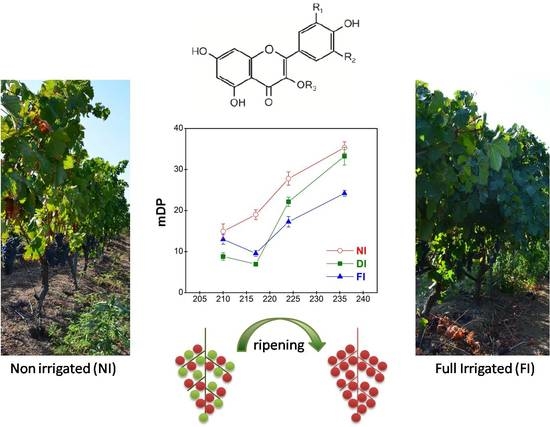
Changes in Tannin Composition of Syrah Grape Skins and Seeds during Fruit Ripening under Contrasting Water Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Vine Water Status and Growth
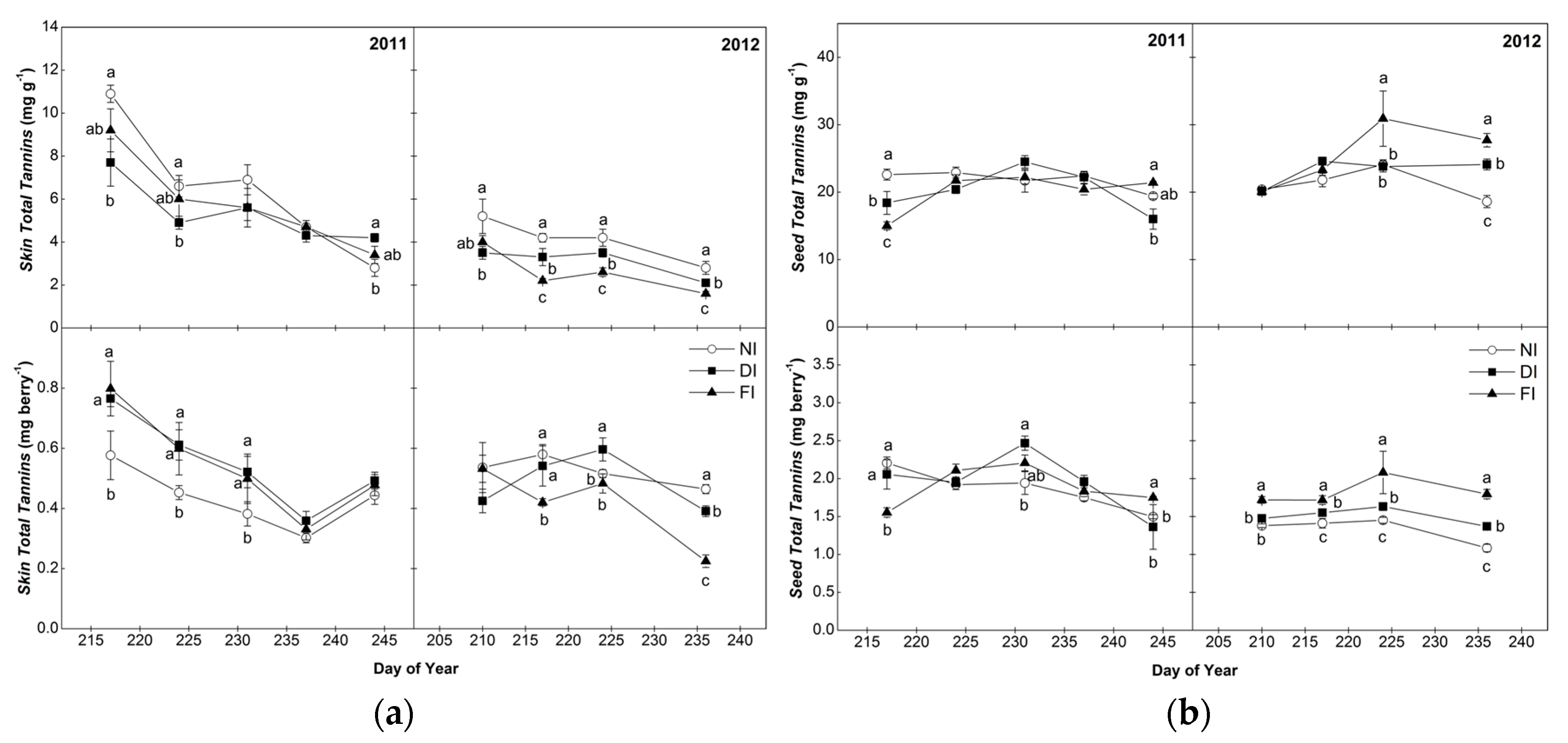
2.2. Total Tannins of Skins and Seeds
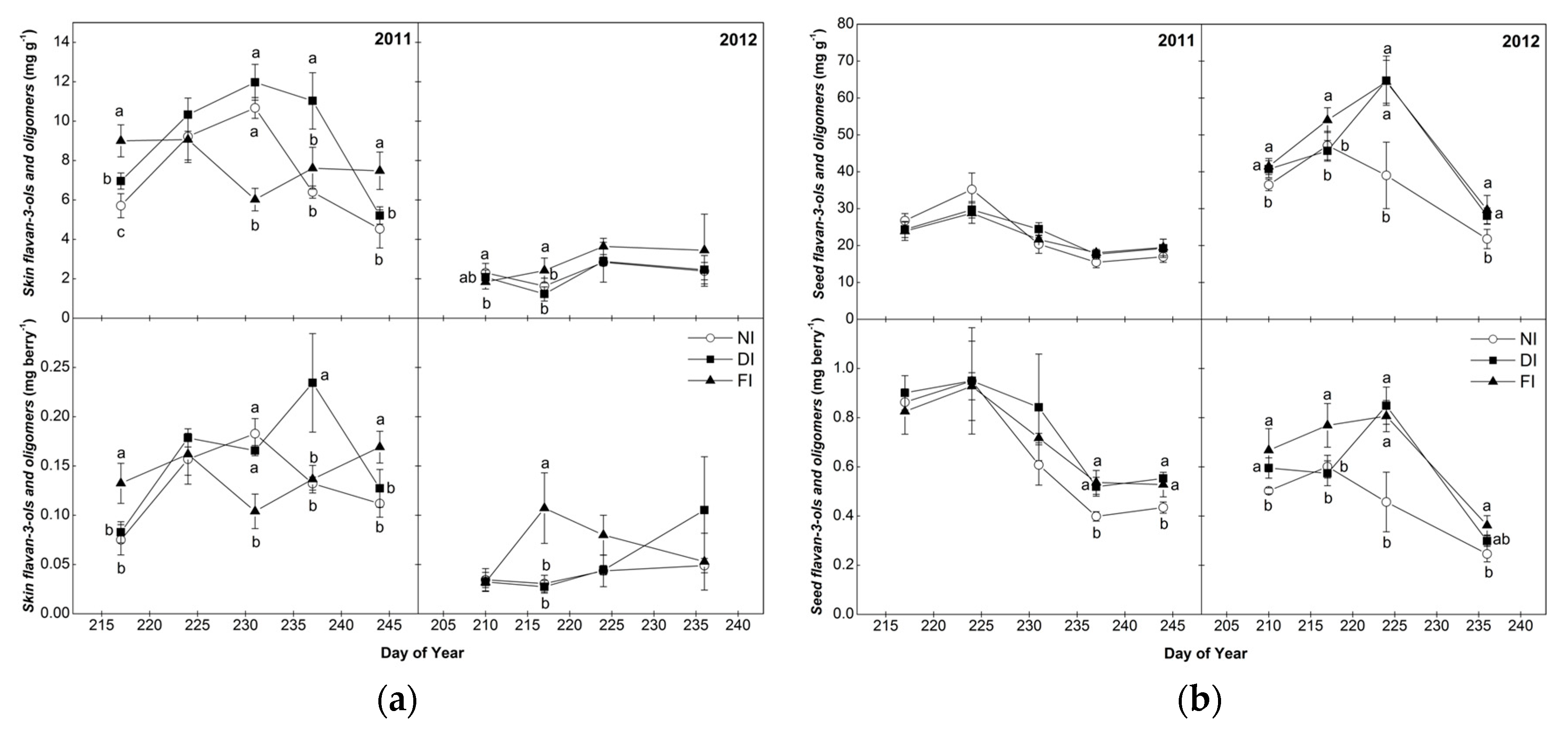
2.3. Flavan-3-ol Monomers and Tannin Oligomers of Skins and Seeds
2.4. Composition of Skin and Seed Tannins
2.4.1. Percentage of Galloylation (%G)
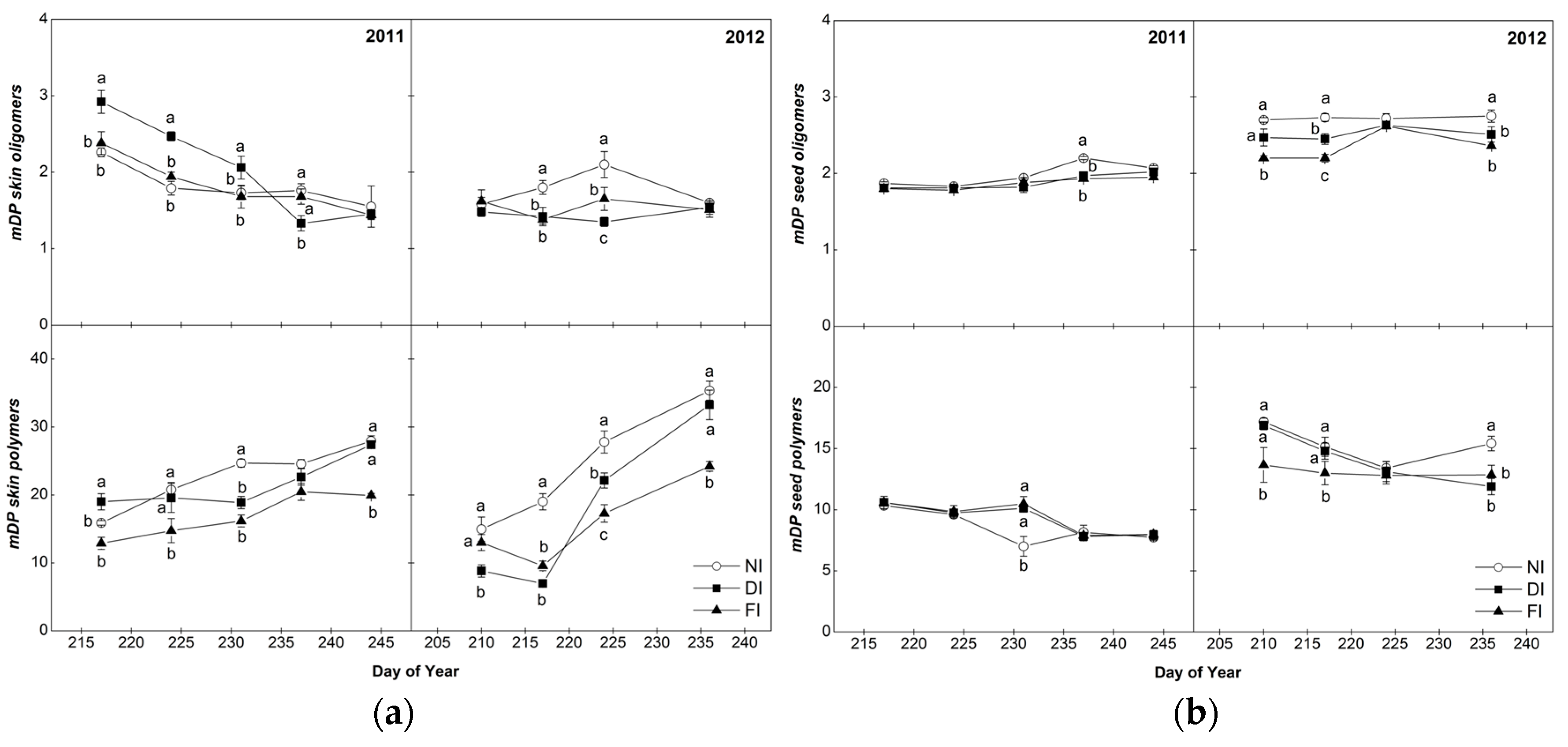
2.4.2. Mean Degree of Polymerization (mDP)
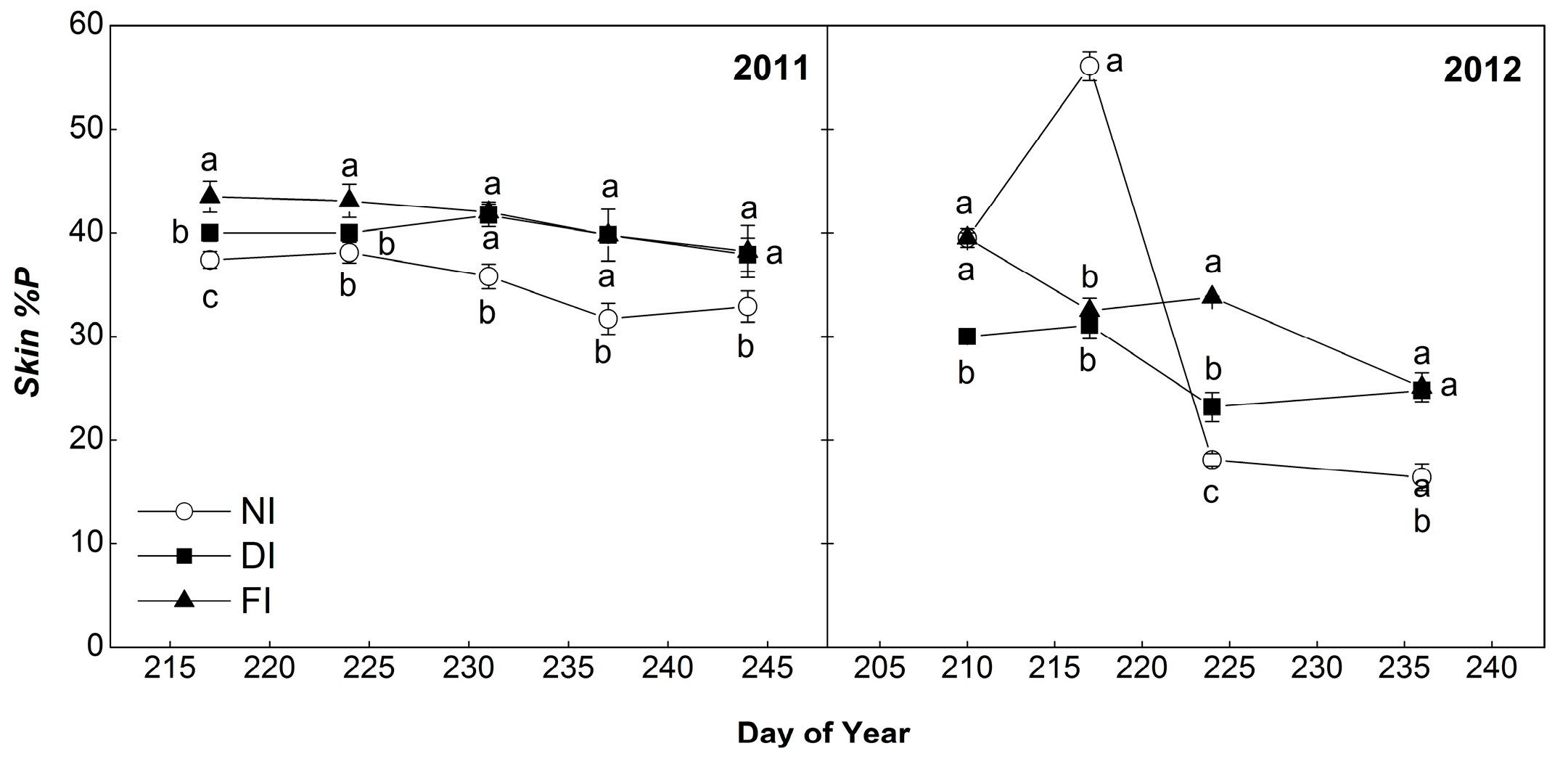
2.4.3. Percentage of Prodelphinidins (%P)
2.4.4. Subunit Composition
3. Materials and Methods
3.1. Chemicals
3.2. Experimental Conditions
3.3. Extraction of Phenolic Compounds from Grape Seeds and Skins
3.4. Grape Tannin Content
3.5. TanninAnalysis
%G = [(ECGe + ECGt)/(Σ terminal subunits + Σ extension subunits)] × 100
%P = [(EGCe + EGCt)/(Σ terminal subunits + Σ extension subunits)] × 100
3.6. Statistics
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chira, K.; Schmauch, G.; Saucier, C.; Fabre, S.; Teissedre, P.L. Grape variety effect on proanthocyanidin composition and sensory perception of skin and seed tannin extracts from Bordeaux wine grapes (Cabernet Sauvignon and Merlot) for two consecutive vintages (2006 and 2007). J. Agric. Food Chem. 2009, 57, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; De Sa, M.; Leonardo, M.; Caldeira, I.; Duarte, F.L.; Spranger, I. Reactivity of polymeric proanthocyanidins toward salivary proteins and their contribution to young red wine astringency. J. Agric. Food Chem. 2013, 61, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Y.X.; Pan, Q.H.; Duan, C.Q.; Shi, Y. Comparison of proanthocyanidins with different polymerization degrees among berry skins of ‘Shiraz’, ‘Cabernet Sauvignon’, and ‘Marselan’. S. Afr. J. Enol. Vitic. 2014, 35, 51–58. [Google Scholar]
- Ćurko, N.; Kovačević Ganić, K.; Gracin, L.; Ðapić, M.; Jourde, M.; Teissedre, P.L. Characterization of seed and skin polyphenolic extracts of two red grape cultivars grown in Croatia and their sensory perception in a wine model medium. Food Chem. 2014, 145, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, M.; Travaglia, F.; Locatelli, M.; Coisson, J.D.; Arlorio, M. Characterization of polymeric skin and seed proanthocyanidins during ripening in six Vitis vinifera L. cv. Food Chem. 2011, 127, 180–187. [Google Scholar] [CrossRef]
