 113.0(4) |
| O(1)–C(1)–C(41) | 125.6(4) | C(43)–C(44)–C(45) | 121.3(5) |
| O(2)–C(2)–C(3) | 108.9(4) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moriuchi-Kawakami, T.; Hisada, Y.; Higashikado, A.; Inoue, T.; Fujimori, K.; Moriuchi, T. Bis(1-pyrenylmethyl)-2-benzyl-2-methyl-malonate as a Cu2+ Ion-Selective Fluoroionophore. Molecules 2017, 22, 1415. https://doi.org/10.3390/molecules22091415
Moriuchi-Kawakami T, Hisada Y, Higashikado A, Inoue T, Fujimori K, Moriuchi T. Bis(1-pyrenylmethyl)-2-benzyl-2-methyl-malonate as a Cu2+ Ion-Selective Fluoroionophore. Molecules. 2017; 22(9):1415. https://doi.org/10.3390/molecules22091415
Chicago/Turabian StyleMoriuchi-Kawakami, Takayo, Youji Hisada, Akihisa Higashikado, Tsubasa Inoue, Keiichi Fujimori, and Toshiyuki Moriuchi. 2017. "Bis(1-pyrenylmethyl)-2-benzyl-2-methyl-malonate as a Cu2+ Ion-Selective Fluoroionophore" Molecules 22, no. 9: 1415. https://doi.org/10.3390/molecules22091415