Identification of Polar Constituents in the Decoction of Juglans mandshurica and in the Medicated Egg Prepared with the Decoction by HPLC-Q-TOF MS2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the Sample Preparation Procedure and the HPLC-Q-TOF-MS Conditions
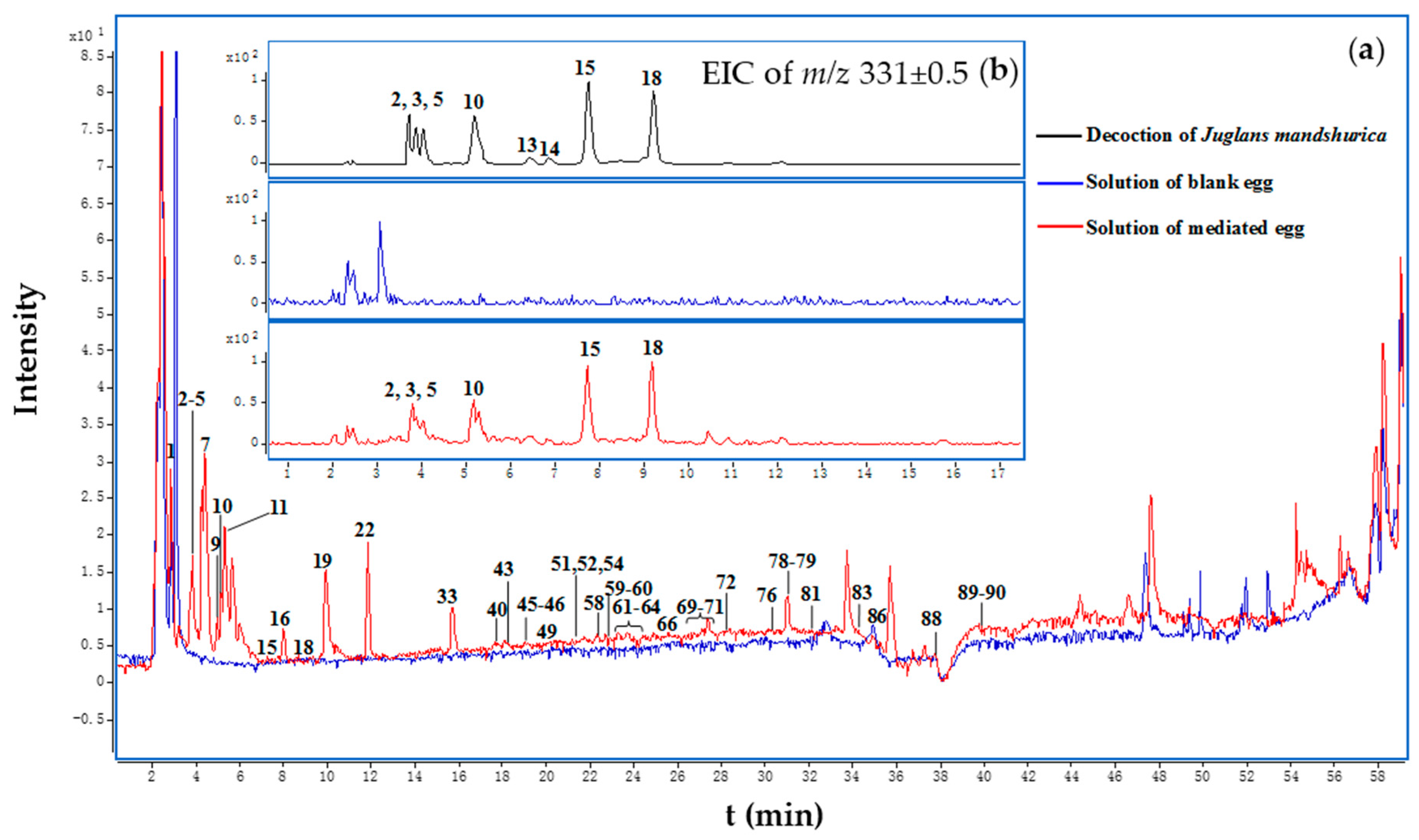
2.2. Identification of Compounds in Juglans Mandshurica Decoction
2.3. Identification of Compounds in Medicated Eggs Decocted with the Juglans Mandshurica Decoction
3. Materials and Methods
3.1. Chemicals
3.2. Plant Materials
3.3. Reference Compounds and the Preparation of Reference Solutions
3.4. Preparation of the Decoction of Juglans Mandshurica
3.5. Preparation of Blank and Medicated Eggs and Blank and Medicated Egg Solutions
3.6. HPLC-Q-TOF-MS2 System and Conditions
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuang, K.R.; Li, P.Q. Juglandaceae. In Flora of China; Scientific Publisher: Beijing, China, 2013; Volume 21, pp. 32–33. [Google Scholar]
- Li, G.; Cui, J.M.; Kwon, Y.; Seo, C.S.; Lee, C.S.; Woo, M.H.; Lee, E.S.; Jahng, Y.; Chang, H.W.; Lee, S.H.; et al. Two new diarylheptanoids from Juglans mandshurica. Bull. Korean Chem. Soc. 2005, 26, 1878–1880. [Google Scholar] [CrossRef]
- Xin, N.; Hasan, M.; Li, W.; Li, Y. Juglans mandshurica Maxim extracts exhibit antitumor activity on HeLa cells in vitro. Mol. Med. Rep. 2014, 9, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.F.; Ji, J.F.; Zhan, M.; Cui, Y.Q.; Zhang, Y.L. Effect of flavones extract from Juglans mandshurica maxim stem-barks on p73-gene expression in hepatocarcinoma cell Bel-7402. Shandong Med. J. 2010, 50, 10–12. [Google Scholar]
- Liu, C.; Zhou, X.M.; Sun, F.F.; Gao, X.Z. Investigation into the anti-tumor activity of Juglans mandshurica Maxim. extracts. J. Shenyang Pharm. Univ. 2013, 30, 799–802. [Google Scholar] [CrossRef]
- Wang, T.M.; Yu, W.J.; Zhu, L.F.; Di, X.; Zhai, Y.J.; Chu, Z.Y.; Zhang, H.; Kang, T.G. Anti-tumor activity of the EtOH extracts of different parts of Juglans mandshurica. Lishizhen Med. Mater. Med. Res. 2017, 28, 570–572. [Google Scholar] [CrossRef]
- Min, B.S.; Kwon, O.K.; Park, B.Y.; Kim, Y.H.; Hattori, M.; Joung, H.; Lee, H.K. Apoptosis-inducing activity of galloylglucoses from Juglans mandshurica in human promyeloid leukemic HL-60 cells. Nat. Prod. Sci. 2004, 10, 48–53. [Google Scholar]
- Min, B.S.; Lee, H.K.; Lee, S.M.; Kim, Y.H.; Bae, K.H.; Otake, T.; Nakamura, N.; Hattori, M. Anti-human immunodeficiency virus-type 1 activity of constituents from Juglans mandshurica. Arch Pharm. Res. 2002, 25, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Min, B.S.; Lee, S.Y.; Kim, J.H.; Lee, J.K.; Kim, T.J.; Kim, D.H.; Kim, Y.H.; Joung, H.; Lee, H.K.; Nakamura, N.; et al. Anti-complement activity of constituents from the stem-bark of Juglans mandshurica. Biol. Pharm. Bull. 2003, 26, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, P.S.; Zou, Z.X.; Zou, H.; Long, H.P.; Tan, L.H.; Liu, R.H.; Wang, Y.K.; Xu, K.P.; Tan, G.S. Three new compounds from the roots of Juglans mandshurica Maxim. Phytochem. Lett. 2017, 20, 40–44. [Google Scholar] [CrossRef]