- Hanlin, R.L.; Downey, M.O. Condensed tannin accumulation and composition in Shiraz and Cabernet Sauvignon grapes during berry development. Am. J. Enol. Vitic. 2009, 60, 13–23. [Google Scholar]
- Gil, M.; Kontoudakis, N.; González, E.; Esteruelas, M.; Fort, F.; Canals, J.M.; Zamora, F. Influence of grape maturity and maceration length on color, polyphenolic composition, and polysaccharide content of Cabernet Sauvignon and Tempranillo wines. J. Agric. Food Chem. 2012, 60, 7988–8001. [Google Scholar] [CrossRef] [PubMed]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. Analysis of tannins in seeds and skins of Shiraz grapes throughout berry development. Aust. J. Grape Wine Res. 2003, 9, 15–27. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Ferrier, J.; Harbertson, J.F.; Gachons, C.P.D. Analysis of tannins in red wine using multiple methods: Correlation with perceived astringency. Am. J. Enol. Vitic. 2006, 57, 481–485. [Google Scholar]
- Mercurio, M.D.; Smith, P.A. Tannin quantification in red grapes and wine: Comparison of polysaccharide and protein based tannin precipitation techniques and their ability to mode wine astringency. J. Agric. Food Chem. 2008, 56, 5528–5537. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.; Francis, L.; Guyot, S.; Marnet, N.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E.J. The mouth-feel properties of grape and apple proanthocyanidins in a wine-like medium. J. Sci. Food Agric. 2003, 83, 564–573. [Google Scholar] [CrossRef]
- Woollmann, N.; Hofmann, T. Compositional and sensory characterization of red wine polymers. J. Agric. Food Chem. 2013, 61, 2045–2061. [Google Scholar] [CrossRef] [PubMed]
- Chira, K.; Pacella, N.; Jourdes, M.; Teissedre, P.L. Chemical and sensory evaluation of Bordeaux wines (Cabernet-Sauvignon and Merlot) and correlation with wine age. Food Chem. 2011, 126, 1971–1977. [Google Scholar] [CrossRef] [PubMed]
- Quijada-Morin, N.; Regueiro, J.; Simal-Gandara, J.; Tomas, E.; Rivas-Gonzalo, J.C.; Escribano-Bailόn, M.T. Relationship between the sensory-determined astringency and the flavanolic composition of red wines. J. Agric. Food Chem. 2012, 60, 12355–12361. [Google Scholar] [CrossRef] [PubMed]
