Discovery of Phylloseptins that Defense against Gram-Positive Bacteria and Inhibit the Proliferation of the Non-Small Cell Lung Cancer Cell Line, from the Skin Secretions of Phyllomedusa Frogs
Abstract
:1. Introduction
2. Results
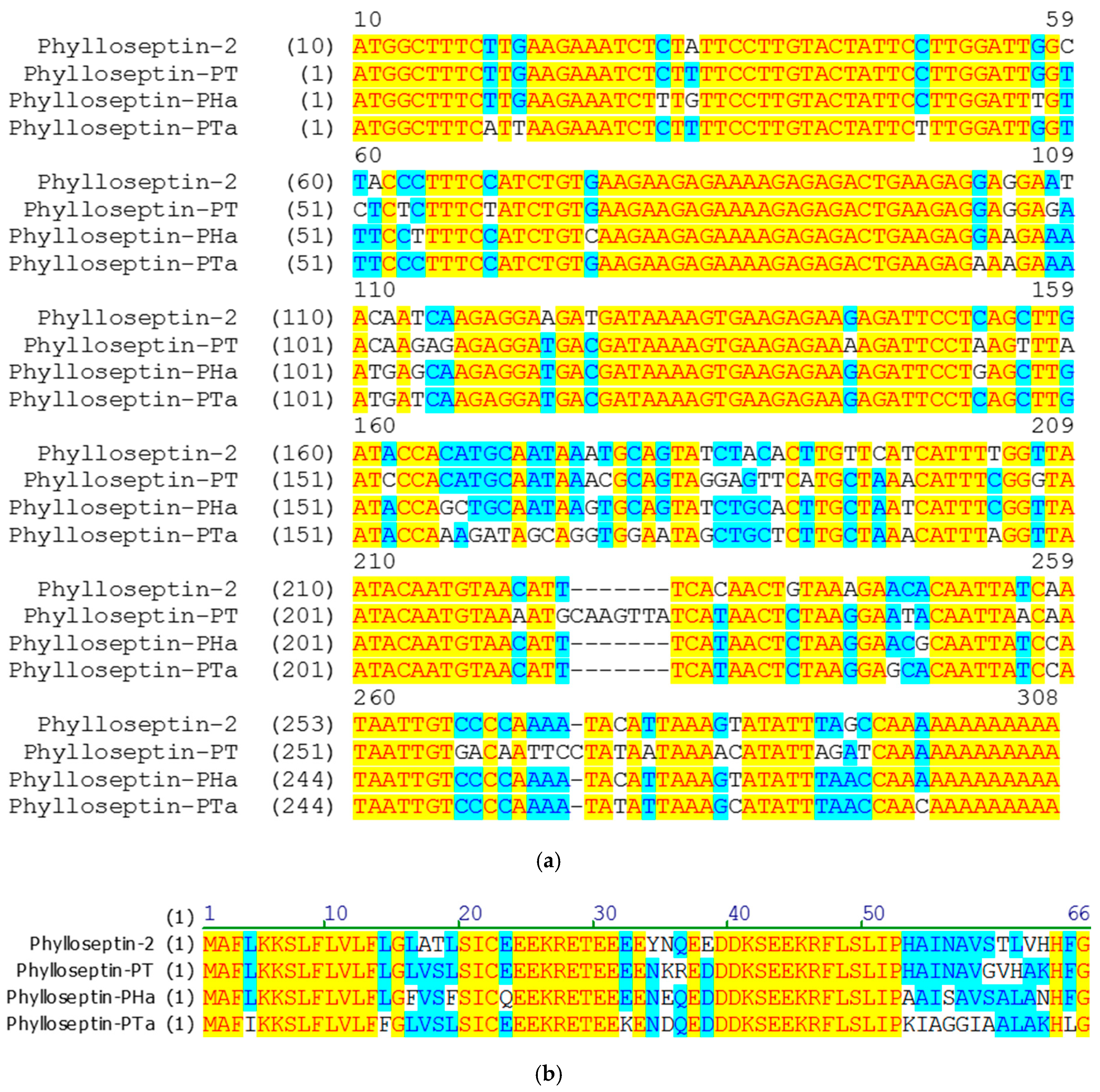
2.1. ‘Shotgun’ Cloning of cDNAs Encoding the Phylloseptin-PTa Precursor from the Skin Secretions of P. tarsius and the Phylloseptin-PHa Precursor from the Skin Secretions of P. hypochondrialis
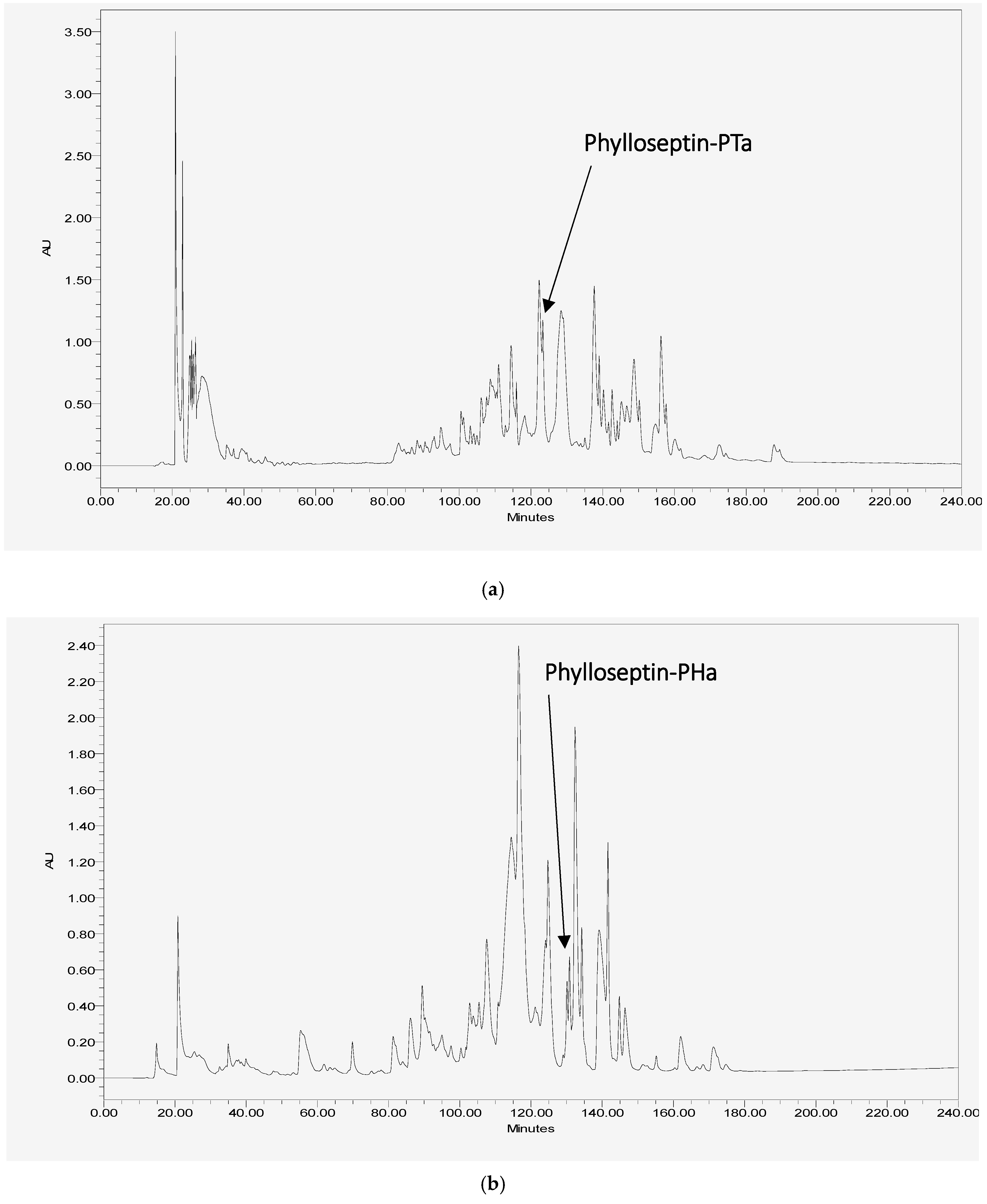
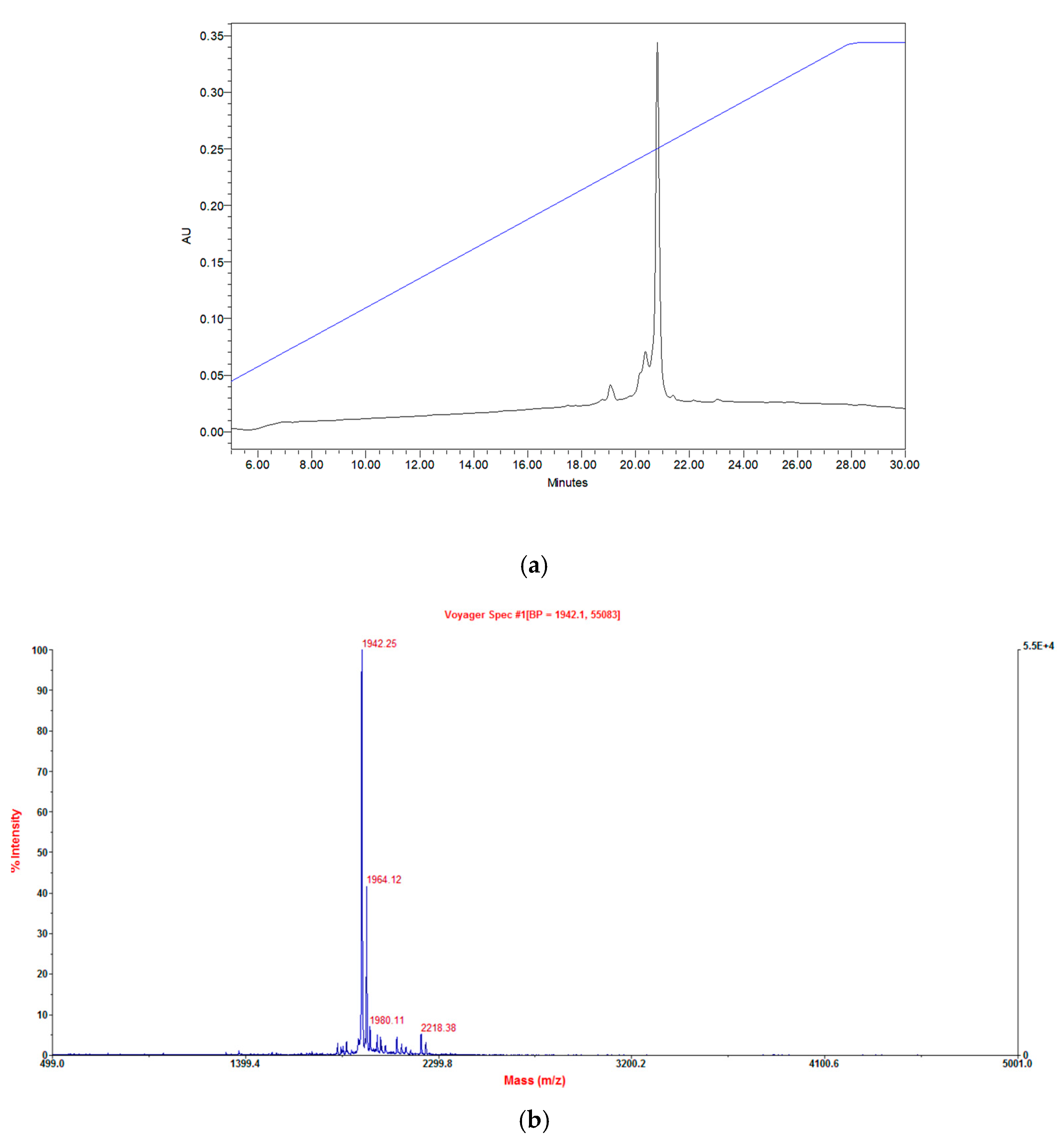
2.2. Isolation and Primary Structural Identification of Phylloseptin-PTa and Phylloseptin-PHa
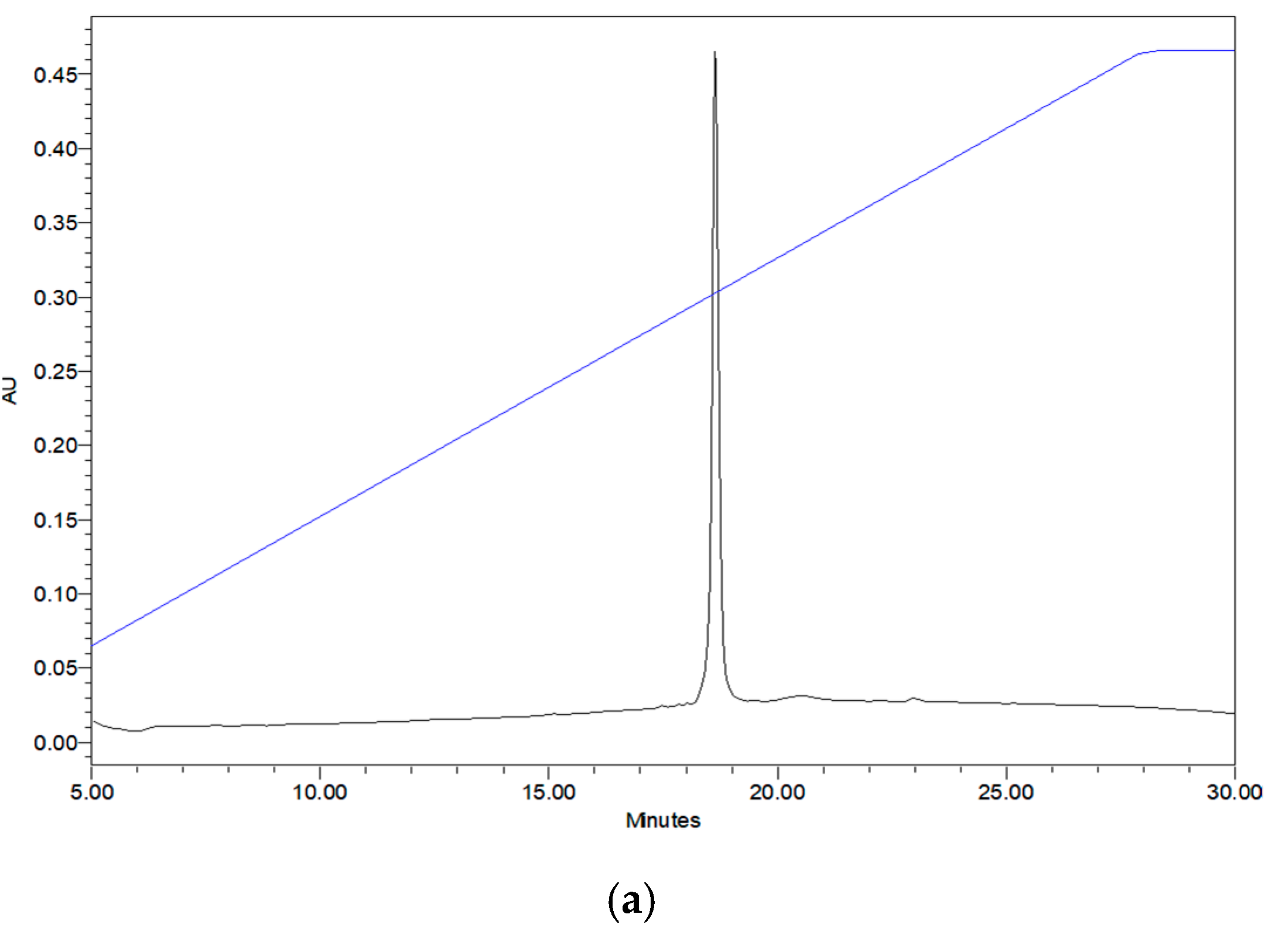
2.3. Verification of Synthetic Phylloseptin-PTa and Phylloseptin-PHa
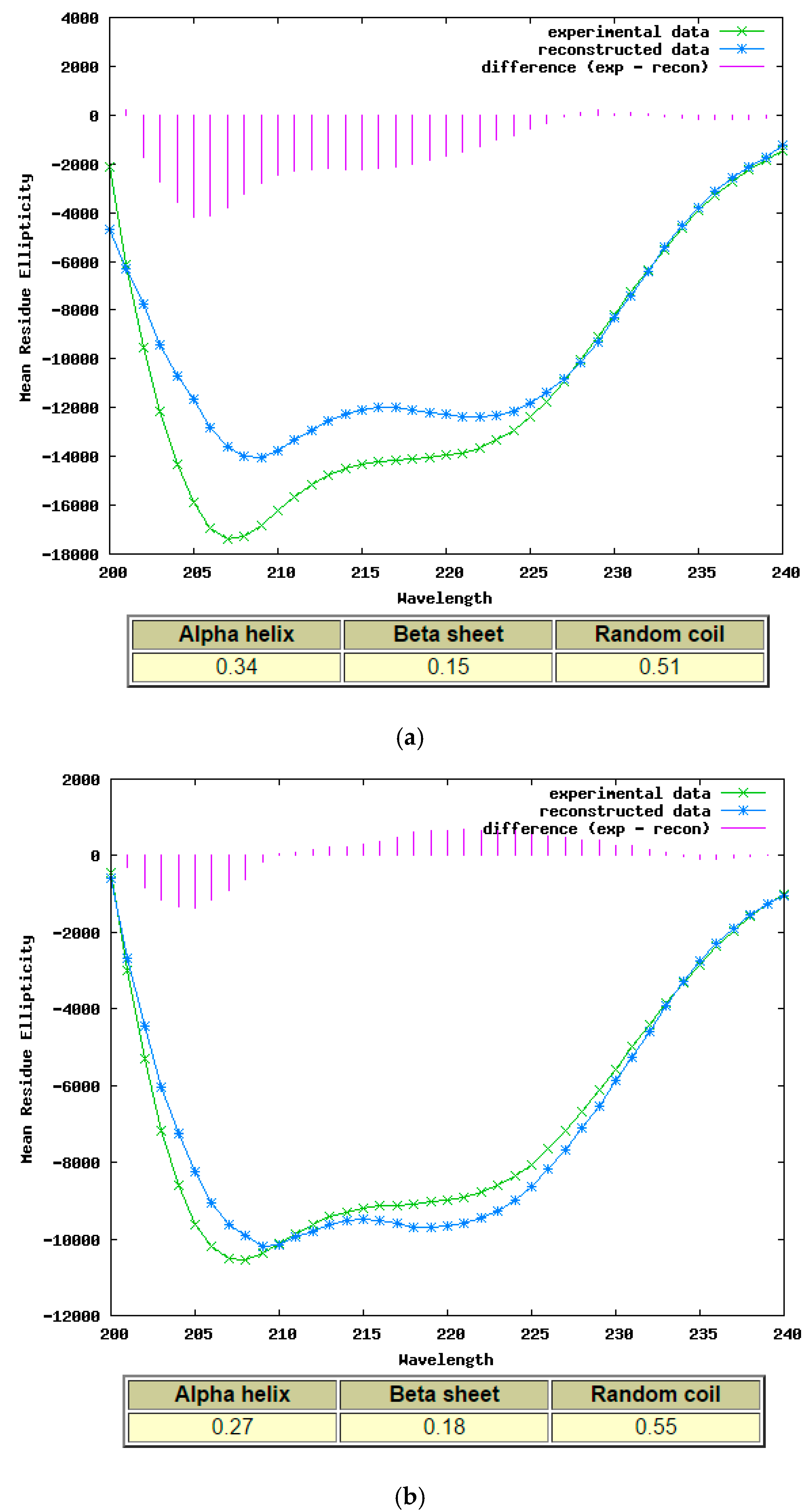
2.4. Secondary Structure Determination of Phylloseptin-PTa and Phylloseptin-PHa by Circular Dichroism
2.5. Antimicrobial and Haemolytic Activities of Synthetic Phylloseptin-PTa and Phylloseptin-PHa
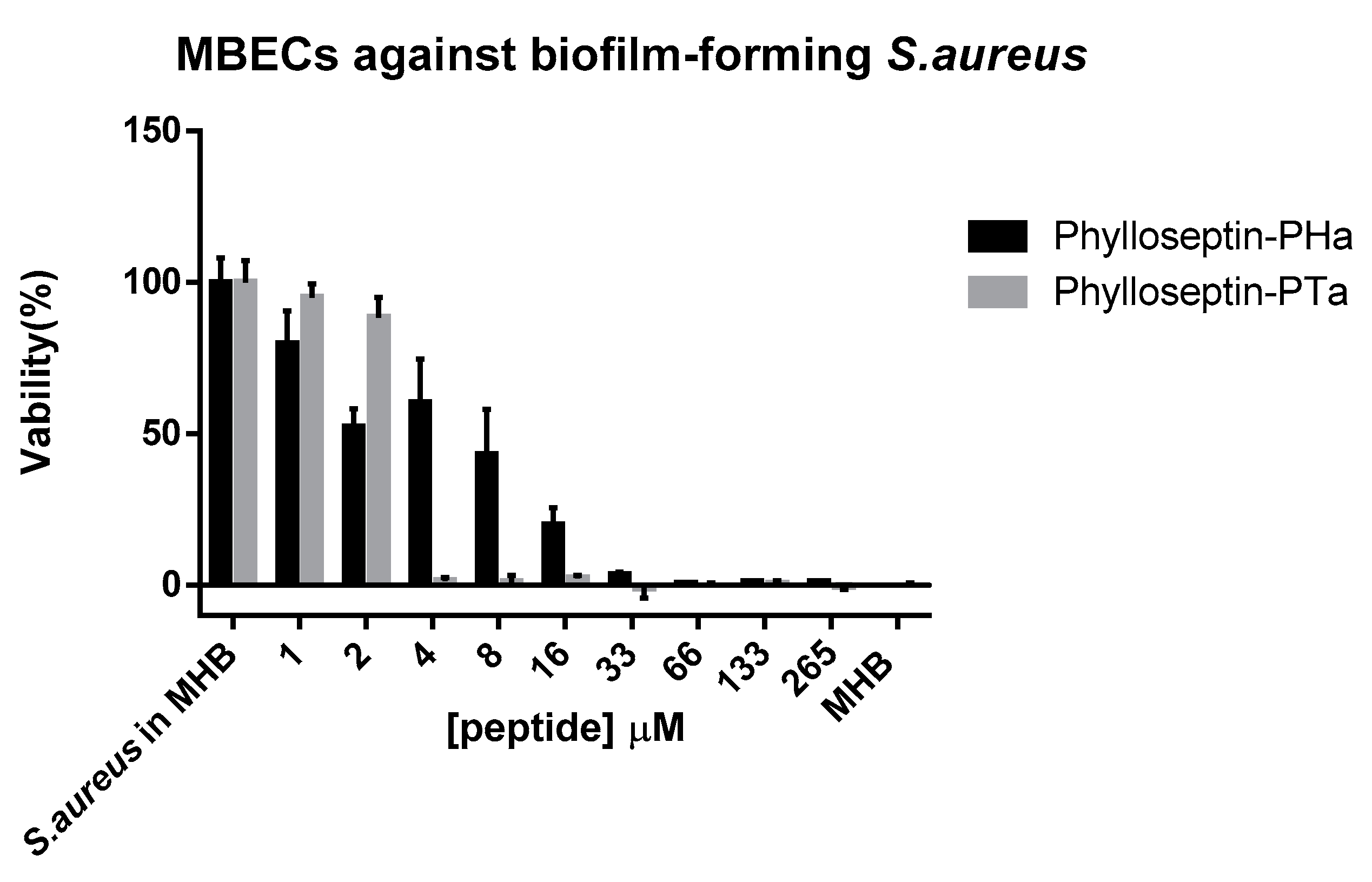
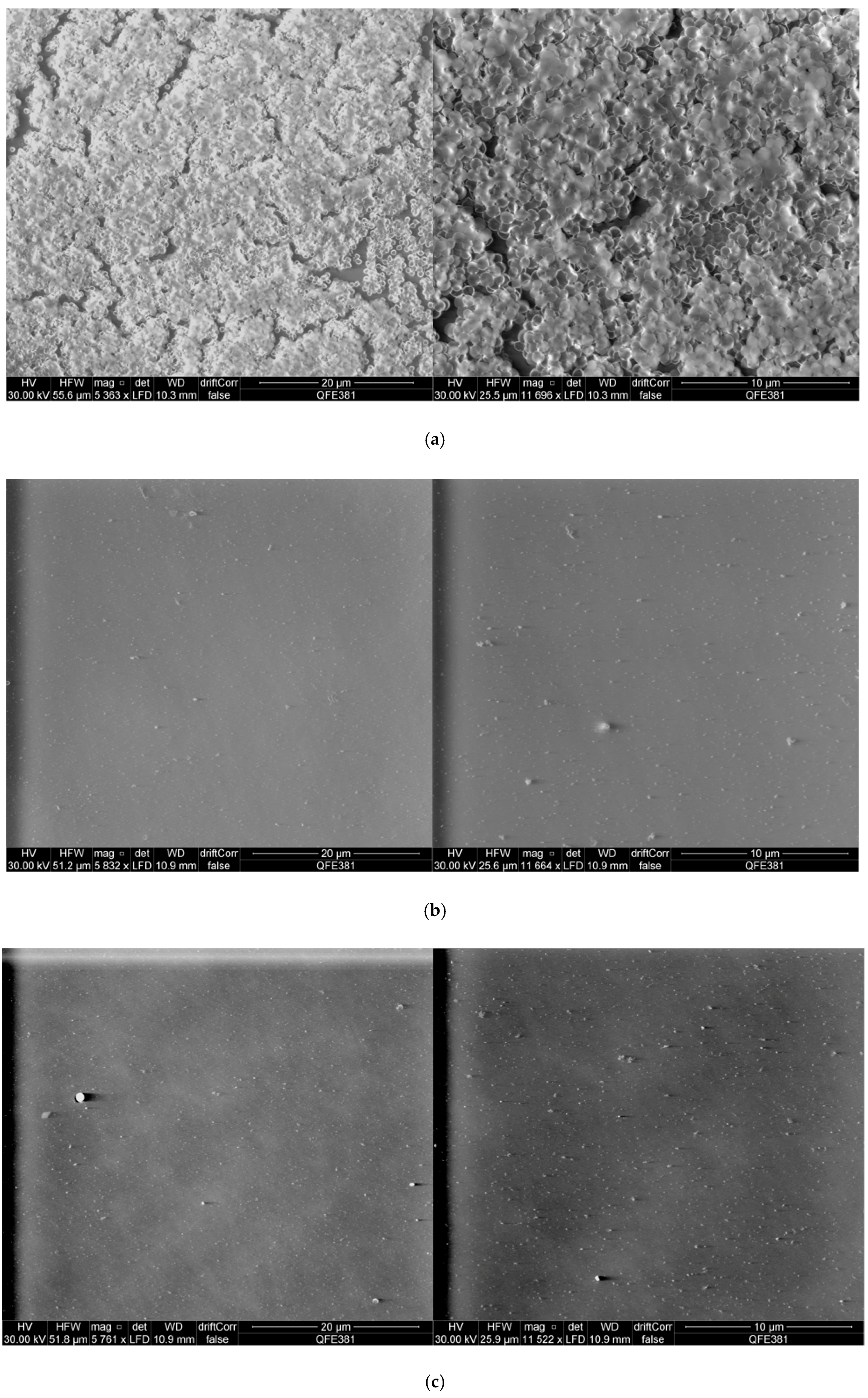
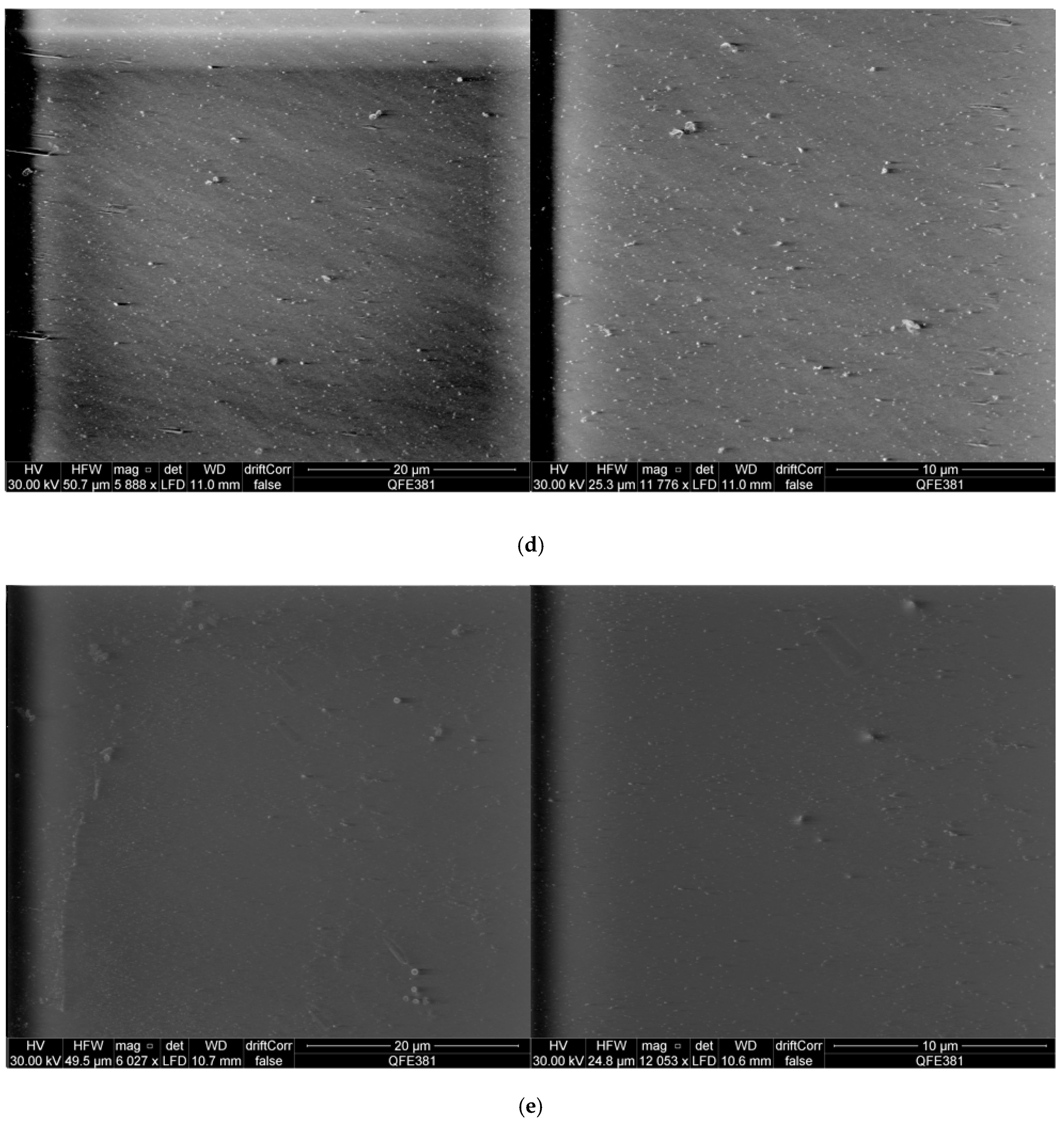
2.6. Scanning Electron Microscopy Observation of S. aureus Biofilms and Peptide Treated S. aureus Biofilms
2.7. Cytostatic Activities of Synthetic Phylloseptin-PTa and Phylloseptin-PHa on Non-Small Cell Lung Cancer Cells H157
2.8. Therapeutic Index of Phylloseptin-PTa and Phylloseptin-PHa
3. Discussion
4. Materials and Methods
4.1. Acquisition and Collection of Skin Secretions of P. tarsius and P. hypochondralis
4.2. ‘Shotgun’ Cloning of Prepropeptide Encoding cDNAs
4.3. Isolation and Identification of Novel Peptides and their Primary Structural Analysis
4.4. Solid-phase Peptide Synthesis of Novel Peptides
4.5. Secondary Structure Determination of Novel Peptides by Circular Dichroism
4.6. Antimicrobial Susceptibility Assays of Novel Peptides
4.7. Anti-Biofilm Activity using S. aureus Biofilm
