New Antifeedant Grayanane Diterpenoids from the Flowers of Pieris formosa
Abstract
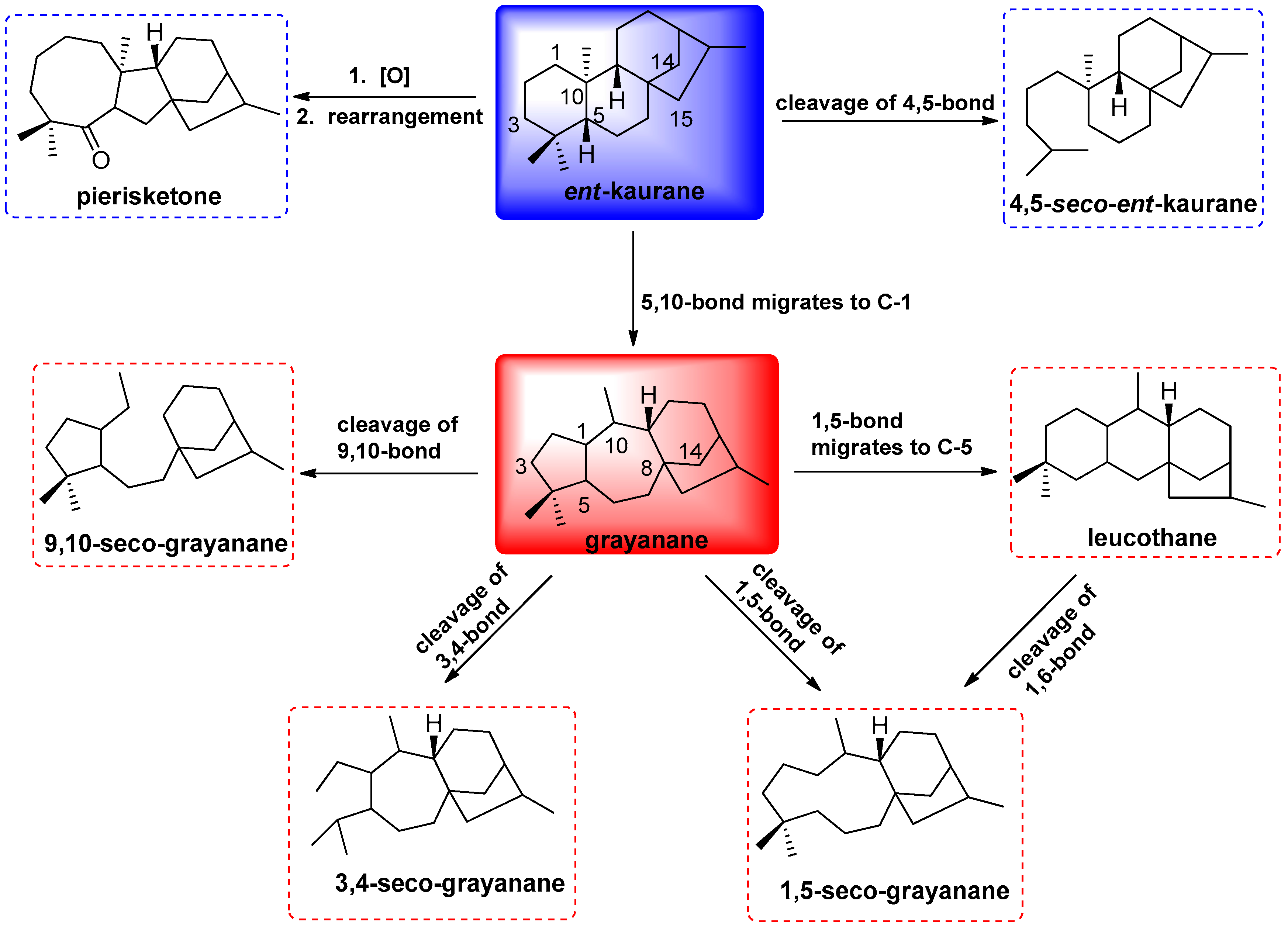
:1. Introduction
2. Results
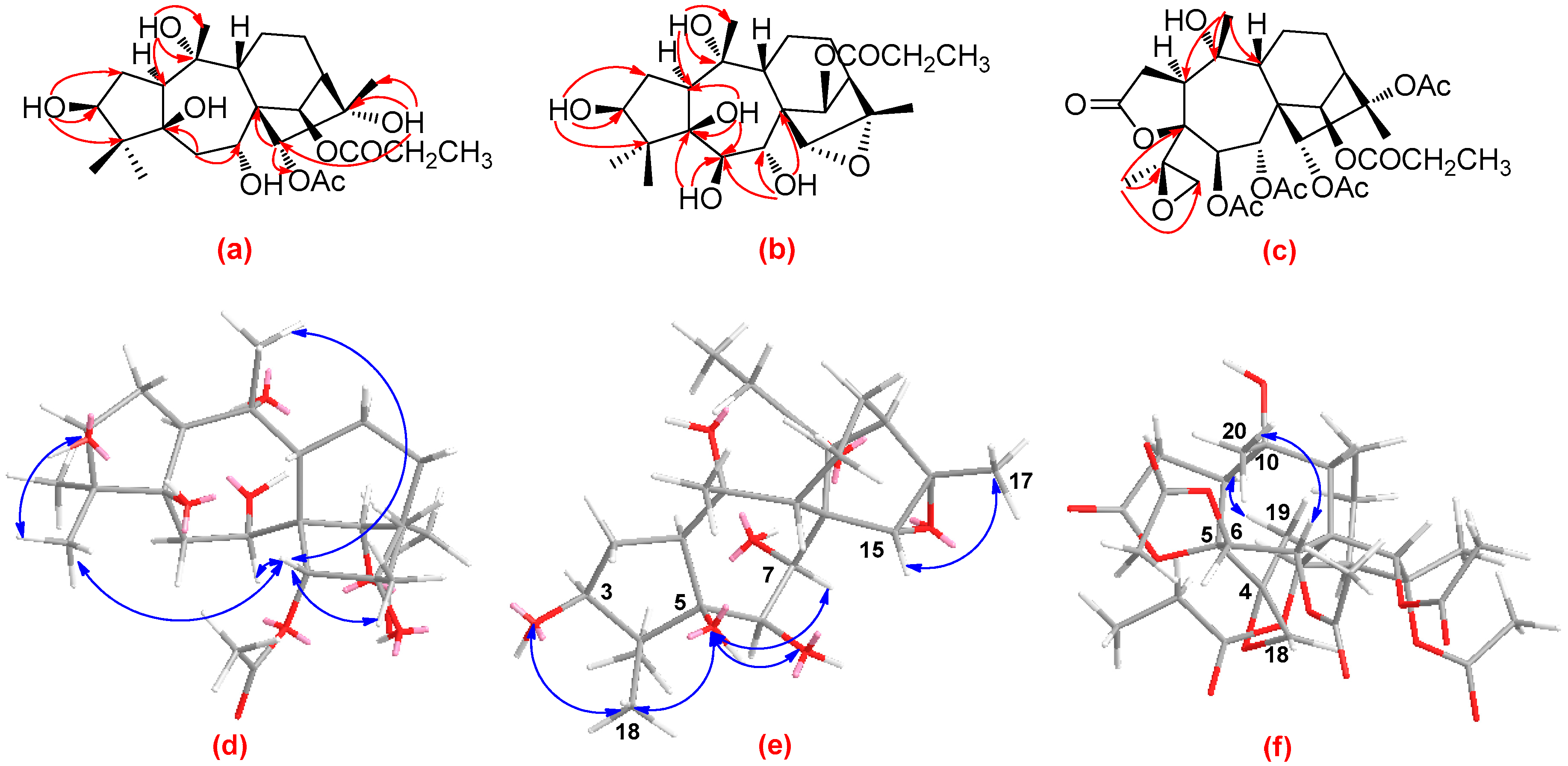
2.1. Structural Elucidation of Compounds 1–3
2.2. Antifeedant Activity of Compounds 1, 3, 4, and 10
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Materials
4.3. Extraction and Isolation
4.4. Antifeedant Activity
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cao, L.; Li, Y.; Li, H.; Liu, D.; Li, R. New 3,4-seco-Grayanane Diterpenoids from the Flowers of Pieris japonica. Chem. Pharm. Bull. 2016, 64, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.B.; Yu, S.S. Grayanoids from the Ericaceae family: Structures, biological activities and mechanism of action. Phytochem. Rev. 2013, 12, 305–325. [Google Scholar] [CrossRef]
- Niu, C.-S.; Li, Y.; Liu, Y.-B.; Ma, S.-G.; Li, L.; Qu, J.; Yu, S.-S. Analgesic diterpenoids from the twigs of Pieris formosa. Tetrahedron 2016, 72, 44–49. [Google Scholar] [CrossRef]
- Chen, X.; Gao, L.; Li, Y.; Li, H.; Liu, D.; Liao, X.; Li, R. Highly Oxygenated Grayanane Diterpenoids from Flowers of Pieris japonica and Their Structure-Activity Relationship of Antifeedant Activity Against Pieris brassicae. J. Agric. Food Chem. 2017, 65, 4456–4463. [Google Scholar] [CrossRef] [PubMed]
- Im, N.-K.; Zhou, W.; Na, M.; Jeong, G.-S. Pierisformoside B exhibits neuroprotective and anti-inflammatory effects in murine hippocampal and microglial cells via the HO-1/Nrf2-mediated pathway. Int. Immunopharmacol. 2015, 24, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.G.; Li, H.M.; Li, H.Z.; Wu, Z.Y.; Li, R.T. New grayanol diterpenoid and new phenolic glucoside from the flowers of Pieris formosa. J. Asian Nat. Prod. Res. 2010, 12, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Lin, S.; Zhu, C.G.; Yang, Y.C.; Li, S.A.; Zhang, J.J.; Chen, X. G.; Shi, J. G. Highly