Enhancement of Glucose Uptake by Meso-Dihydroguaiaretic Acid through GLUT4 Up-Regulation in 3T3-L1 Adipocytes
Abstract
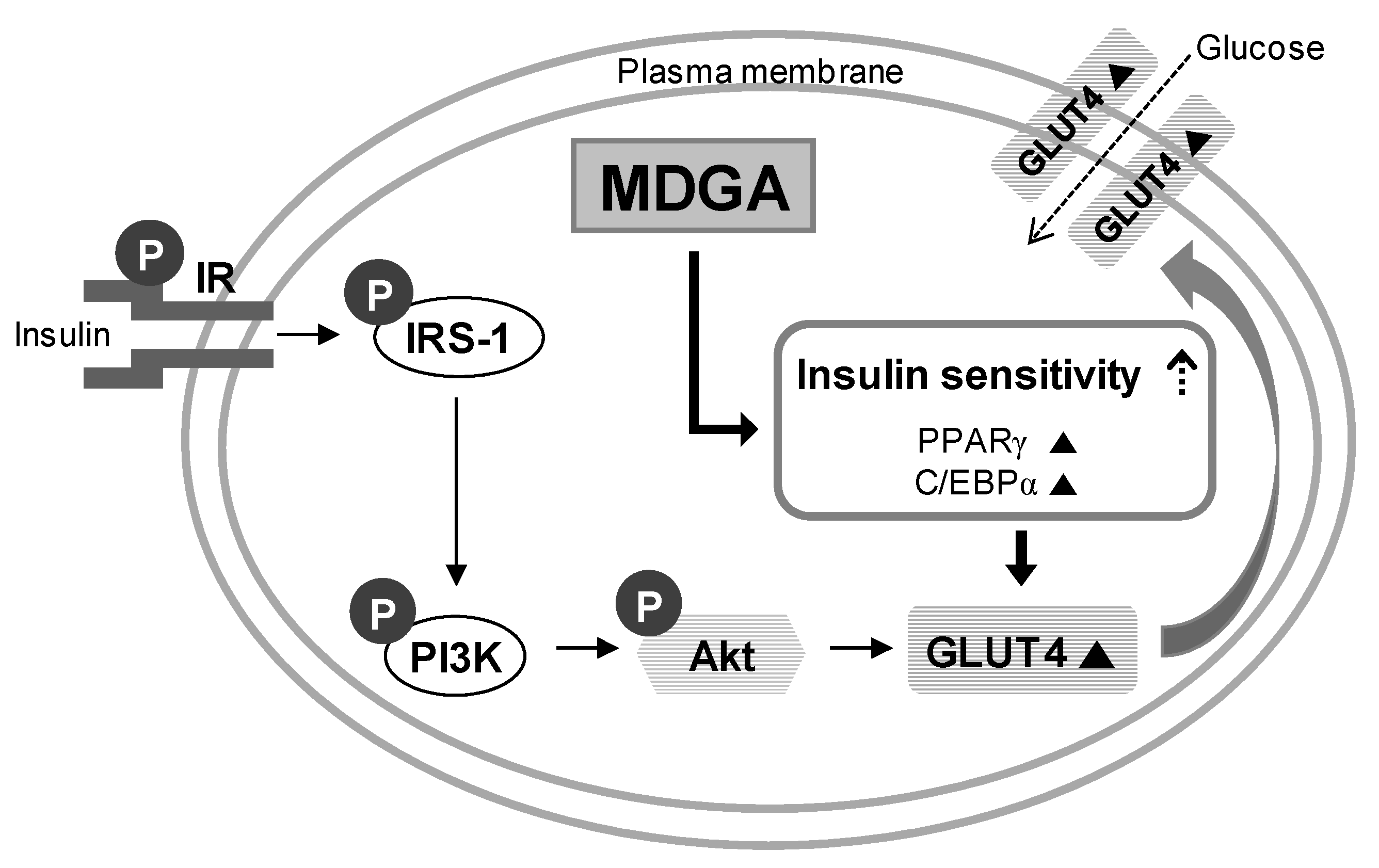
:1. Introduction
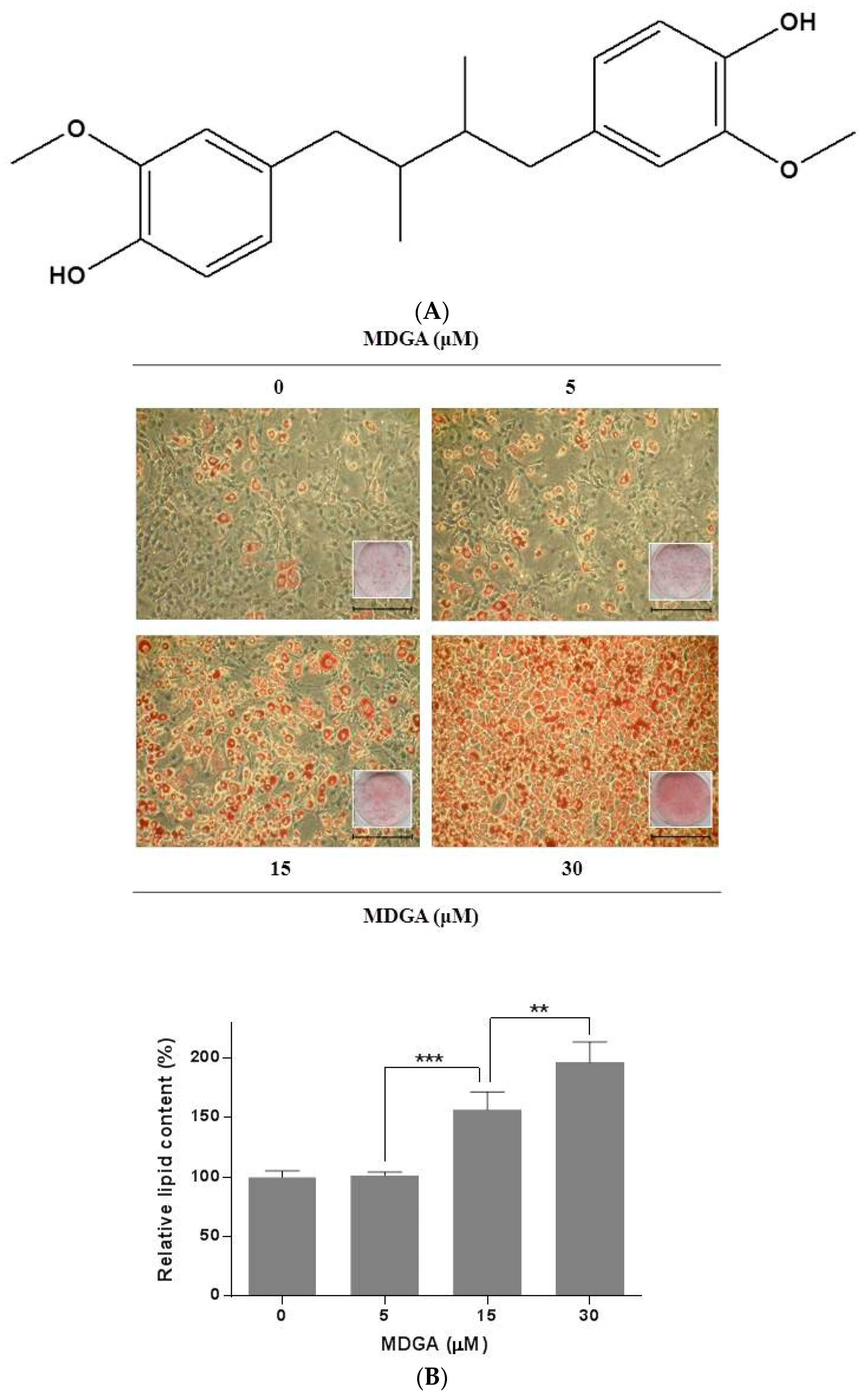
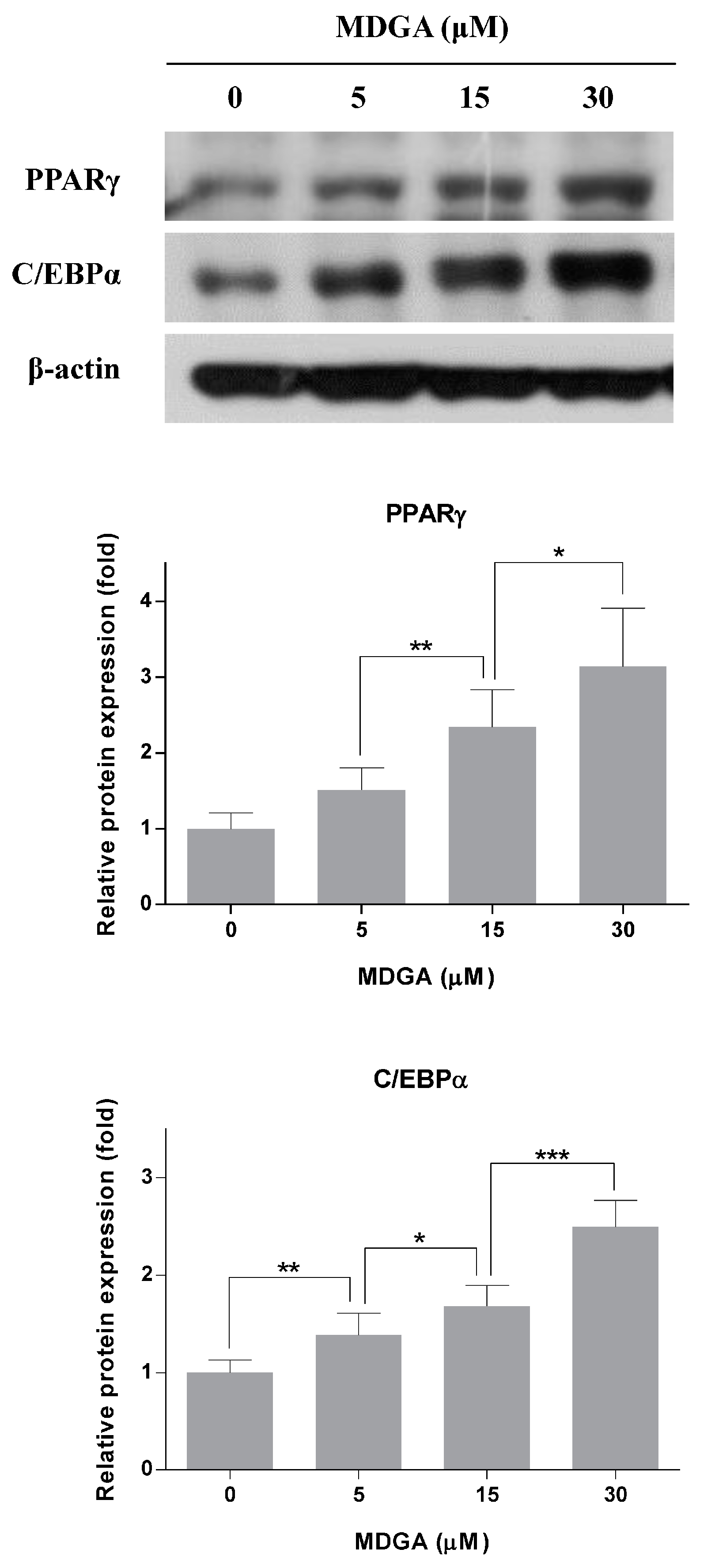
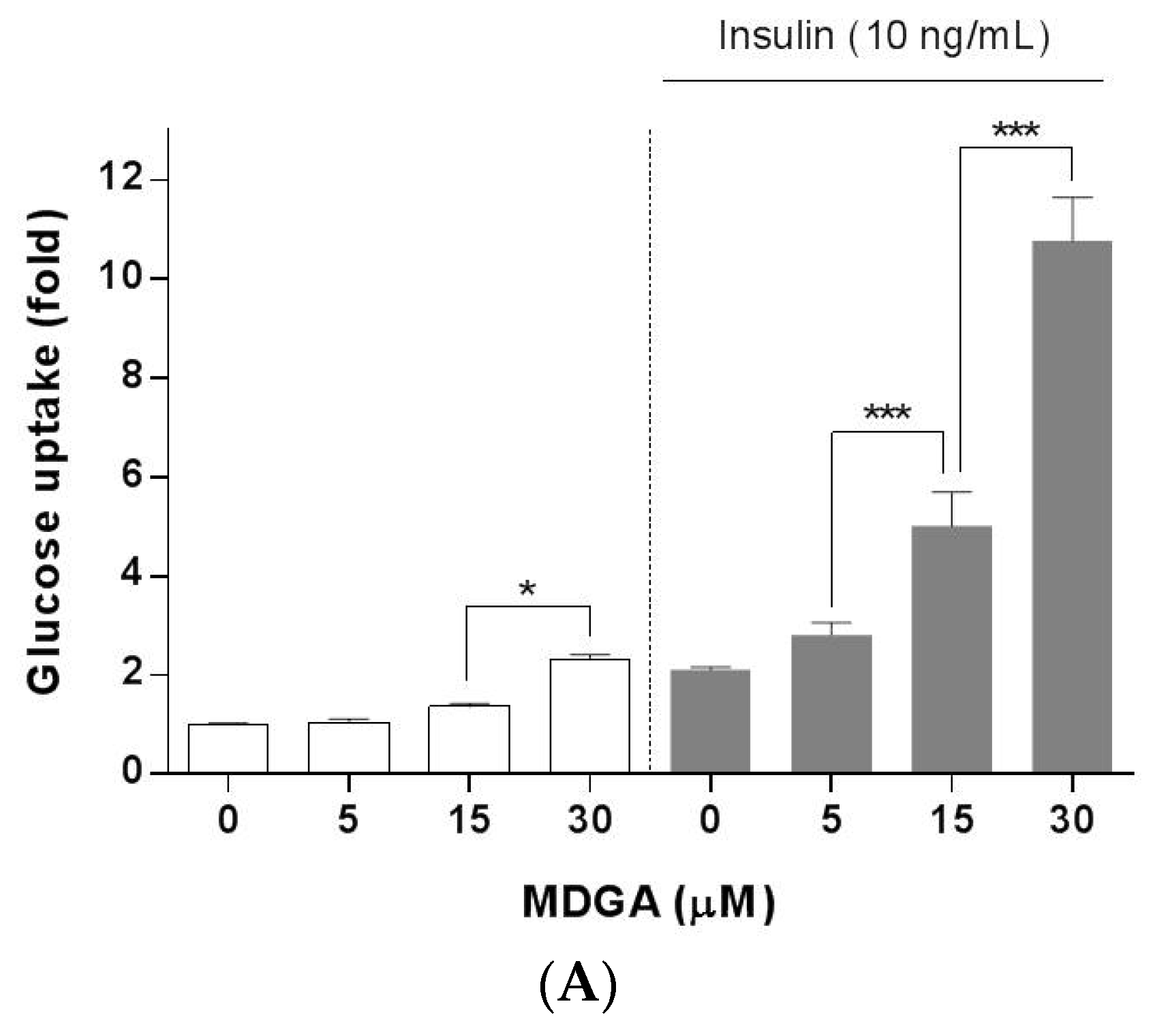
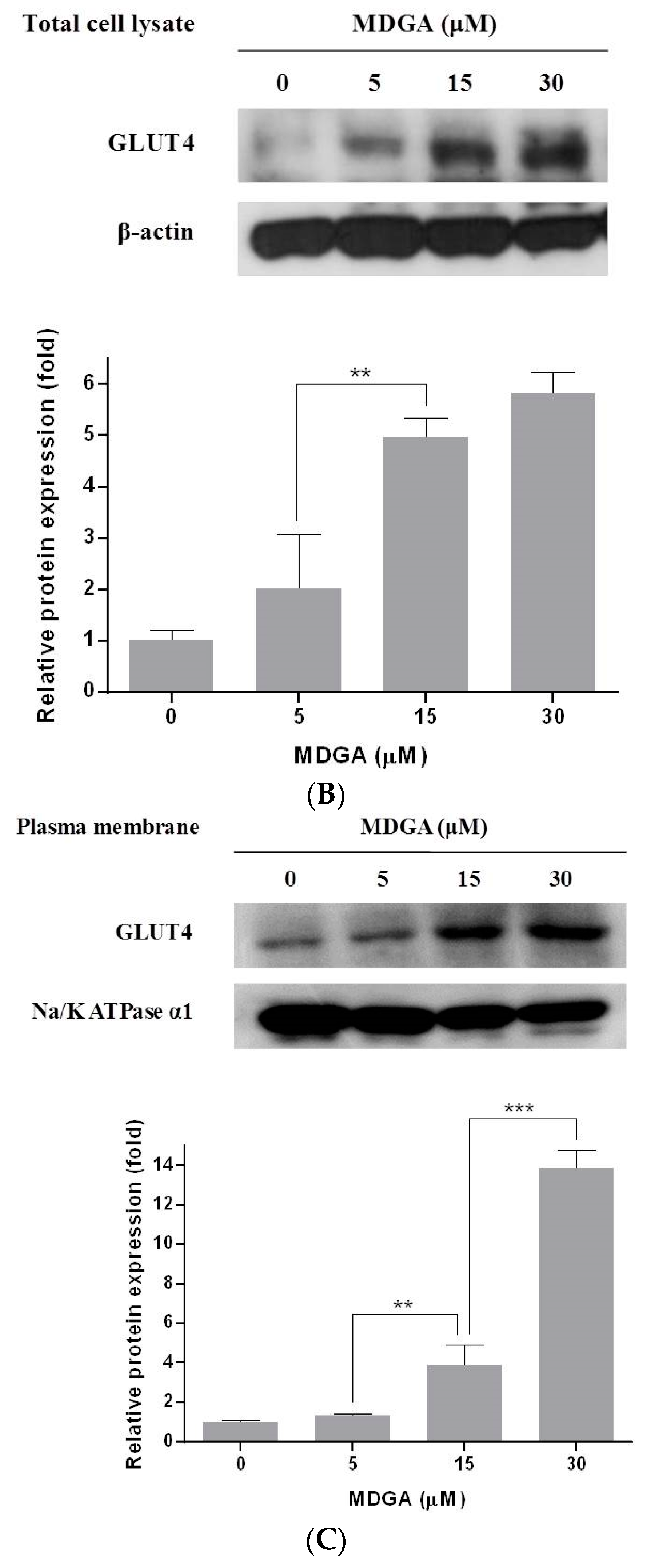
2. Results and Discussion
3. Materials and Methods
3.1. Reagents
3.2. Cell Culture and Adipocyte Differentiation Induction
3.3. Oil Red O Staining
3.4. Glucose Uptake Assay
3.5. Extraction of Plasma Membrane Protein
3.6. Western Blot Analysis
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Govers, R. Molecular mechanisms of GLUT4 regulation in adipocytes. Diabetes Metab. 2014, 40, 400–410. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33 (Suppl. 1), S62–S69. [Google Scholar]