- Chen, G.; Pi, X.M.; Yu, C.Y. A new naphthalenone isolated from the green walnut husks of Juglans mandshurica Maxim. Nat. Prod. Res. 2015, 29, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, K.P.; Zou, Z.X.; Zou, H.; Long, H.P.; Tan, L.H.; Liu, R.H.; Wang, Y.K.; Xu, P.S.; Tan, G.S. Two new compounds from the green peel of Juglans mandshurica. J. Asian Nat. Prod. Res. 2017, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.L.; Lin, H.; Bao, Y.L.; Sun, L.G.; Zhao, W.; Hou, Y.Q.; Song, Z.B.; Tian, S.Y.; Li, Y.X.; Liu, B. JA a new type of polyunsaturated fatty acid isolated from Juglans mandshurica Maxim, limits the survival and induces apoptosis of heptocarcinoma cells. Apoptosis 2016, 21, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, Y.W.; Zheng, L.H.; Meng, X.Y.; Bao, Y.L.; Wu, Y.; Yu, C.L.; Huang, Y.X.; Li, Y.X. Anthracene and Anthraquinone Derivatives from the Stem Bark of Juglans mandshurica Maxim. Helv. Chim. Acta 2011, 94, 1488–1495. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Yang, B.Y.; Liu, Z.X.; Jiang, Y.Q.; Liu, Y.X.; Fu, L.; Wang, X.L.; Kuang, H.X. Cytotoxicity of triterpenes from green walnut husks of Juglans mandshurica Maxim in HepG-2 cancer cells. Molecules 2015, 20, 19252–19262. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, N.; Yoo, G.; Park, J.H.; Kim, S.H. Four new neolignans from the fruits of Juglans mandshurica Maxim. Planta Med. 2016, 81, S1–S381. [Google Scholar] [CrossRef]
- Cheng, Z.Y.; Yao, G.D.; Guo, R.; Huang, X.X.; Song, S.J. Phenylpropanoids from Juglans mandshurica exhibit cytotoxicities on liver cancer cell lines through apoptosis induction. Bioorg. Med. Chem. Lett. 2017, 27, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.L.; Yu, X.F.; Qu, S.C.; Sui, D.Y. Juglone, isolated from Juglans mandshurica Maxim, induces apoptosis via down-regulation of AR expression in human prostate cancer LNCaP cells. Bioorg. Med. Chem. Lett. 2013, 23, 3631–3634. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.L.; Yu, X.F.; Qu, S.C.; Qu, X.R.; Jiang, Y.F.; Sui, D.Y. Juglone, from Juglans mandshurica Maxim, inhibits growth and induces apoptosis in human leukemia cell HL-60 through a reactive oxygen species-dependent mechanism. Food Chem. Toxicol. 2012, 50, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Barathi, S.; Vardhini, R.D.S.; Chitra, P.; Arulselvi, P.I. Cytotoxic effect of juglone on human peripheral blood lymphocytes. Asian J. Pharm. Clin. Res. 2013, 6, 178–186. [Google Scholar]
- Paulsen, M.T.; Ljungman, M. The natural toxin juglone causes degradation of p53 and induces rapid H2AX phosphorylation and cell death in human fibroblasts. Toxicol. Appl. Pharmacol. 2005, 209, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aynehchi, Y.; Dehpour, A.R.; Mahmoodian, M. Juglone: The echtiotoxic principle of Pterocarya fraxinifolia. Phytochemistry 1973, 12, 3001–3002. [Google Scholar] [CrossRef]
- Patricia, S. A tribute to You you Tu and artemisinin. Travel Med. Infect. Dis. 2015, 13, 513–514. [Google Scholar] [CrossRef]
- Yu, X.H.; Liu, L.L.; Cong, S.; Wang, J.; Yan, P.Y. Effect of the decoction of Juglans Maxim’s branch on cytokines, telomerase and cath-D levels in S180 tumor-bearing mice. J. Zhejiang Univ. Tradit. Chin. Med. 2015, 39, 139–143. [Google Scholar] [CrossRef]
- Dong, Y.Y.; Luo, L. The therapic effects of Juglans mandshurica branch on esophageal cancer. Zhongguo Minjian Liaofa 2000, 8, 46. [Google Scholar]
- Wang, T.M.; Yu, W.J.; Fu, Y.; Di, X.; Zhai, Y.J.; Zhang, H.; Chen, H.B. Inhibitory effect of the eggs decocted with branches of Juglans mandshurica on solid tumor of murine H22 hepatocarcinoma cell in mice. Drugs Clin. 2017, 32, 365–369. [Google Scholar] [CrossRef]
- Wang, T.M.; Yu, W.J.; Zhu, L.F.; Di, X.; Zhai, Y.J.; Chu, Z.Y.; Zhang, H.; Kang, T.G. Anti-tumor activity of the EtOH extracts of different parts of Juglans mandshurica. Northwest Pharm. J. (in press).