4.8. Scanning Electron Microscopy Observation of Complete S. aureus Biofilms and S. aureus Biofilms Treated with Phylloseptin-PTa and Phylloseptin-PHa
4.9. Anticancer Activity Assays of Phylloseptin-PTa and Phylloseptin-PHa
4.10. Cytotoxicity Evaluations of Phylloseptin-PTa and Phylloseptin-PHa
4.11. Haemolysis Evaluations of Phylloseptin-PTa and Phylloseptin-PHa
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CL | cardiolipin |
| EDT | 1,2-ethonedithiol |
| MBEC | minimum biofilm eradication concentration |
| PC | phosphatidylcholine |
| PE | phosphatidylethanolamine |
| PG | phosphatidylglycerol |
| PS | phosphatidylserine |
| RP-HPLC | reversed-phase high performance liquid chromatography |
| TFA | trifluoroacetic acid |
| TFE | trifluoroethanol |
| TIS | thioanisole |
References
- Toledo, R.D.; Jared, C. Cutaneous granular glands and amphibian venoms. Comp. Biochem. Physiol. A Physiol. 1995, 111, 1–29. [Google Scholar] [CrossRef]
- Pukala, T.L.; Bowie, J.H.; Maselli, V.M.; Musgrave, I.F.; Tyler, M.J. Host-defence peptides from the glandular secretions of amphibians: structure and activity. Nat. Prod. Rep. 2006, 23, 368–393. [Google Scholar] [CrossRef] [PubMed]
- Bowie, J.H.; Separovic, F.; Tyler, M.J. Host-defense peptides of Australian anurans. Part 2. Structure, activity, mechanism of action, and evolutionary significance. Peptides 2012, 37, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Erspamer, V.; Melchiorri, P.; Erspamer, G.F.; Montecucchi, P.; De Castiglione, R. Phyllomedusa skin: A huge factory and store-house of a variety of active peptides. Peptides 1985, 6, 7–12. [Google Scholar] [CrossRef]
- Amiche, M.; Ladram, A.; Nicolas, P. A consistent nomenclature of antimicrobial peptides isolated from frogs of the subfamily Phyllomedusinae. Peptides 2008, 29, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