Acylated Diterpenoids with a New 3,4-Secograyanane Skeleton from the Flower Buds of Rhododendron molle. Org. Lett. 2010, 12, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Li, H.Z.; Wang, W.G.; Li, H.M.; Chen, R.; Li, R.T.; Luo, H.R. Lyonin A, a new 9,10-Secograyanotoxin from Lyonia ovalifolia. Chem. Biodivers. 2011, 8, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.-B.; Zhang, J.-J.; Li, Y.-H.; Jiang, J.-D.; Yu, S.-S.; Ma, S.-G.; Qu, J.; Lv, H.-N. Mollolide A, a Diterpenoid with a new 1, 10:2,3-disecograyanane skeleton from the roots of Rhododendron molle. Org. Lett. 2013, 15, 3074–3077. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Q.; Chen, S.-N.; Cheng, K.-F.; Li, C.-J.; Qin, G.-W. Diterpene glucosides from Pieris formosa. Phytochemistry 2000, 54, 847–852. [Google Scholar] [CrossRef]
- Burke, J.W.; Doskotch, R.W.; Ni, C.Z.; Clardy, J. Kalmanol, a pharmacologically active diterpenoid with a new ring skeleton from Kalmia angustifolia L. J. Am. Chem. Soc. 1989, 111, 5831–5833. [Google Scholar] [CrossRef]
- Zhou, S.-Z.; Yao, S.; Tang, C.; Ke, C.; Li, L.; Lin, G.; Ye, Y. Diterpenoids from the flowers of Rhododendron molle. J. Nat. Prod. 2014, 77, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, Y.; Zhan, G.; Shu, P.; Sa, R.; Lei, L.; Xiang, M.; Xue, Y.; Luo, Z.; Wan, Q. Micranthanone A, a new diterpene with an unprecedented carbon skeleton from Rhododendron micranthum. Org. Lett. 2013, 15, 3094–3097. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.-B.; Liu, Y.-L.; Wang, C.; Wu, L.-Q.; Li, L.; Ma, S.-G.; Qu, J.; Yu, S.-S. Mollanol A, a diterpenoid with a new C-nor-D-homograyanane skeleton from the fruits of Rhododendron molle. Org. Lett. 2014, 16, 4320–4323. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.-B.; Yan, H.