- Wang, T.M.; Liu, L.; Di, X.; Xu, L.; Kang, T.G.; Chen, H.B. Influence of different drying methods on juglone content in stem of Juglans mandshurica. Chin. J. Hosp. Pharm. 2011, 31, 1461–1463. [Google Scholar]
- Wang, T.M.; Yu, W.J.; Di, X.; Xu, Y.Y.; Zhang, H.; Chu, Z.Y.; Zhai, Y.J. In vivo anti-tumor activity of Juglans mandshurica water extract with or without juglone. Northwest Pharm. J. 2017, in press. [Google Scholar]
- Wolfender, J.L.; Marti, G.; Thomas, A.; Bertrand, S. Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, T.M.; Di, X.; Zhang, H.; Chu, Y.; Zhai, Y.J. Optimization of the extraction technology of drug-contained eggs cooked with branches of Juglans mandshurica. Chin. J. Ethnomed. Ethnopharm. 2016, 25, 24–28. [Google Scholar]
- Barreto, J.C.; Trevisan, M.T.S.; Hull, W.E.; Erben, G.; Brito, E.S.D.; Pfundstein, B.; Wüertele, G.; Spiegelhalder, B.; Owen, R.W. Characterization and Quantitation of Polyphenolic Compounds in Bark, Kernel, Leaves, and Peel of Mango (Mangifera indica L.). J. Agric. Food Chem. 2008, 56, 5599–5610. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.M.; Liu, J.; Yi, T.; Zhai, Y.J.; Zhang, H.; Chen, H.B.; Cai, S.Q.; Kang, T.G.; Zhao, Z.Z. Multi-constituent identification in root, branch, and leaf extracts of Juglans mandshurica using ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.H.; Huang, J.W.; Liu, Y.H.; Yuan, K. Separation, antioxidant and antimicrobial activities of chemical constituents from exocarp of Juglans mandshurica Maxim. Asian J. Chem. 2013, 25, 3361–3365. [Google Scholar]
- Han, L.K.; Li, W.; Narimatsu, S.; Liu, L.J.; Fu, H.W.; Okuda, H.; Koike, K. Inhibitory effects of compounds isolated from fruit of Juglans mandshurica on pancreatic lipase. J. Nat. Med. 2007, 61, 184–186. [Google Scholar] [CrossRef]
- Machida, K.; Yogiashi, Y.; Matsuda, S.; Suzuki, A.; Kikuchi, M. A new phenolic glycoside syringate from the bark of Juglans mandshurica MAXIM. var. sieboldiana MAKINO. J. Nat. Med. 2009, 63, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.H.; Du, X.W.; Sun, G.D.; Meng, Y.L.; Wang, W.M. Comparison of the chemical profiles of fresh-raw and dry-processed Juglans mandshurica. J. Sep. Sci. 2017, 40, 646–662. [Google Scholar] [CrossRef] [PubMed]
- Min, B.S.; Nakamura, N.; Miyashiro, H.; Kim, Y.H.; Hattori, M. Inhibition of human immunodeficiency virus type 1 reverse transcriptase and ribonuclease H activities by constituents of Juglans mandshurica. Chem. Pharm. Bull. 2000, 48, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.L.; Pu, Y.Q.; Zhang, T.; Tao, J.S. Determination of hydrolysate gallic acid in Yinzhihuang Injection by HPLC. Chin. Tradit. Herb Drugs 2011, 42, 288–290. [Google Scholar]
- Liu, L.J.; Li, W.; Koike, K.; Zhang, S.J.; Nikaido, T. New α-tetralonyl glucosides from the fruit of Juglans mandshurica. Chem. Pharm. Bull. 2004, 52, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.L.; Song, Z.Q.; Fang, G.Z.; Li, S.Z.; Yuan, H.J. Extraction of Juglone from Bark of Juglans mandshurica Maxim. by Vacuum Distillation. Chem. Ind. For. Prod. 2007, 27, 113–115. [Google Scholar]
- Al-Sayed, E.; Singab, A.N.; Ayoub, N.; Martiskainen, O.; Sinkkonen, J.; Pihlaja, K. HPLC-PDA-ESI-MS/MS profiling and chemopreventive potential of Eucalyptus gomphocephala DC. Food Chem. 2012, 133, 1017–1024. [Google Scholar] [CrossRef]
- Gomez-Caravaca, A.M.; Verardo, V.; Segura-Carretero, A.; Caboni, M.F.; Fernandez-Gutierrez, A. Development of a rapid method to determine phenolic and other polar compounds in walnut by capillary electrophoresis-electrospray ionization time-of-flight mass spectrometry. J. Chromatogr. A 2008, 1209, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Reham, H.M.; María, D.M.C.; Mohamed, R.E.G.; Azza, R.A.M.; Essam, A.S.; Antonio, S.C. Profiling of phenolic and other compounds from Egyptian cultivars of chickpea (Cicer arietinum L.) and antioxidant activity: A comparative study. RSC Adv. 2015, 5, 17751–17767. [Google Scholar] [CrossRef]