- Pyron, R.A.; Wiens, J.J. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol. Phylogenet. Evol. 2011, 61, 543–583. [Google Scholar] [CrossRef] [PubMed]
- Jackway, R.J.; Pukala, T.L.; Donnellan, S.C.; Sherman, P.J.; Tyler, M.J.; Bowie, J.H. Skin peptide and cDNA profiling of Australian anurans: Genus and species identification and evolutionary trends. Peptides 2011, 32, 161–172. [Google Scholar] [CrossRef] [PubMed]
- König, E.; Bininda-Emonds, O.R.; Shaw, C. The diversity and evolution of anuran skin peptides. Peptides 2015, 63, 96–117. [Google Scholar] [CrossRef] [PubMed]
- Vanhoye, D.; Bruston, F.; Nicolas, P.; Amiche, M. Antimicrobial peptides from hylid and ranin frogs originated from a 150-million-year-old ancestral precursor with a conserved signal peptide but a hypermutable antimicrobial domain. Eur. J. Biochem. 2003, 270, 2068–2081. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo Calderon, L.; Alexandre de Almeida, E.S.; Ciancaglini, P.; Stábeli, R.G. Antimicrobial peptides from Phyllomedusa frogs: From biomolecular diversity to potential nanotechnologic medical applications. Amino Acids 2011, 40, 29–49. [Google Scholar] [CrossRef] [PubMed]
- Mura, M.; Wang, J.; Zhou, Y.; Pinna, M.; Zvelindovsky, A.V.; Dennison, S.R.; Phoenix, D.A. The effect of amidation on the behaviour of antimicrobial peptides. Eur. Biophys. J. 2016, 45, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Wan, J.; Roginski, H.; Lee, A.; Shiell, B.; Michalski, W.; Coventry, M. Comparison of the effects of acylation and amidation on the antimicrobial and antiviral properties of lactoferrin. Lett. Appl. Microbiol. 2007, 44, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Ma, C.; Zhou, M.; Xi, X.; Li, L.; Wu, D.; Wang, L.; Lin, C.; Lopez, J.C.; Chen, T. Phylloseptin-PBa—A novel broad-spectrum antimicrobial peptide from the skin secretion of the peruvian purple-sided leaf frog (phyllomedusa baltea) which exhibits cancer cell