- Kyraleou, M.; Kotseridis, Y.; Koundouras, S.; Chira, K.; Teissedre, P.L.; Kallithraka, S. Effect of irrigation regime on perceived astringency and proanthocyanidin composition of skins and seeds of Vitis vinifera L. cv. Syrah grapes under semiarid conditions. Food Chem. 2016, 203, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Chira, K.; Zeng, L.; Le Floch, A.; Pechamat, L.; Jourdes, M.; Teissedre, P.L. Compositional and sensory characterization of grape proanthocyanins and oak wood ellegitannin. Tetraedron 2015, 71, 2999–3006. [Google Scholar] [CrossRef]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A.C. Bitterness and astringency of flavan-3-ol monomers, dimers and trimers. J. Sci. Food Agric. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Brossaud, F.; Cheynier, V.; Noble, A.C. Bitterness and astringency of grape and wine polyphenols. Aust. J. Grape Wine Res. 2001, 7, 33–39. [Google Scholar] [CrossRef]
- Nunan, K.J.; Sims, I.M.; Bacic, A.; Robinson, S.P.; Fincher, G.B. Changes in cell wall composition during ripening of grape berries. Plant Physiol. 1998, 118, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Cadot, Y.; Minana-Castello, M.T.; Chevalier, M. Anatomical, histological, and histochemical changes in grape seeds from Vitis vinifera L. cv Cabernet franc during fruit development. J. Agric. Food Chem. 2006, 29, 9206–9215. [Google Scholar] [CrossRef] [PubMed]
- Ollé, D.; Guiraud, J.L.; Souquet, J.M.; Terrier, N.; Ageorges, A.; Cheynier, V.; Verries, C. Effect of pre- and post-veraison water deficit on proanthocyanidin and anthocyanin accumulation during Shiraz berry development. Aust. J. Grape Wine Res. 2011, 17, 90–100. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Kennedy, J.A.; Adams, D.O. Tannin in seeds and skins of Cabernet Sauvignon, Syrah and Pinot noir berries during ripening. Am. J. Enol. Vitic. 2002, 53, 54–59. [Google Scholar]
- Kennedy, J.A.; Hayasaka, Y.; Vidal, S.; Waters, E.J.; Jones, G.P. Composition of grape skin proanthocyanidins at different stages of berry development. J. Agric. Food Chem. 2001, 49, 5348–5355. [Google Scholar] [CrossRef] [PubMed]
- Kallithraka, S.; Tsoutsouras, E.; Tzourou, E.; Lanaridis, P. Principal phenolic compounds in Greek red wines. Food Chem. 2006, 99, 784–793. [Google Scholar] [CrossRef]
- Koundouras, S.; Marinos, V.; Gkoulioti, A.; Kotseridis, Y.; van Leeuwen, C. Influence of vineyard location and vine water status on fruit maturation of nonirrigated cv. Agiorgitiko (Vitis vinifera L.). Effects on wine phenolic and aroma components. J. Agric. Food Chem. 2006, 54, 5077–5086. [Google Scholar] [CrossRef] [PubMed]