- Yao, D.L.; Zhang, C.H.; Luo, J.; Jin, M.; Zheng, M.S.; Cui, J.M.; Son, J.K.; Li, G. Chemical constituents from the leaves of Juglans mandshurica. Arch. Pharm. Res. 2015, 38, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Jiang, Y.Q.; Meng, Y.; Liu, Y.X.; Liu, Z.X.; Xiao, H.B.; Wang, X.L.; Zhou, Y.Y. Chemical constituents in n-butanol extracts from epicarp of green fruit of Juglans mandshurica. Chin. Tradit. Herb Drugs 2015, 46, 481–485. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, K.S.; Son, J.K.; Je, G.H.; Lee, J.S.; Lee, C.H.; Cheong, C.J. Cytotoxic Compounds from the Roots of Juglans mandshurica. J. Nat. Prod. 1998, 61, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Li, W.; Koike, K.; Nikaido, T. Two new naphthalenyl glucosides and a new phenylbutyric acid glucoside from the fruit of Juglans mandshurica. Heterocycles 2004, 63, 1429–1436. [Google Scholar] [CrossRef]
- Shi, J.H.; Wang, J.H.; Yuan, Z.; Che, D.; Song, Y.R.; Li, X. Chemical constituents of Juglans mandshurica Maxim. J. Shenyang Pharm. Univ. 2006, 23, 501–504. [Google Scholar] [CrossRef]
- Pedro, M.; Luca, C.; Chiara, D.A.; Gianni, G.; Cristina, G.V.; Renato, B.; Alan, C.; Daniele, D.R. Rapid and Comprehensive Evaluation of (Poly) phenolic Compounds in Pomegranate (Punica granatum L.) Juice by UHPLC-MSn. Molecules 2012, 17, 14821–14840. [Google Scholar] [CrossRef]
- Liu, L.J.; Satou, T.; Koike, K.; Li, W.; Nikaido, T. Studies on the cytotoxicity of compounds from fruits of Juglans mandshurica. Nat. Med. 2004, 58, 226–229. [Google Scholar]
- Li, J.; Sun, J.X.; Yu, H.Y.; Chen, Z.Y.; Zhao, X.Y.; Ruan, H.L. Diarylheptanoids from the root bark of Juglans cathayensis. Chin. Chem. Lett. 2013, 24, 521–523. [Google Scholar] [CrossRef]
- Li, G.; Lee, S.Y.; Lee, K.S.; Lee, S.W.; Kim, S.H.; Lee, S.H.; Lee, C.S.; Woo, M.H.; Son, J.K. DNA topoisomerases I and II inhibitory activity of constituents isolated from Juglans mandshurica. Arch. Pharm. Res. 2003, 26, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.F.; Wang, H. Study on microwave hydrolysis for the analysis of amino acid in egg by HPLC performance liquid chromatography. Chin. J. Chromatogr. 1997, 15, 138–140. [Google Scholar]
- Wang, T.M.; Wang, R.F.; Chen, H.B.; Shang, M.Y.; Cai, S.Q. Alkyl and phenolic glycosides from Saussurea stella. Fitoterapia 2013, 88, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Hatano, T.; Okuda, T.; Memon, M.U.; Shingu, T.; Kenichiro, I. Spectral and chromatographic analyses of tannins. I. 13C Nuclear magnetic resonance spectra of hydrolyzable tannins. Chem. Pharm. Bull. 1984, 32, 1790–1799. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Peak | tR (min) | m/z 1, [M − H]− | Formula | Major Fragments (Negative-Ion Mode) 2 | Identification 3 | Absorbed by Medicated Eggs | |||
|---|---|---|---|---|---|---|---|---|---|
| Measured | Mass Error (ppm) | Peak Area in Medicated Eggs Solution (×103) | Peak Area in Decocotion (×103) | Absorption Ratio (%) 4 | |||||
| 1 | 3.04 | 133.0143 | 4.51 | C4H6O5 | 115.0025 (14), 89.0258 (9), 71.0139 (100) | Malic acid | 794 | 18,028 | 2.0 |
| 2 | 3.71 | 331.0677 | 3.62 | C13H16O10 | 211.0278 (18), 169.0135 (81), 125.0238 (100), 124.0154 (60), 107.0132 (34), 89.0247 (18) | Mono-O-galloyl-glucose | 41 | 1526 | 1.2 |
| 3 | 3.84 | 331.0672 | 2.11 | C13H16O10 | 211.0225 (41), 169.0132 (78), 125.0233 (60), 124.0165 (100), 107.0138 (56), 89.0259 (7) | Mono-O-galloyl-glucose | 20 | 870 | 1.0 |