- Camp, H.S.; Ren, D.; Leff, T. Adipogenesis and fat-cell function in obesity and diabetes. Trends Mol. Med. 2002, 8, 442–447. [Google Scholar] [CrossRef]
- Gregoire, F.M. Adipocyte differentiation: From fibroblast to endocrine cell. Exp. Biol. Med. 2001, 226, 997–1002. [Google Scholar] [CrossRef]
- Ntambi, J.M.; Young-Cheul, K. Adipocyte differentiation and gene expression. J. Nutr. 2000, 130, 3122S–3126S. [Google Scholar] [PubMed]
- Smith, P.J.; Wise, L.S.; Berkowitz, R.; Wan, C.; Rubin, C.S. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J. Biol. Chem. 1988, 263, 9402–9408. [Google Scholar] [PubMed]
- Yu, Y.U.; Kang, S.Y.; Park, H.Y.; Sung, S.H.; Lee, E.J.; Kim, S.Y.; Kim, Y.C. Antioxidant lignans from Machilus thunbergii protect CCl4-injured primary cultures of rat hepatocytes. J. Pharm. Pharmacol. 2000, 52, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Seo, C.S.; Cho, E.S.; Kim, T.I.; Won, Y.S.; Kwon, H.J.; Son, J.K.; Son, H.Y. Meso-dihydroguaiaretic acid attenuates airway inflammation and mucus hypersecretion in an ovalbumin-induced murine model of asthma. Int. Immunopharmacol. 