- Kyraleou, Μ.; Kallithraka, S.; Koundouras, S.; Chira, K.; Haroutounian, S.; Spinthiropoulou, H.; Kotseridis, Y. Effect of vine training system on the phenolic composition of red grapes (Vitis vinifera L. cv. Xinomavro). J. Int. Sci. Vigne Vin 2015, 49, 71–84. [Google Scholar] [CrossRef]
- Santesteban, L.G.; Miranda, C.; Royo, J.B. Regulated deficit irrigation effects on growth, yield, grape quality and individual anthocyanin composition in Vitis vinifera L. cv. ‘Tempranillo’. Agric. Water Manag. 2011, 98, 1171–1179. [Google Scholar] [CrossRef]
- Romero, P.; Fernández-Fernández, J.I.; Martines-Cutillas, A. Physiological thresholds for efficient regulated deficit-irrigation management in winegrapes grown under semiarid conditions. Am. J. Enol. Vitic. 2010, 61, 300–312. [Google Scholar]
- Kyraleou, M.; Koundouras, S.; Kallithraka, S.; Theodorou, N.; Proxenia, N.; Kotseridis, Y. Effect of irrigation regime on anthocyanin content and antioxidant activity of Vitis vinifera L. cv. Syrah grapes under semiarid conditions. J. Sci. Food Agric. 2016, 96, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Matthews, M.A.; Waterhouse, A.L. Effect of maturity and vine water status on grape skin and wine flavonoids. Am. J. Enol. Vitic. 2002, 53, 268–274. [Google Scholar]
- Bonada, M.; Jeffery, D.W.; Petrie, P.R.; Moran, M.A.; Sadras, V.O. Impact of elevated temperature and water deficit on the chemical and sensory profiles of Barossa Shiraz grapes and wines. Aust. J. Grape Wine Res. 2015, 21, 240–253. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Matthews, M.A.; Waterhouse, A.L. Changes in grape seed polyphenols during fruit ripening. Phytochemistry 2000, 55, 77–85. [Google Scholar] [CrossRef]
- Chacón, J.L.; Garcia, E.; Martinez, J.; Romero, R.; Gomez, S. Impact of the vine water status on the berry and seed phenolic composition of‘Merlot’ (Vitis vinifera L.) cultivated in a warm climate: Consequence for the style of wine. Vitis 2009, 48, 7–9. [Google Scholar]
- Genebra, T.; Santos, R.R.; Francisco, R.; Pinto-Marijuan, M.; Brossa, R.; Serra, A.T.; Duarte, C.M.M.; Chaves, M.M.; Zarrouk, O. Proanthocyanidin accumulation and biosynthesis are modulated by the irrigation regime in Tempranillo seeds. Int. J. Mol. Sci. 2014, 15, 11862–11877. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, O.; Francisco, R.; Pinto-Marijuan, M.; Brossa, R.; Santos, R.R.; Pinheiro, C.; Costa, J.M.; Lopes, C.; Chaves, M.M. Impact of irrigation regime on berry development and flavonoids composition in Aragonez (Syn. Tempranillo) grapevine. Agric. Water Manag. 2012, 114, 18–29. [Google Scholar] [CrossRef]