| 4 | 3.86 | 167.0204 | −0.60 | C5H4N4O3 | 124.0144 (100), 96.0200 (49), 69.0098 (30) | Uric acid | 1163 | 331 | 159.7 |
| 5 | 4.05 | 331.0668 | 0.91 | C13H16O10 | 211.0216 (10), 169.0112 (100), 125.0234 (89), 124.0160 (91), 107.0120 (36) | Mono-O-galloyl-glucose | 28 | 1094 | 1.2 |
| 6 | 4.18 | 481.0626 | 1.66 | C20H18O14 | 300.9978 (100), 275.0194 (38), 257.0072 (14) | Mono-O-HHDP-glucose 5 | |||
| 7 | 4.30 | 191.0199 | 3.66 | C6H8O7 | 111.0088 (72), 87.0087 (100), 85.0291 (49), 67.0184 (29), 57.0346 (33) | Citric acid | 9267 | 25,918 | 16.3 |
| 8 | 4.68 | 481.0626 | 1.66 | C20H18O14 | 300.9948 (100), 275.0205 (34), 257.0117 (13) | Mono-O-HHDP-glucose 5 | |||
| 9 | 4.97 | 243.0617 | 0.00 | C9H12N2O6 | 152.0329 (16), 110.0244 (100) | Uridine | 282 | 738 | 17.4 |
| 10 | 5.23 | 331.0676 | 3.32 | C13H16O10 | 271.0466 (8), 211.0237 (26), 169.0122 (56), 125.0230 (67), 124.0155 (53), 107.0112 (20), 59.0136 (100) | Mono-O-galloyl-glucose | 161 | 3721 | 2.0 |
| 11 | 5.44 | 180.0668 | 3.89 | C9H11N1O3 | 163.0387 (46), 119.0496 (100), 93.0347 (39) | Tyrosine | 3755 | 100 | 1706.8 |
| 12 | 5.60 | 481.0626 | 1.66 | C20H18O14 | 300.9983 (100), 275.0184 (57), 257.0088 (14) | Mono-O-HHDP-glucose 5 | |||
| 13 | 6.49 | 331.0678 | 3.93 | C13H16O10 | 271.0520 (13), 211.0289 (33), 169.0171 (94), 125.0266 (18), 124.0154 (36), 107.0208 (19), 59.0153 (100) | Mono-O-galloyl-glucose | |||
| 14 | 6.91 | 331.0664 | −0.30 | C13H16O10 | 169.0145 (79), 124.0203 (36), 107.0140 (9) | Mono-O-galloyl-glucose | |||
| 15 | 7.75 | 331.0676 | 3.32 | C13H16O10 | 271.0431 (39), 211.0238 (45), 169.0129 (32), 125.0225 (40), 124.0160 (100), 107.0137 (16) | Mono-O-galloyl-glucose | 214 | 5022 | 1.9 |
| 16 6 | 8.04 | 169.0150 | 7.69 | C7H6O5 | 125.0238 (100), 124.0162 (11), 107.0134 (5), 97.0285 (10), 79.0186 (21) | Gallic acid | 1862 | 23,485 | 3.6 |
| 17 | 9.13 | 483.0779 | 0.83 | C20H20O14 | 331.0655 (17), 313.0516 (14), 271.0472 (6), 169.0139 (100), 125.0237 (85), 107.0118 (13) | Di-O-galloyl-glucose | |||
| 18 | 9.22 | 331.0687 | 6.65 | C13H16O10 | 271.0464 (37), 211.0237 (54), 169.0137 (47), 125.0233 (57), 124.0162 (100), 107.0142 (25) | Mono-O-galloyl-glucose | 185 | 3901 | 2.2 |
| 19 | 10.06 | 164.0714 | 0.00 | C9H11N1O2 | 147.0445 (28), 103.0551 (100), 91.0565 (14), 72.0093 (55) | Phenylalanine | 2962 | 118 | 1141.0 |
| 20 | 10.06 | 483.0775 | 0.00 | C20H20O14 | 331.0746 (8), 313.0608 (6), 271.0466 (5), 169.0142 (94), 125.0225 (100) | Di-O-galloyl-glucose | |||
| 21 | 10.64 | 483.0777 | 0.41 | C20H20O14 | 331.0697 (8), 211.0259 (6), 169.0129 (74), 125.0248 (100) | Di-O-galloyl-glucose | |||
| 22 | 11.82 | 218.1030 | 0.92 | C9H17N1O5 | 146.0814 (52), 88.0405 (100) | Pantothenic acid | 626 | 327 | 87.0 |
| 23 | 12.24 | 483.0789 | 2.90 | C20H20O14 | 331.0710 (14), 313.0564 (20), 271.0411 (12), 211.0240 (19), 169.0134 (99), 125.0249 (100), 124.0166 (9) | Di-O-galloyl-glucose | |||
| 24 | 12.62 | 329.0881 | 2.43 | C14H18O9 | 167.0304 (55), 152.0190 (66), 123.0436 (64), 108.0208 (100) | Mono-O-vanilloyl-glucose | |||
| 25 | 13.29 | 483.0791 | 3.31 | C20H20O14 | 211.0283 (29), 169.0138 (100), 125.0223 (43), 124.0134 (14) | Di-O-galloyl-glucose | |||
| 26 | 13.33 | 345.0829 | 2.03 | C14H18O10 | 183.0261 (23), 138.0315 (100) | Methylgalloyl-O-glucose | |||
| 27 | 13.37 | 359.0983 | 1.39 | C15H20O10 | 344.0747 (75), 197.0379 (33), 166.9989 (20), 137.0251 (100) | Mono-O-syringoyl-glucose | |||
| 28 | 14.84 | 345.0812 | −2.90 | C14H18O10 | 138.0304 (100) | Methylgalloyl-O-glucose | |||
| 29 | 14.93 | 483.0796 | 4.35 | C20H20O14 | 271.0465 (41), 211.0243 (69), 169.0135 (100), 125.0236 (47), 124.0157 (69) | Di-O-galloyl-glucose | |||
| 30 | 15.18 | 359.0979 | 0.28 | C15H20O10 | 344.0754 (48), 197.0475 (79), 182.0225 (26), 166.9989 (13), 152.0455 (50), 137.0251 (84), 123.0089 (100) | Mono-O-syringoyl-glucose | |||