2016, 31, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.C.; Park, S.; Lee, K.Y.; Je, Y.T.; Yin, H.Q.; Choi, Y.J.; Sung, S.H.; Park, S.J.; Park, H.J.; Shin, K.J.; et al. LXR-alpha antagonist meso-dihydroguaiaretic acid attenuates high-fat diet-induced nonalcoholic fatty liver. Biochem. Pharmacol. 2014, 90, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kim, K.J.; Kim, D.; Lee, K.E.; Hwang, J.K. Meso-dihydroguaiaretic acid inhibits hepatic lipid accumulation by activating AMP-activated protein kinase in human HepG2 cells. Biol. Pharm. Bull. 2011, 34, 1628–1630. [Google Scholar] [CrossRef] [PubMed]
- Song, M.C.; Kim, E.C.; Kim, W.J.; Kim, T.J. Meso-dihydroguaiaretic acid inhibits rat aortic vascular smooth muscle cell proliferation by suppressing phosphorylation of platelet-derived growth factor receptor beta. Eur. J. Pharmacol. 2014, 744, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Mbata, O.; Abo El-Magd, N.F.; El-Remessy, A.B. Obesity, metabolic syndrome and diabetic retinopathy: Beyond hyperglycemia. World J. Diabetes 2017, 8, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol. 2000, 16, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Jeong, H.J.; Kang, T.H.; Shin, H.M.; Oh, S.T.; Choi, Y.; Jeon, S. Meso-dihydroguaiaretic acid induces apoptosis and inhibits cell migration via p38 activation and EGFR/Src/intergrin beta3 downregulation in breast cancer cells. Life Sci. 2015, 141, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Spiegelman, B.M. PPAR-gamma: Adipogenic regulator and thiazolidinedione receptor. Diabetes 1998, 47, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Pessin, J.E. Insulin regulation of glucose uptake: A complex interplay of intracellular signalling pathways. Diabetologia 2002, 45, 1475–1483. [Google Scholar] [PubMed]