- Mavromatis, T. Changes in exceptional hydrological and meteorological weekly event frequencies in Greece. Clim. Chang. 2012, 110, 249–267. [Google Scholar] [CrossRef]
- Οbreque-Slier, E.; Peña-Neira, A.; Lopez-Solís, R.; Zamora-Marín, F.; Ricardo-da Silva, M.A.; Laureano, O. Comparative study of the phenolic composition of seeds and skins from Carménère and Cabernet Sauvignon Grape varieties (Vitis vinifera L.) during ripening. J. Agric. Food Chem. 2010, 58, 3591–3599. [Google Scholar]
- Casassa, L.F.; Keller, M.; Harbertson, J.F. Regulated deficit irrigation alters anthocyanins, tannins and sensory properties of Cabernet Sauvignon grapes and wines. Molecules 2015, 20, 7820–7844. [Google Scholar] [CrossRef] [PubMed]
- Matthews, M.A.; Kriedemann, P.E. Water deficit, yield, and berry size as factors for composition and sensory attributes of red wine. In Proceedings of the Australian Society of Viticulture and Oenology ‘Finishing the Job’—Optimal Ripening of Cabernet Sauvignon and Shiraz, Adelaide, Australia, 21 July 2006; pp. 46–54. [Google Scholar]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J.C. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am. J. Enol. Vitic. 2002, 53, 171–182. [Google Scholar]
- Pastor del Rio, J.L.; Kennedy, J.A. Development of proanthocyanidins in Vitis vinifera L. cv. Pinot noir grapes and extraction into wine. Am. J. Enol. Vitic. 2006, 57, 125–132. [Google Scholar]
- Kennedy, J.A.; Troup, G.J.; Pilbrow, J.R.; Hutton, D.R.; Hewitt, D.; Hunter, C.R.; Ristic, R.; Iland, P.G.; Jones, G.P. Development of seed polyphenols in berries from Vitis vinifera L. cv. Shiraz. Aust. J. Grape Wine Res. 2000, 6, 244–254. [Google Scholar] [CrossRef]
- Ristic, R.; Downey, M.O.; Iland, P.G.; Bindon, K.; Francis, I.L.; Herderich, M.J.; Robinson, S.P. Exclusion of sunlight from Shiraz grapes alters wine colour, tannin and sensory properties. Aust. J. Grape Wine Res. 2007, 13, 53–65. [Google Scholar] [CrossRef]
- Rinaldi, A.; Jourdes, M.; Teissedre, P.L.; Moio, L.A. Preliminary characterization of Aglianico (Vitis vinifera L. cv.) grape proanthocyanidins and evaluation of their reactivity towards salivary proteins. Food Chem. 2014, 164, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Quijada-Morin, N.; Williams, P.; Rivas-Gonzalo, J.C.; Doco, T.; Escribano-Bailόn, M.T. Polyphenolic, polysaccharide and oligosaccharide composition of Tempranillo red wines and their relationship with the perceived astringeny. Food Chem. 2014, 154, 44–51. [Google Scholar] [CrossRef] [PubMed]