-M.; Liu, Y.-L.; Li, Y.-H.; Lv, H.-N.; Ma, S.-G.; Qu, J.; Yu, S.-S. Rhodomollins A and B, two Diterpenoids with an Unprecedented Backbone from the Fruits of Rhododendron molle. Sci. Rep. 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Q.; Qin, G.-W.; Chen, S.-N.; Li, C.-J. Three diterpene glucosides and a diphenylamine derivative from Pieris formosa. Fitoterapia 2001, 72, 779–787. [Google Scholar] [CrossRef]
- Niu, C.-S.; Li, Y.; Liu, Y.-B.; Ma, S.-G.; Liu, F.; Li, L.; Xu, S.; Wang, X.-J.; Wang, R.-B.; Qu, J. Pierisketolide A and Pierisketones B and C, Three Diterpenes with an Unusual Carbon Skeleton from the Roots of Pieris formosa. Org. Lett. 2017, 19, 906–909. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhan, G.; Zhang, H.; Zhang, Q.; Li, Y.; Xue, Y.; Yao, G. Rhodomollanol A, a Highly Oxygenated Diterpenoid with a 5/7/5/5 Tetracyclic Carbon Skeleton from the Leaves of Rhododendron molle. Org. Lett. 2017, 19, 3935–3938. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Q.; Chen, S.N.; Qin, G.W.; Cheng, K.F. Grayanane diterpenoids from Pieris formosa. J. Nat. Prod. 1998, 61, 1473–1475. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-H.; Niu, X.-M.; Luo, Q.; Xie, M.-J.; Luo, S.-H.; Zhou, Y.-Y.; Li, S.-H. Novel polyesterified 3,4-seco-grayanane diterpenoids as antifeedants from Pieris formosa. Org. Lett. 2010, 12, 2426–2429. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Q.; Ding, B.Y.; Zhao, W.M.; Qin, G.W. Grayanane diterpenoids from Pieris formosa. Chin. Chem. Lett. 1998, 9, 465–467. [Google Scholar]
- Wang, L.-Q.; Ding, B.-Y.; Qin, G.-W.; Lin, G.; Cheng, K.-F. Grayanoids from Pieris formosa. Phytochemistry 1998, 49, 2045–2048. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Li, H.Z.; Wang, W.G.; Li, X.L.; Li, R.T. Secopieristoxins A and B, two unusual diterpenoids with a new 9,10-secograyanane skeleton from the fruits of Pieris formosa. Phytochem. Lett. 2012, 5, 87–90. [Google Scholar] [CrossRef]