cytotoxicity. Toxins (Basel) 2015, 7, 5182–5193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, N.; Li, L.; Wu, D.; Gao, Y.; Xi, X.; Zhou, M.; Wang, L.; Chen, T.; Shaw, C. Discovery of novel bacterial cell-penetrating phylloseptins in defensive skin secretions of the south american hylid frogs, phyllomedusa duellmani and phyllomedusa coelestis. Toxins (Basel) 2016, 8, 255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhou, M.; Wang, L.; McGrath, S.; Chen, T.; Chen, X.; Shaw, C. Phylloseptin-1 (PSN-1) from phyllomedusa sauvagei skin secretion: A novel broad-spectrum antimicrobial peptide with antibiofilm activity. Mol. Immunol. 2010, 47, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.R.S.; Silva, L.P.; Rodrigues, M.I.S.; Prates, M.V.; Brand, G.D.; Lacava, B.M.; Azevedo, R.B.; Bocca, A.L.; Albuquerque, S.; Bloch, C. Phylloseptins: A novel class of anti-bacterial and anti-protozoan peptides from the Phyllomedusa genus. Peptides 2005, 26, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Raja, Z.; André, S.; Piesse, C.; Sereno, D.; Nicolas, P.; Foulon, T.; Oury, B.; Ladram, A. Correction: Structure, antimicrobial activities and mode of interaction with membranes of bovel phylloseptins from the painted-belly leaf frog, Phyllomedusa sauvagii. PLoS ONE 2013, 8, e70782. [Google Scholar] [CrossRef]
- Pinto, E.G.; Pimenta, D.C.; Antoniazzi, M.M.; Jared, C.; Tempone, A.G. Antimicrobial peptides isolated from Phyllomedusa nordestina (Amphibia) alter the permeability of plasma membrane of Leishmania and Trypanosoma cruzi. Exp. Parasitol. 2013, 135, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, D.; Xi, X.; Wu, Y.; Ma, C.; Zhou, M.; Wang, L.; Yang, M.; Chen, T.; Shaw, C. Identification and characterisation of the Antimicrobial Peptide, Phylloseptin-PT, from the Skin Secretion of Phyllomedusa tarsius, and Comparison of Activity with Designed, Cationicity-Enhanced Analogues and Diastereomers. Molecules 2016, 21, 1667. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhou, M.; Gagliardo, R.; Walker, B.; Shaw, C. Elements of the granular gland peptidome and transcriptome persist in air-dried skin of the South American orange-legged leaf frog, Phyllomedusa hypocondrialis. Peptides 2006, 27, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Jacob, B.; Rajasekaran, G.; Kim, E.Y.; Park, I.-S.; Bang, J.-K.; Shin, S.Y. The stereochemical effect of SMAP-29 and SMAP-18 on bacterial selectivity, membrane interaction and anti-inflammatory activity. Amino Acids 2016, 48, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Mojsoska, B.; Carretero, G.; Larsen, S.; Mateiu, R.V.; Jenssen, H. Peptoids successfully inhibit the growth of gram negative E. coli causing substantial membrane damage. Sci. Rep. 2017, 7, 42332. [Google Scholar] [CrossRef] [PubMed]
- Schmidtchen, A.; Pasupuleti, M.; Malmsten, M. Effect of hydrophobic modifications in antimicrobial peptides. Adv. Colloid Interface Sci. 2014, 205, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.L.; Heath, G.R.; Johnson, B.R.; Abdel-Rahman, M.A.; Strong, P.N.; Evans, S.D.; Miller, K. Phospholipid dependent mechanism of smp24, an α-helical antimicrobial peptide from scorpion venom. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2737–2744. [Google Scholar] [CrossRef] [PubMed]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta Biomembr. 2016, 1858, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Papo, N.; Shai, Y. Host defense peptides as new weapons in cancer treatment. CMLS Cell. Mol. Life Sci. 2005, 62, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Veiga, A.S.; Castanho, M.A. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K. Control of cell selectivity of antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2009, 1788, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Park, M.J.; Ye, S.