- McRae, J.M.; Schulkin, A.; Cassara, S.; Holt, H.; Smith, P. Sensory properties of wine tannin fractions: Implications for in-mouth sensory properties. J. Agric. Food Chem. 2013, 61, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Waffo-Teguo, P.; Jourdes, M.; Li, H.; Teissedre, P.-L. Chemical affinity between tannin size and salivary protein binding abilities: Implications for wine astringency. PLoS ONE 2016, 11, e0161095. [Google Scholar] [CrossRef] [PubMed]
- Quijada-Morin, N.; Hernandez-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Extractability of low molecular mass flavanols and flavonols from red grape skins. Relationship to cell wall composition at different ripeness stages. J. Agric. Food Chem. 2015, 63, 7654–7662. [Google Scholar] [CrossRef] [PubMed]
- Harbertson, J.F.; Picciotto, E.A.; Adams, D.O. Measurement of polymeric pigments in grape berry extracts and wines using a protein precipitation assay combined with bisulfite bleaching. Am. J. Enol. Vitic. 2003, 54, 301–306. [Google Scholar]
- Kennedy, J.A.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Drinkine, J. Oxydation du vin:Rôle de L’acétaldéhyde et de L’acideglyoxylique; Thèse Doctorat, Université de Bordeaux 2: Bordeaux, France, 2005; p. 156. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
| Day of 2011 | Day of 2012 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 217 | 224 | 231 | 237 | 244 2 | 210 | 217 | 224 | 236 2 | ||
| Skins | ||||||||||
| Oligomers | NI | 4.88 ± 0.15 a 1 | 6.74 ± 0.20 a | 8.17 ± 0.68 a | 6.35 ± 0.30 b | 6.74 ± 1.10 b | 6.52 ± 0.24 ab | 4.98 ± 0.44 a | 4.20 ± 1.96 b | 3.77 ± 0.58 c |
| DI | 3.55 ± 0.15 b | 3.02 ± 0.16 b | 8.03 ± 0.62 a | 3.73 ± 0.11 c | 8.63 ± 0.32 a | 4.55 ± 0.31 b | 4.25 ± 0.12 a | 13.55 ± 1.26 a | 5.44 ± 0.55 b | |
| FI | 4.23 ± 0.30 ab | 6.67 ± 0.32 a | 6.05 ± 1.00 b | 9.51 ± 0.49 a | 8.04 ± 0.50 a | 8.38 ± 1.39 a | 6.11 ± 1.18 a | 9.47 ± 1.72 ab | 8.97 ± 3.22 a | |
| Polymers | NI | 0.19 ± 0.01 b | 0.29 ± 0.03 a | 0.34 ± 0.01 a | 0.16 ± 0.03 b | 0.25 ± 0.00 a | 0.18 ± 0.02 a | 0.19 ± 0.03 a | 0.36 ± 0.02 a | 0.31 ± 0.02 b |
| DI | 0.23 ± 0.01 b | 0.21 ± 0.03 a | 0.21 ± 0.03 b | 0.17 ± 0.00 b | 0.23 ± 0.02 a | 0.34 ± 0.18 a | 0.18 ± 0.03 a | 0.31 ± 0.00 a | 0.36 ± 0.02 a | |