- Wang, W.G.; Wu, Z.Y.; Chen, R.; Li, H.Z.; Li, H.M.; Li, Y.D.; Li, R.T.; Luo, H.R. Pierisformotoxins A–D, Polyesterified Grayanane Diterpenoids from Pieris formosa and Their cAMP-Decreasing Activities. Chem. Biodivers. 2013, 10, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Li, Y.D.; Wu, G.S.; Luo, H.R.; Li, H.M.; Li, R.T. Three New Highly Acylated 3,4-seco-Grayanane Diterpenoids from the Fruits of Pieris formosa. Chem. Pharm. Bull. 2011, 59, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Hikino, H.; Ogura, M.; Fushiya, S.; Konno, C.; Takemoto, T. Stereostructure of asebotoxin VI, VIII, and IX, toxins of Pieris japonica. Chem. Pharm. Bull. 1977, 25, 523–524. [Google Scholar] [CrossRef]
- Wang, S.; Lin, S.; Zhu, C.; Yang, Y.; Li, S.; Zhang, J.; Chen, X.; Shi, J. Highly acylated diterpenoids with a new 3,4-secograyanane skeleton from the flower buds of Rhododendron molle. Org. Lett. 2010, 12, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Li, H.M.; Li, Y.D.; Li, H.Z.; Li, R.T. Highly Acylated 3,4-Secograyanane Diterpenoids from the Fruits of Pieris formosa. Helv. Chim. Acta 2011, 94, 1283–1289. [Google Scholar] [CrossRef]
- Katai, M.; Fujiwara, M.; Terai, T.; Meguri, H. Studies on the Constituents of the Leaves of Pieris japonica D. DON. Chem. Pharm. Bull. 1980, 28, 3124–3126. [Google Scholar] [CrossRef]
- Zhou, C.; Li, X.; Li, H.; Li, R. Chemical constituents from the leaves of Craibiodendron yunnanens. Biochem. Syst. Ecol. 2012, 45, 179–182. [Google Scholar] [CrossRef]