| 31 | 15.31 | 353.0864 | −2.55 | C16H18O9 | 191.0571 (100), 179.0288 (40), 135.0440 (78), 117.0370 (25) | Caffeoyl quinic acid | |||
| 32 | 15.68 | 633.0759 | 4.90 | C27H22O18 | 481.0643 (8), 463.0506 (7), 300.9996 (100), 275.0192 (27), 169.0113 (7) | HHDP-galloyl-O-glucose 5 | |||
| 33 | 15.81 | 203.0819 | −0.98 | C11H12N2O2 | 142.0642 (29), 116.0500 (100), 74.0249 (24) | Tryptophan | 1443 | 178 | 368.5 |
| 34 | 15.98 | 483.0793 | 3.73 | C20H20O14 | 423.0526 (34), 331.0733 (5), 313.0620 (11), 271.0455 (32), 211.0238 (52), 169.0135 (100), 125.0234 (86) | Di-O-galloyl-glucose | |||
| 35 | 16.44 | 329.0875 | 0.61 | C14H18O9 | 269.0721 (19), 209.0434 (35), 167.0343 (41), 152.0111 (15), 123.0435 (24), 59.0136 (100) | Mono-O-vanilloyl-glucose | |||
| 36 | 16.48 | 483.0787 | 2.48 | C20H20O14 | 313.0563 (6), 271.0452 (17), 211.0233 (41), 169.0134 (100), 125.0241 (62) | Di-O-galloyl-glucose | |||
| 37 | 17.41 | 483.0799 | 4.97 | C20H20O14 | 423.0596 (23), 331.0711 (6), 313.0509 (5), 271.0449 (26), 211.0246 (21), 169.0128 (100), 125.0246 (55) | Di-O-galloyl-glucose | |||
| 38 7 | 17.83 | 357.1183 | −0.84 | C16H22O9 | 177.0541 (8), 133.0659 (100) | Juglanoside H | |||
| 39 | 17.83 | 483.0787 | 2.48 | C20H20O14 | 331.0651 (6), 313.0562 (14), 271.0452 (24), 211.0245 (29), 169.0142 (100), 125.0240 (68), 107.0138 (12) | Di-O-galloyl-glucose | |||
| 40 | 18.12 | 359.0990 | 3.34 | C15H20O10 | 299.0759 (8), 239.0560 (25), 197.0448 (54), 182.0209 (12), 166.9973 (5), 152.0465 (15), 137.0239 (24), 59.0135 (100) | Mono-O-syringoyl-glucose | 105 | 3282 | 1.5 |
| 41 | 18.50 | 483.0798 | 4.76 | C20H20O14 | 313.0615 (14), 271.0435 (51), 211.0239 (77), 169.0127 (100), 125.0237 (71), 107.0144 (15) | Di-O-galloyl-glucose | |||
| 42 | 19.13 | 511.1098 | 1.96 | C22H24O14 | 467.1314 (15), 327.0605 (14), 313.0528 (40), 197.0495 (10), 182.0226 (14), 169.0138 (67), 125.0228 (100), 124.0172 (31), 107.0121 (18) | Syringoyl-galloyl-O-glucose | |||
| 43 | 19.25 | 483.1141 | 0.41 | C21H24O13 | 327.0770 (8), 313.0573 (6), 297.0666 (11), 169.0473 (6), 169.0129 (78), 154.0254 (14), 139.0041 (8), 125.0228 (70), 124.0160 (100), 107.0134 (13) | Hydroxy-dimethoxyphenol galloyl-glucoside | 51 | 785 | 3.0 |
| 44 | 19.51 | 483.0767 | −1.66 | C20H20O14 | 331.0609 (9), 313.0567 (58), 271.0450 (39), 211.0244 (25), 169.0130 (100), 125.0250 (47) | Di-O-galloyl-glucose | |||
| 45 6 | 19.59 | 353.0880 | 1.98 | C16H18O9 | 191.0547 (100), 135.0437 (8), 93.0353 (22) | Chlorogenic acid | 26 | 798 | 1.5 |
| 46 | 19.67 | 359.0994 | 4.46 | C15H20O10 | 197.0430 (29), 182.0203 (8), 166.9953 (5), 152.0455 (24), 137.0237 (42), 59.0138 (100) | Mono-O-syringoyl-glucose | 72 | 1953 | 1.7 |
| 47 | 19.97 | 635.0859 | −3.94 | C27H24O18 | 483.0814 (10), 465.0776 (10), 313.0566 (10), 295.0513 (8), 271.0492 (6), 169.0134 (100), 125.0257 (11) | Tri-O-galloyl-glucose | |||
| 48 | 20.47 | 281.0669 | 2.85 | C13H14O7 | 163.0402 (100), 119.0492 (100), 75.0084 (33) | Coumaroyl threonic acid | |||
| 49 | 20.51 | 533.1494 8 | −2.25 | C21H28O13 | 193.04490 (100), 175.0377 (42) | Trihydroxytetralone pentosyl-hexoside | 13 | 259 | 2.4 |
| 50 | 20.60 | 453.1051 | 3.97 | C20H22O12 | 313.0523 (27), 169.0121 (23), 139.0410 (14), 125.0229 (27), 124.0160 (100), 97.0300 (8) | hydroxy-methoxyphenol galloylglucoside | |||
| 51 | 21.27 | 291.0139 | −0.69 | C13H8O8 | 247.0245 (100), 219.0288 (13), 191.0341 (37), 173.0250 (14), 145.0280 (21) | Brevifolin carboxylic acid | 59 | 1330 | 2.0 |
| 52 | 21.48 | 533.1516 8 | 1.88 | C21H28O13− | 193.0505 (100), 175.0409 (24) | Trihydroxytetralone pentosyl-hexoside | 16 | 203 | 3.4 |
| 53 | 21.56 | 339.1076 | −1.18 | C16H20O8 | 159.0430 (93), 115.0552 (100) | Dihydroxytetralone hexoside | |||