| FI | 0.28 ± 0.02 a | 0.22 ± 0.02 a | 0.13 ± 0.00 c | 0.32 ± 0.03 a | 0.26 ± 0.01 a | 0.15 ± 0.01 a | 0.18 ± 0.03 a | 0.42 ± 0.09 a | 0.23 ± 0.03 c | |
| Seeds | ||||||||||
| Oligomers | NI | 16.19 ± 0.34 a | 14.93 ± 0.39 a | 12.51 ± 0.22 a | 11.88 ± 0.26 a | 10.25 ± 0.25 a | 7.72 ± 0.06 a | 7.90 ± 0.12 a | 7.12 ± 0.16 a | 6.91 ± 0.15 a |
| DI | 15.55 ± 0.28 a | 14.26 ± 0.21 a | 11.60 ± 0.96 a | 11.52 ± 0.15 ab | 10.70 ± 0.21 a | 7.77 ± 0.19 a | 7.79 ± 0.11 a | 7.13 ± 0.23 a | 6.41 ± 0.03 b | |
| FI | 16.06 ± 0.50 a | 14.48 ± 0.23 a | 13.20 ± 0.10 a | 10.64 ± 0.39 b | 10.24 ± 0.05 a | 6.98 ± 0.27 a | 7.06 ± 0.12 b | 6.85 ± 0.62 a | 6.25 ± 0.08 b | |
| Polymers | NI | 5.07 ± 0.19 a | 4.69 ± 0.05 a | 4.57 ± 0.43 a | 5.26 ± 0.18 a | 5.20 ± 0.08 a | 2.18 ± 0.02 a | 2.13 ± 0.07 a | 2.18 ± 0.04 ab | 2.36 ± 0.05 a |
| DI | 5.16 ± 0.14 a | 4.74 ± 0.14 a | 4.16 ± 0.39 a | 5.36 ± 0.18 a | 5.25 ± 0.16 a | 2.28 ± 0.10 a | 2.17 ± 0.15 a | 2.28 ± 0.03 a | 2.36 ± 0.04 a | |
| FI | 5.16 ± 0.14 a | 4.78 ± 0.17 a | 4.84 ± 0.19 a | 4.91 ± 0.12 a | 5.17 ± 0.08 a | 2.70 ± 0.44 a | 2.18 ± 0.05 a | 2.11 ± 0.03 b | 2.31 ± 0.09 a | |
| Day of 2011 | Day of 2012 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 217 | 224 | 231 | 237 | 244 | 210 | 217 | 224 | 236 | ||
| Oligomers | ||||||||||
| Ct | NI | 20.7 ± 0.56 b 1 | 11.1 ± 0.38 c | 10.9 ± 0.17 c | 8.1 ± 0.50 a | 6.5 ± 0.52 a | 10.3 ± 0.56 a | 8.1 ± 0.38 a | 9.1 ± 0.17 a | 5.1 ± 0.50 b |
| DI | 22.1 ± 0.44 a | 16.7 ± 0.34 a | 13.3 ± 0.73 b | 2.9 ± 0.07 b | 5.9 ± 0.30 a | 7.6 ± 0.44 b | 6.6 ± 0.34 b | 6.9 ± 0.73 b | 6.1 ± 0.07 a | |
| FI | 20.8 ± 0.52 b | 13.7 ± 0.47 b | 17.2 ± 1.26 a | 8.0 ± 0.34 a | 4.6 ± 0.34 b | 6.8 ± 0.52 b | 6.3 ± 0.47 b | 5.8 ± 1.26 b | 6.6 ± 0.34 a | |
| ECt | NI | 70.6 ± 0.76 a | 79.7 ± 1.26 a | 76.3 ± 0.15 a | 83.8 ± 1.61 a | 85.6 ± 3.63 a | 80.3 ± 0.76 b | 83.9 ± 1.26 b | 81.7 ± 0.15 a | 89.5 ± 1.61 a |
| DI | 69.8 ± 0.75 a | 82.0 ± 1.35 a | 72.9 ± 1.41 b | 85.5 ± 0.68 a | 83.5 ± 0.99 a | 86.4 ± 0.75 a | 87.9 ± 1.35 a | 75.4 ± 1.41 b | 86.1 ± 0.68 a | |
| FI | 71.8 ± 1.68 a | 75.9 ± 1.93 b | 74.8 ±1.34 ab | 78.7 ± 1.07 b | 86.6 ± 2.11 a | 80.3 ± 1.68 b | 85.8 ± 1.93 ab | 78.9 ± 1.34 ab | 80.6 ± 1.07 b | |
| ECGt | NI | 8.7 ± 0.13 a | 9.2 ± 0.20 a | 12.8 ± 0.68 a | 8.1 ± 0.26 c | 7.9 ± 1.15 b | 9.4 ± 0.13 b | 8.0 ± 0.20 a | 9.2 ± 0.68 b | 5.4 ± 0.26 b |