- Li, C.H.; Jing, S.X.; Luo, S.H.; Shi, W.; Hua, J.; Liu, Y.; Li, X.N.; Schneider, B.; Gershenzon, J.; Li, S.H. Peltate Glandular Trichomes of Colquhounia coccinea var. mollis Harbor a New Class of Defensive Sesterterpenoids. Org. Lett. 2013, 15, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Liu, Y.; Hua, J.; Luo, S.H.; Li, S.H. Peltate glandular trichomes of Colquhounia seguinii harbor new defensive clerodane diterpenoids. J. Integr. Plant Biol. 2014, 56, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.H.; Luo, Q.A.; Niu, X.M.; Xie, M.J.; Zhao, X.; Schneider, B.; Gershenzon, J.; Li, S.H. Glandular Trichomes of Leucosceptrum canum Harbor Defensive Sesterterpenoids. Angew. Chem. Int. Edit. 2010, 49, 4471–4475. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-M.; Zhang, C.-C.; Zhang, Q.; Shafiq, N.; Pescitelli, G.; Li, D.-W.; Gao, J.-M. Wightianines A-E, Dihydro-beta-agarofuran Sesquiterpenes from Parnassia wightiana, and Their Antifungal and Insecticidal Activities. J. Agric. Food Chem. 2014, 62, 6669–6676. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–13 are available from the authors. |
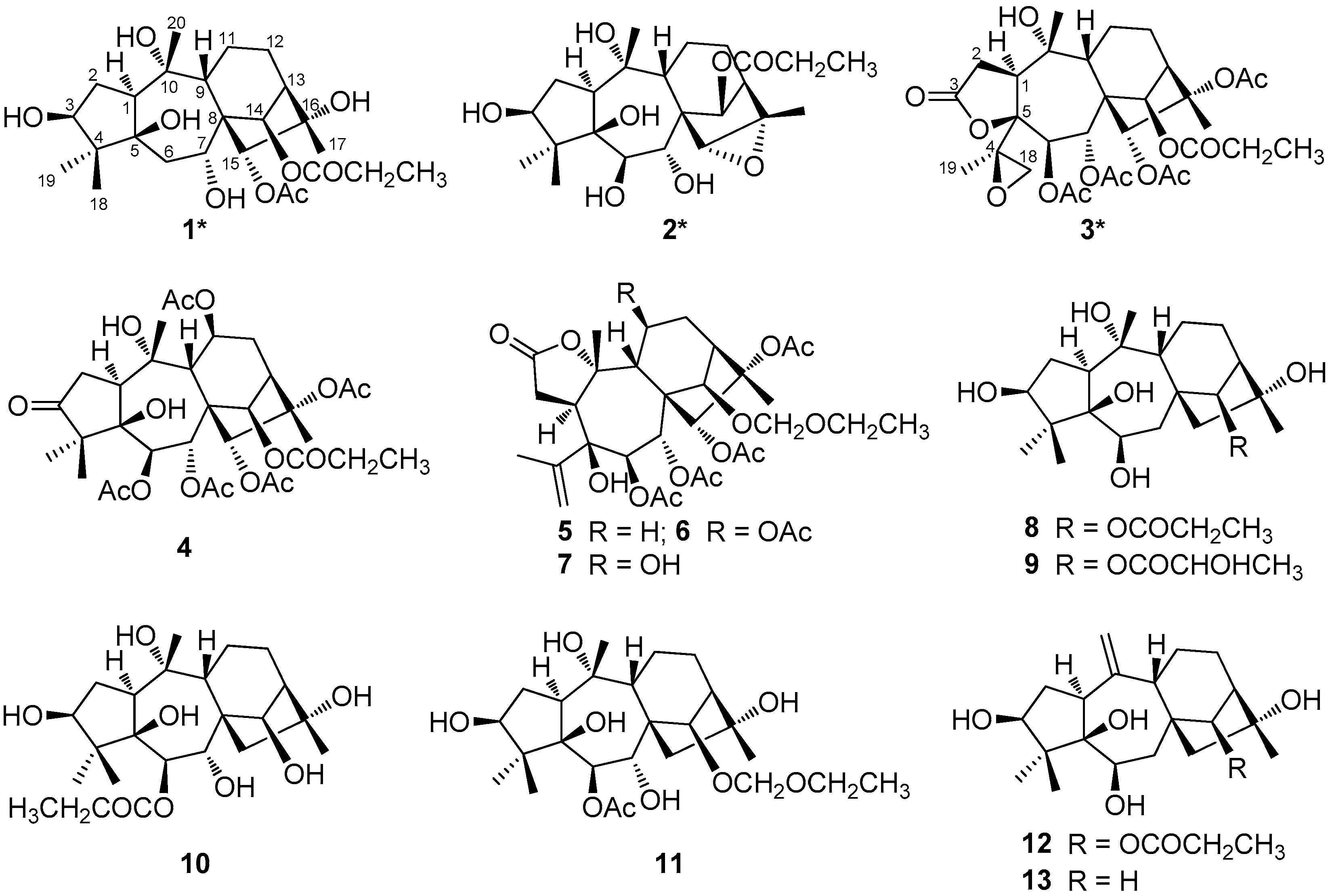
| No. | 1 a | 2 b | 3 | |||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 1 | 2.74 dd (11.6, 5.2) | 50.9 d | 2.77 m | 50.9 d | 3.08 m | 52.6 d |
| 2a 2b | 2.06 m 2.27 m | 35.4 t | 2.05 m 2.22 m | 35.9 t | 2.47 m 2.73 dd (18.9, 11.8) | 32.8 t |
| 3 | 3.60 t (5.0) | 82.5 d | 3.53 dd (1.5, 5.1) | 83.0 d | 174.0 s | |
| 4 | 51.4 s | 52.3 s | 62.5 s | |||
| 5 | 84.1 s | 83.1 s | 87.5 s | |||
| 6 | 2.05 m (2H) | 34.0 t | 3.91 overlap | 78.2 d | 6.04 d (9.6) | 69.8 d |
| 7 | 3.62 t (5.0) | 72.4 d | 3.41 overlap | 78.3 d | 5.23 d (9.7) | 68.7 d |
| 8 | 54.5 s | 53.8 s | 56.1 s | |||
| 9 | 2.08 m | 54.6 d | 2.08 m | 49.9 d | 2.32 m | 47.0 d |
| 10 | 78.4 s | 77.7 s | 77.4 s | |||
| 11a 11b | 1.58 m 1.90 m | 21.8 t | 1.71 m 1.81 m | 21.6 t | 1.98 m 2.03 m | 21.1 t |
| 12a 12b | 1.58 m 2.23 m | 27.0 t | 1.68 m 2.35 m | 28.1 t | 1.74 m 2.08 m | 25.8 t |
| 13 | 2.05 overlap | 52.9 d | 2.24 m | 47.6 d | 3.10 overlap | 46.4 d |