| 54 | 21.73 | 533.1520 8 | 2.63 | C21H28O13 | 193.0502 (100), 175.0386 (30) | Trihydroxytetralone pentosyl-hexoside | 48 | 623 | 3.5 |
| 55 | 21.81 | 401.1084 8 | 0.00 | C16H20O9 | 193.0506 (100), 175.0390 (42) | Trihydroxytetralone hexoside | |||
| 56 | 21.90 | 635.0888 | 0.63 | C27H24O18 | 483.0850 (52), 465.0636 (45), 169.0140 (100), 125.0244 (11) | Tri-O-galloyl-glucose | |||
| 57 | 22.11 | 281.0661 | 0.00 | C13H14O7 | 163.0390 (23), 119.0501 (100), 117.0208 (56), 75.0103 (42) | Coumaroyl threonic acid | |||
| 58 | 22.40 | 481.0998 | 3.33 | C21H22O13 | 313.0551 (53), 169.0143 (100), 167.0323 (38), 152.0104 (45), 125.0234 (63), 124.0149 (18) | Vanilloyl-O-galloyl-glucose | 45 | 710 | 2.9 |
| 59 | 22.86 | 297.0614 | 1.35 | C13H14O8 | 179.0301 (26), 161.0284 (16), 135.0284 (96), 107.0497 (23), 75.0077 (100) | Caffeoyl threonic acid | 30 | 564 | 2.4 |
| 60 6 | 22.86 | 197.0444 | −3.04 | C9H10O5 | 182.0228 (14), 166.9941 (100), 123.0085 (65), 95.0135 (34), 61.9890 (53) | Syringic acid | 86 | 479 | 8.2 |
| 61 | 23.41 | 467.0812 | −3.00 | C20H20O13 | 423.0881 (62), 315.0710 (23), 313.0557 (33), 169.0144 (69), 153.0205 (27), 152.0107 (97), 125.0231 (78), 109.0281 (33), 108.0209 (100) | Dihydroxybenzoic acid galloyl-glucoside | 29 | 869 | 1.5 |
| 62 | 23.83 | 177.0552 | 0.00 | C10H10O3 | 159.0444 (97), 131.0495 (5), 115.0547 (100) | Dihydroxytetralone | 158 | 3117 | 2.3 |
| 63 | 23.83 | 337.0930 | 2.08 | C16H18O8 | 191.0543 (71), 163.0404 (21), 145.0323 (9), 119.0505 (21), 93.0336 (100) | Coumaroyl quinic acid | 31 | 892 | 1.6 |
| 64 | 24.67 | 469.1361 | 3.20 | C21H26O12 | 175.0397 (100) | Trihydroxynaphthalene pentosyl-hexoside | 43 | 766 | 2.5 |
| 65 | 25.09 | 469.1342 | −0.85 | C21H26O12 | 175.0391 (100) | Trihydroxynaphthalene pentosyl-hexoside | |||
| 66 | 25.64 | 385.1148 8 | 3.38 | C16H20O8 | 339.1042 (27), 177.0532 (15), 159.0437 (100) | Dihydroxytetralone hexoside | 62 | 571 | 5.0 |
| 67 6 | 25.82 | 337.0916 | −2.08 | C16H18O8 | 176.0446 (16), 175.0390 (100) | 1,4,8-Trihydroxynaphthalene 1-O-β-d-glucoside | |||
| 68 | 25.91 | 635.0859 | −3.94 | C27H24O18 | 465.0638 (69), 313.0571 (67), 169.0141 (100), 125.0223 (12), 124.0127 (18) | Tri-O-galloyl-glucose | |||
| 69 | 26.77 | 193.0501 | C10H10O4 | 175.0382 (100), 157.0277 (30), 147.0479 (11), 131.0458 (18) | Trihydroxytetralone | 102 | 2745 | 1.7 | |
| 70 | 27.32 | 281.0666 | 1.78 | C13H14O7 | 163.0408 (62), 135.0289 (14), 119.0494 (100), 117.0329 (26), 75.0079 (9) | Coumaroyl threonic acid | 232 | 3414 | 3.1 |
| 71 6 | 27.88 | 163.0400 | 3.07 | C9H8O3 | 119.0492 (100) | p-Coumaric acid | 108 | 582 | 8.4 |
| 72 | 28.11 | 511.1453 | 0.20 | C23H28O13 | 341.0830 (11), 327.0719 (12), 197.0480 (14), 182.0204 (10), 169.0462 (27), 154.0245 (13), 153.0187 (100) | Hydroxy-dimethoxyphenol syringoyl-glucoside | 73 | 1060 | 3.1 |
| 73 | 28.91 | 507.1152 | 2.56 | C23H24O13 | 313.0579 (48), 193.0500 (37), 175.0394 (33), 169.0117 (100), 157.0293 (8), 125.0233 (31) | Trihydroxytetralone galloyl-hexoside | |||
| 74 | 29.29 | 311.0761 | −1.93 | C14H16O8 | 193.0466 (14), 149.0554 (11), 134.0359 (100), 117.0334 (21), 75.0080 (6) | Feruloyl threonic acid | |||
| 75 | 30.05 | 403.1613 | 2.23 | C18H28O10 | 223.0982 (22), 179.1072 (100), 161.0975 (16) | Glucopyranose, 1-[10-hydrogen (2E,4E)-8-hydroxy-2,7-dimethyl-2,4-decadienedioate] | |||
| 76 6 | 30.30 | 300.9989 | 1.66 | C14H6O8 | 283.9921 (15), 271.9869 (8), 257.0076 (12), 245.0128 (9) | Ellagic acid | 220 | 2775 | 3.6 |
| 77 | 30.34 | 511.1074 | −2.74 | C22H24O14 | 197.0448 (14), 182.0280 (14), 169.0150 (20), 168.0060 (40), 149.9941 (100), 138.0327 (12), 125.0228 (26), 124.0165 (18) | Syringoyl-galloyl-O-glucose | |||