-K.; Kim, C.-W.; Kim, Y.-N. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am. J. Pathol. 2006, 168, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur. J. Pharmacol. 2009, 625, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Tyler, M.J.; Stone, D.J.; Bowie, J.H. A novel method for the release and collection of dermal, glandular secretions from the skin of frogs. J. Pharmacol. Toxicol. Methods 1992, 28, 199–200. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B.A. Protein secondary structure analyses from circular dichroism spectroscopy: Methods and reference databases. Biopolymers 2008, 89, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, L.; Wallace, B.A. Dichroweb: An online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004, 32, W668–W673. [Google Scholar] [CrossRef] [PubMed]
- Lobley, A.; Whitmore, L.; Wallace, B.A. Dichroweb: An interactive website for the analysis of protein secondary structure from circular dichroism spectra. Bioinformatics 2002, 18, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; Chacón, P.; Merelo, J.J.; Morán, F. Evaluation of secondary structure of proteins from UV circular dichroism using an unsupervised learning neural network. Protein Eng. Des. Sel. 1993, 6, 383–390. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds phylloseptin-PTa and phylloseptin-PHa are available from the authors. |

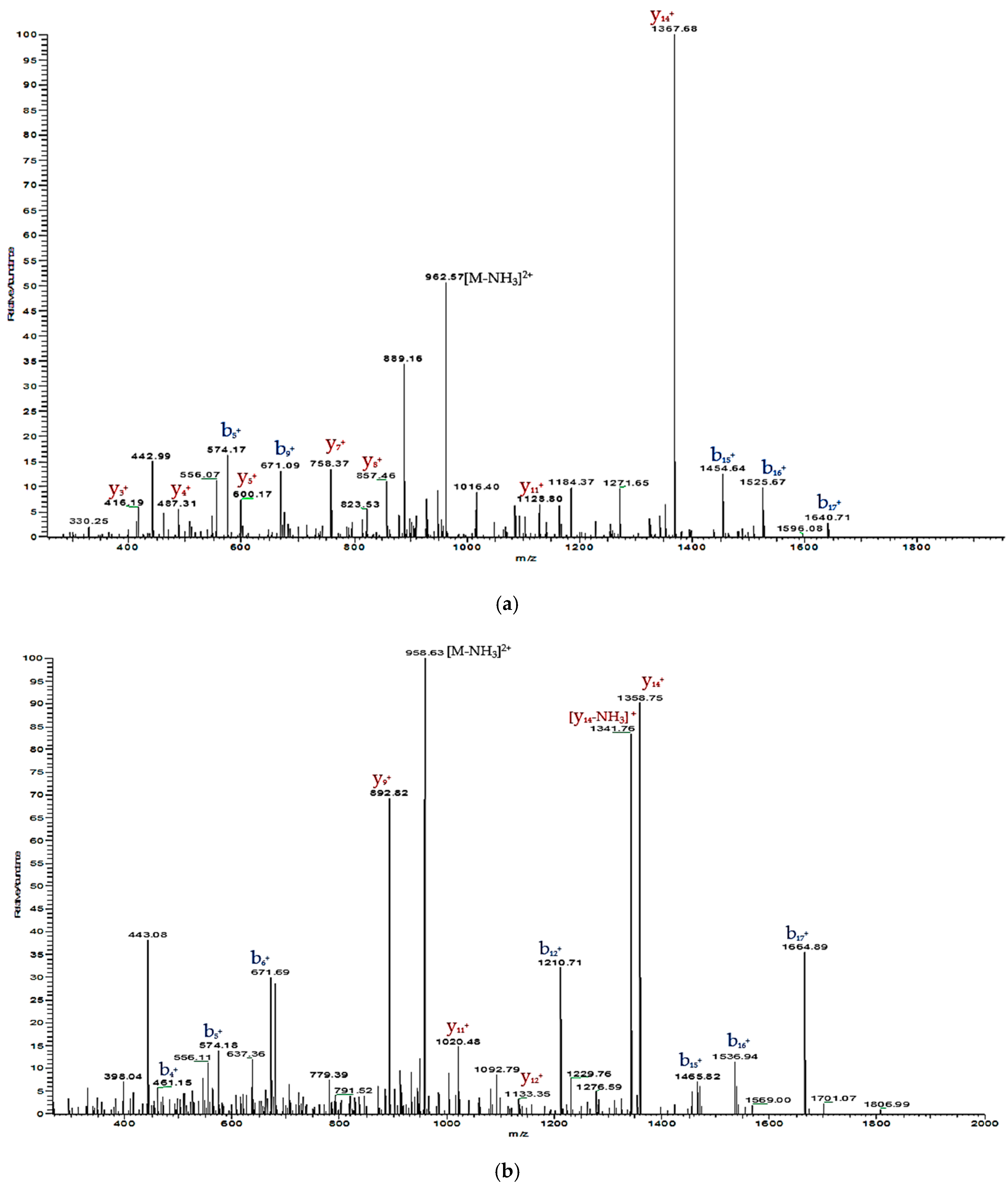


| #1 | b(1+) | b(2+) | Seq. | y(1+) | y(2+) | #2 |
|---|---|---|---|---|---|---|
| (a) | ||||||
| 1 | 148.08 | 74.54 | F | 19 | ||
| 2 | 261.16 | 131.08 | L | 1785.15 | 893.08 | 18 |
| 3 | 348.19 | 174.60 | S | 1672.06 | 836.54 | 17 |
| 4 | 461.15 | 231.14 | L | 1585.03 | 793.02 | 16 |
| 5 | 574.18 | 287.68 | I | 1471.95 | 736.48 | 15 |
| 6 | 671.69 | 336.21 | P | 1358.75 | 679.94 | 14 |
| 7 | 799.51 | 400.26 | K | 1261.81 | 631.41 | 13 |
| 8 | 912.59 | 456.80 | I | 1133.71 | 567.36 | 12 |