| 14 | 5.59 s | 82.1 d | 5.52 s | 76.4 d | 6.43 s | 79.3 d |
| 15 | 4.93 s | 85.0 d | 3.26 s | 68.6 d | 5.27 s | 86.2 d |
| 16 | 79.6 s | 61.3 s | 88.4 s | |||
| 17 | 1.27 s (3H) | 22.8 q | 1.45 s (3H) | 14.6 q | 1.55 s (3H) | 19.4 q |
| 18 | 1.11 s (3H) | 18.5 q | 1.23 s (3H) | 19.3 q | 2.49 d (5.0) 2.98 d (4.9) | 52.8 t |
| 19 | 0.91 s (3H) | 23.0 q | 0.97 s (3H) | 23.2 q | 1.34 s (3H) | 17.4 q |
| 20 | 1.41 s (3H) | 27.8 q | 1.41 s (3H) | 28.2 q | 1.51 s (3H) | 33.6 q |
| 6-OAc | 2.02 s (3H) | 20.6 q | ||||
| 169.4 s | ||||||
| 7-OAc | 2.10 s (3H) | 21.6 q | ||||
| 169.8 s | ||||||
| 14-OPr | 1.11 t (3H, 7.6) 2.85 m (2H) | 9.6 q | 1.08 t (3H, 7.6) 2.27 m (2H) | 9.5 q | 1.08 t (3H, 7.5) 2.36 m (2H) | 9.1 q |
| 28.4 t | 28.5 t | 28.2 t | ||||
| 173.4 s | 173.8 s | 174.0 s | ||||
| 15-OAc | 2.10 s (3H) | 21.0 q | 1.98 s (3H) | 20.8 q | ||
| 170.3 s | 171.6 s | |||||
| 16-OAc | 1.98 s (3H) | 22.7 q | ||||
| 169.8 s | ||||||
| Compound | Molecular Formula | m/z | EC50 (µg/cm2) |
|---|---|---|---|
| 1 | C25H40O9 | 484 | 10.91 |
| 3 | C31H42O14 | 638 | 33.89 |
| 4 | C33H46O15 | 682 | NA |
| 10 | C23H38O8 | 442 | 6.58 |
| Neem oil | - | - | 3.71 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-H.; Luo, S.-H.; Li, S.-H.; Gao, J.-M. New Antifeedant Grayanane Diterpenoids from the Flowers of Pieris formosa. Molecules 2017, 22, 1431. https://doi.org/10.3390/molecules22091431
Li C-H, Luo S-H, Li S-H, Gao J-M. New Antifeedant Grayanane Diterpenoids from the Flowers of Pieris formosa. Molecules. 2017; 22(9):1431. https://doi.org/10.3390/molecules22091431
Chicago/Turabian StyleLi, Chun-Huan, Shi-Hong Luo, Sheng-Hong Li, and Jin-Ming Gao. 2017. "New Antifeedant Grayanane Diterpenoids from the Flowers of Pieris formosa" Molecules 22, no. 9: 1431. https://doi.org/10.3390/molecules22091431