| 78 | 30.38 | 481.1357 | 2.29 | C22H26O12 | 341.0879 (40), 197.0433 (16), 182.0264 (8), 152.0482 (33) , 138.0316 (80), 123.0084 (100) | Hydroxy-methoxyphenol syringoyl-glucopyranoside | 42 | 427 | 4.5 |
| 79 | 31.10 | 509.1296 | 0.20 | C23H26O13 | 341.0851 (14), 327.0708 (30), 197.0458 (46), 167.0349 (100), 152.0126 (44), 137.0244 (28), 123.0442 (15), 108.0197 (26) | Vanilloyl-O-syingoyl-glucose | 51 | 680 | 3.4 |
| 80 | 31.31 | 539.2120 8 | −1.67 | C25H34O10 | 493.2079 (100), 361.1590 (66), 179.0692 (34), 165.0559 (32) | Secoisolariciresinol pentoside | |||
| 81 | 31.89 | 495.1161 | 4.44 | C22H24O13 | 451.1234 (8), 327.0654 (13), 183.0281 (19), 169.0136 (8), 152.0119 (100), 138.0324 (56), 108.0210 (100) | Vanilloyl-methylgalloyl-O-glucose | 93 | 1211 | 3.5 |
| 82 | 32.23 | 433.0783 | 2.77 | C20H18O11 | 287.0436 (7), 281.0692 (7), 169.0144 (39), 163.0391 (72), 135.0306 (100), 125.0224 (66), 119.0480 (68), 117.0180 (8), 107.0131 (40), 75.0085 (48) | Coumaroyl-galloyl-threonic acid | |||
| 83 | 33.99 | 361.1660 | 2.49 | C20H26O6 | 343.1534 (16), 179.0698 (80), 165.0545 (31), 163.0760 (36), 145.0644 (76), 135.0434 (49), 121.0293 (62), 107.0499 (63), 93.0352 (71) | Secoisolariciresinol | 182 | 2705 | 3.1 |
| 84 | 34.06 | 491.1183 | −1.43 | C23H24O12 | 313.0604 (9), 211.0225 (38), 177.0617 (10), 169.0158 (94), 159.0436 (61), 125.0225 (69), 124.0159 (100) | Dihydroxytetralone galloy-hexoside | |||
| 85 6 | 34.37 | 447.0920 | −1.57 | C21H20O11 | 301.0338 (100), 151.0017 (71) | Quercetin-3-O-α-l-rhamnoside | |||
| 86 7 | 34.96 | 391.1757 | 0.00 | C21H28O7 | 193.0861 (43), 175.0776 (57), 161.0558 (76), 135.0457 (100), 123.0461 (66) | Juglanol B | 94 | 886 | 4.8 |
| 87 7 | 35.61 | 619.1292 | −1.13 | C28H28O16 | 325.0337 (85), 324.0248 (100) | 2,3,7,11,12-pentahydroxy-6-oxabenzo[α]anthracen-5-one pentosyl-hexoside | |||
| 88 | 37.77 | 535.1457 | 0.93 | C25H28O13 | 341.0897 (35), 197.0454 (20), 193.0490 (100), 175.0405 (64), 137.0231 (13), 125.0212 (8) | Trihydroxytetralone syringoyl-hexoside | 88 | 1230 | 3.3 |
| 89 7 | 39.96 | 551.2145 8 | 2.90 | C26H34O10− | 505.1992 (18), 343.1520 (28), 325.1420 (79), 307.1235 (9), 89.0246 (100) | Jugcathayenoside | 28 | 249 | 5.1 |
| 90 7 | 41.05 | 549.1990 8 | 3.28 | C26H32O10− | 503.1977 (6), 341.1364 (18), 323.1282 (100), 295.1367 (9) | Juglaside A | 56 | 865 | 2.9 |
| 91 7 | 41.47 | 311.0193 | 0.32 | C16H8O7 | 267.0289 (52), 223.0364 (34), 195.0462 (100), 167.0454 (27) | Hydroxyanthraquinone dicarboxylic acid | |||
| 92 7 | 43.95 | 343.1550 | 1.46 | C20H24O5 | 179.0705 (100), 121.0297 (49) | Anhydrosecoisolariciresinol (or its isomer) | |||
| 93 7 | 45.34 | 267.0302 | 3.37 | C15H8O5 | 223.0402 (33), 195.0440 (100) | Hydroxyanthraquinone carboxylic acid | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.-M.; Fu, Y.; Yu, W.-J.; Chen, C.; Di, X.; Zhang, H.; Zhai, Y.-J.; Chu, Z.-Y.; Kang, T.-G.; Chen, H.-B. Identification of Polar Constituents in the Decoction of Juglans mandshurica and in the Medicated Egg Prepared with the Decoction by HPLC-Q-TOF MS2. Molecules 2017, 22, 1452. https://doi.org/10.3390/molecules22091452
Wang T-M, Fu Y, Yu W-J, Chen C, Di X, Zhang H, Zhai Y-J, Chu Z-Y, Kang T-G, Chen H-B. Identification of Polar Constituents in the Decoction of Juglans mandshurica and in the Medicated Egg Prepared with the Decoction by HPLC-Q-TOF MS2. Molecules. 2017; 22(9):1452. https://doi.org/10.3390/molecules22091452
Chicago/Turabian StyleWang, Tian-Min, Ying Fu, Wen-Jie Yu, Chen Chen, Xue Di, Hui Zhang, Yan-Jun Zhai, Zheng-Yun Chu, Ting-Guo Kang, and Hu-Biao Chen. 2017. "Identification of Polar Constituents in the Decoction of Juglans mandshurica and in the Medicated Egg Prepared with the Decoction by HPLC-Q-TOF MS2" Molecules 22, no. 9: 1452. https://doi.org/10.3390/molecules22091452