| 9 | 983.63 | 492.32 | A | 1020.71 | 510.82 | 11 |
| 10 | 1040.65 | 520.83 | G | 949.59 | 475.30 | 10 |
| 11 | 1097.67 | 549.34 | G | 892.82 | 446.79 | 9 |
| 12 | 1210.71 | 605.88 | I | 835.55 | 418.28 | 8 |
| 13 | 1281.79 | 641.40 | A | 722.47 | 361.74 | 7 |
| 14 | 1352.83 | 676.92 | A | 651.43 | 326.22 | 6 |
| 15 | 1465.82 | 733.46 | L | 580.39 | 290.70 | 5 |
| 16 | 1536.94 | 768.98 | A | 467.31 | 234.16 | 4 |
| 17 | 1664.89 | 833.03 | K | 396.27 | 198.64 | 3 |
| 18 | 1802.11 | 901.56 | H | 268.18 | 134.59 | 2 |
| 19 | L-Amidated | 131.12 | 66.06 | 1 | ||
| (b) | ||||||
| 1 | 148.08 | 74.54 | F | 19 | ||
| 2 | 261.16 | 131.08 | L | 1794.03 | 897.52 | 18 |
| 3 | 348.19 | 174.60 | S | 1680.94 | 840.98 | 17 |
| 4 | 461.28 | 231.14 | L | 1593.91 | 797.46 | 16 |
| 5 | 574.17 | 287.68 | I | 1480.83 | 740.92 | 15 |
| 6 | 671.09 | 336.21 | P | 1367.68 | 684.38 | 14 |
| 7 | 742.45 | 371.73 | A | 1270.69 | 635.85 | 13 |
| 8 | 813.49 | 407.25 | A | 1199.65 | 600.33 | 12 |
| 9 | 926.57 | 463.79 | I | 1128.80 | 564.81 | 11 |
| 10 | 1013.60 | 507.31 | S | 1015.53 | 508.27 | 10 |
| 11 | 1084.64 | 542.82 | A | 928.50 | 464.75 | 9 |
| 12 | 1183.71 | 592.36 | V | 857.46 | 429.24 | 8 |
| 13 | 1270.74 | 635.87 | S | 758.37 | 379.70 | 7 |
| 14 | 1341.78 | 671.39 | A | 671.36 | 336.18 | 6 |
| 15 | 1454.64 | 727.93 | L | 600.17 | 300.67 | 5 |
| 16 | 1525.67 | 763.45 | A | 487.31 | 244.12 | 4 |
| 17 | 1640.71 | 820.47 | N | 416.19 | 208.61 | 3 |
| 18 | 1777.00 | 889.00 | H | 302.16 | 151.58 | 2 |
| 19 | F-Amidated | 165.10 | 83.05 | 1 | ||
| MIC (µg∙mL−1/ µM) | Haemolysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Peptide | S. aureus NCTC 10788 | MRSA NCTC 12493 | E. faecalis NCTC 12697 | E. coli NCTC 10418 | C. albicans NCYC 1467 | % lysis at MIC 1 | HC10 (μM) | HC50 (μM) |
| Phylloseptin-PTa | 8/4.14 | 8/4.14 | 32/16.56 | 32/16.56 | 4/2.07 | 4.42% | 7.79 | 22.8 |