- Watson, R.T.; Kanzaki, M.; Pessin, J.E. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr. Rev. 2004, 25, 177–204. [Google Scholar] [CrossRef] [PubMed]
- Cushman, S.W.; Wardzala, L.J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J. Biol. Chem. 1980, 255, 4758–4762. [Google Scholar]
- Ma, C.J.; Sung, S.H.; Kim, Y.C. Neuroprotective lignans from the bark of Machilus thunbergii. Planta Med. 2004, 70, 79–80. [Google Scholar] [PubMed]
- Choi, K.M.; Kim, W.; Hong, J.T.; Yoo, H.S. Dodeca-2(E),4(E)-dienoic acid isobutylamide enhances glucose uptake in 3T3-L1 cells via activation of Akt signaling. Mol. Cell. Biochem. 2017, 426, 9–15. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not Available. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.; Choi, K.-M.; Jung, W.-B.; Jeong, H.; Kim, G.-Y.; Lee, J.H.; Lee, M.K.; Hong, J.T.; Roh, Y.-S.; Sung, S.-H.; et al. Enhancement of Glucose Uptake by Meso-Dihydroguaiaretic Acid through GLUT4 Up-Regulation in 3T3-L1 Adipocytes. Molecules 2017, 22, 1423. https://doi.org/10.3390/molecules22091423
Lee A, Choi K-M, Jung W-B, Jeong H, Kim G-Y, Lee JH, Lee MK, Hong JT, Roh Y-S, Sung S-H, et al. Enhancement of Glucose Uptake by Meso-Dihydroguaiaretic Acid through GLUT4 Up-Regulation in 3T3-L1 Adipocytes. Molecules. 2017; 22(9):1423. https://doi.org/10.3390/molecules22091423
Chicago/Turabian StyleLee, Anna, Kyeong-Mi Choi, Won-Beom Jung, Heejin Jeong, Ga-Yeong Kim, Ju Hyun Lee, Mi Kyeong Lee, Jin Tae Hong, Yoon-Seok Roh, Sang-Hyun Sung, and et al. 2017. "Enhancement of Glucose Uptake by Meso-Dihydroguaiaretic Acid through GLUT4 Up-Regulation in 3T3-L1 Adipocytes" Molecules 22, no. 9: 1423. https://doi.org/10.3390/molecules22091423