| Phylloseptin-PHa | 64/32.97 | 64/32.97 | 512/263.78 | >512/>263.78 | 256/131.89 | 2.52% | 76.5 | 109.5 |
| TI on Gram-Positive Bacteria | TI on S. aureus Biofilm | |
|---|---|---|
| Peptide | HC10 | HC10 |
| /GM | /MBEC | |
| Phylloseptin-PTa | 1.19 | 1.88 |
| Phylloseptin-PHa | 1.66 | 3.32 |
| MIC (µg∙mL−1/µM) | ||||||
|---|---|---|---|---|---|---|
| Phylloseptin-PTa | Phylloseptin-PHa | Phylloseptin-Du | Phylloseptin-Co | Phylloseptin-PBa | Phylloseptin-PT | |
| S. aureus | 8/4.14 | 64/32.97 | 8/3.90 | 8/4.06 | 8/3.99 | 512/264.33 |
| C. albicans | 4/2.07 | 256/131.89 | 16/7.81 | 16/8.12 | 8/3.99 | 512/264.33 |
| E. coli | 32/16.56 | >512/>263.78 | 128/62.45 | 128/64.93 | 128/63.84 | >512/>264.33 |
| MBEC (µg∙mL−1/µM) | ||||||
| S. aureus | 8/4.14 | 64/32.97 | 16/7.81 | 16/8.12 | nr 3 | nr 3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Wu, Q.; Li, L.; Xi, X.; Wu, D.; Zhou, M.; Chen, T.; Shaw, C.; Wang, L. Discovery of Phylloseptins that Defense against Gram-Positive Bacteria and Inhibit the Proliferation of the Non-Small Cell Lung Cancer Cell Line, from the Skin Secretions of Phyllomedusa Frogs. Molecules 2017, 22, 1428. https://doi.org/10.3390/molecules22091428
Liu J, Wu Q, Li L, Xi X, Wu D, Zhou M, Chen T, Shaw C, Wang L. Discovery of Phylloseptins that Defense against Gram-Positive Bacteria and Inhibit the Proliferation of the Non-Small Cell Lung Cancer Cell Line, from the Skin Secretions of Phyllomedusa Frogs. Molecules. 2017; 22(9):1428. https://doi.org/10.3390/molecules22091428
Chicago/Turabian StyleLiu, Jia, Qing Wu, Lei Li, Xinping Xi, Di Wu, Mei Zhou, Tianbao Chen, Chris Shaw, and Lei Wang. 2017. "Discovery of Phylloseptins that Defense against Gram-Positive Bacteria and Inhibit the Proliferation of the Non-Small Cell Lung Cancer Cell Line, from the Skin Secretions of Phyllomedusa Frogs" Molecules 22, no. 9: 1428. https://doi.org/10.3390/molecules22091428