| DI | 8.1 ± 0.13 b | 1.3 ± 0.13 b | 13.8 ± 0.63 a | 11.6 ± 0.13 b | 10.6 ± 0.32 a | 6.0 ± 0.13 c | 5.5 ± 0.13 c | 17.8 ± 1.63 a | 7.7 ± 0.13 b | |
| FI | 7.5 ± 0.20 c | 10.4 ± 0.31 a | 7.9 ± 0.68 b | 13.3 ± 0.43 a | 8.8 ± 0.49 b | 12.9 ± 0.20 a | 7.9 ± 0.31 b | 15.3 ± 0.68 a | 12.8 ± 0.43 a | |
| Ce | NI | 3.5 ± 0.05 a | 2.1 ± 0.12 c | 2.6 ± 0.06 a | 3.1 ± 0.09 b | 2.6 ± 0.00 a | 4.9 ± 0.05 a | 2.8 ± 0.12 c | 4.7 ± 0.06 c | 4.9 ± 0.09 b |
| DI | 3.7 ± 0.06 a | 5.4 ± 0.08 a | 2.6 ± 0.05 a | 10.3 ± 0.03 a | 0.0 ± 0.00 b | 4.1 ± 0.06 b | 3.3 ± 0.08 b | 6.0 ± 0.05 b | 4.5 ± 0.03 b | |
| FI | 3.6 ± 0.10 a | 3.6 ± 0.08 b | 2.0 ± 1.90 a | 3.0 ± 0.03 b | 0.0 ± 0.00 b | 4.9 ± 0.10 a | 4.8 ± 0.08 a | 9.5 ± 1.90 a | 11.4 ± 0.03 a | |
| ECe | NI | 93.8 ± 0.63 a | 92.6 ± 1.46 a | 92.9 ± 0.63 a | 90.5 ± 2.10 a | 89.4 ± 1.41 a | 93.2 ± 0.63 a | 95.2 ± 1.46 a | 94.3 ± 0.63 a | 93.5 ± 2.10 a |
| DI | 94.4 ± 2.27 a | 81.3 ± 4.39 b | 93.3 ± 2.24 a | 77.0 ± 1.25 b | 91.6 ± 1.46 a | 94.1 ± 2.27 a | 95.0 ± 4.39 a | 92.1 ± 2.24 a | 94.0 ± 1.25 a | |
| FI | 93.5 ± 1.17 a | 92.2 ± 1.10 a | 93.1 ± 2.33 a | 90.9 ± 0.95 a | 87.7 ± 1.53 a | 93.5 ± 1.17 a | 93.5 ± 1.10 a | 89.3 ± 2.33 a | 87.2 ± 0.95 b | |
| ECGe | NI | 2.7 ± 0.02 a | 5.3 ± 0.33 b | 4.5 ± 0.01 a | 6.5 ± 0.08 b | 8.0 ± 0.08 b | 1.9 ± 0.02 a | 2.0 ± 0.03 a | 0.9 ± 0.01 c | 1.6 ± 0.08 a |
| DI | 1.9 ± 0.03 b | 13.3 ± 0.06 a | 4.1 ± 0.02 a | 12.6 ± 0.02 a | 8.4 ± 0.04 b | 1.8 ± 0.03 a | 1.7 ± 0.06 b | 2.0 ± 0.02 a | 1.5 ± 0.02 a | |
| FI | 2.9 ± 0.12 a | 4.2 ± 0.66 b | 4.9 ± 0.32 a | 6.1 ± 0.09 b | 12.3 ± 0.06 a | 1.7 ± 0.12 a | 1.7 ± 0.07 b | 1.2 ± 0.32 b | 1.3 ± 0.09 b | |
| Polymers | ||||||||||
| Ct | NI | 21.1 ± 0.10 a | 18.6 ± 0.05 a | 16.7 ± 0.09 b | 16.7 ± 0.07 b | 28.5 ± 0.13 b | 5.3 ± 0.10 a | 4.7 ± 0.05 a | 4.2 ± 0.09 b | 4.2 ± 0.07 c |
| DI | 20.0 ± 0.04 a | 18.9 ± 0.06 a | 20.1 ± 0.05 a | 22.5 ± 0.04 a | 48.7 ± 0.08 a | 5.0 ± 0.04 a | 4.7 ± 0.06 a | 5.0 ± 0.05 a | 5.6 ± 0.04 a | |
| FI | 20.6 ± 0.16 a | 13.5 ± 0.05 b | 17.3 ± 0.04 b | 19.9 ± 0.10 b | 24.3 ± 0.02 b | 5.1 ± 0.16 a | 3.4 ± 0.05 b | 4.3 ± 0.04 b | 5.0 ± 0.10 b | |
| ECt | NI | 78.9 ± 0.10 a | 81.4 ± 0.26 b | 83.3 ± 0.17 a | 83.3 ± 0.17 a | 71.5 ± 0.11 a | 94.7 ± 0.10 a | 95.3 ± 0.26 a | 95.8 ± 0.17 a | 95.8 ± 0.17 a |
| DI | 80.0 ± 0.15 a | 81.1 ± 0.43 b | 79.9 ± 0.18 b | 77.5 ± 0.20 b | 51.3 ± 0.05 b | 95.0 ± 0.15 a | 95.3 ± 0.43 a | 95.0 ± 0.18 a | 94.4 ± 0.20 a | |
| FI | 79.4 ± 0.18 a | 86.5 ± 0.52 a | 82.7 ± 0.18 a | 80.1 ± 0.23 a | 75.7 ± 0.07 a | 94.9 ± 0.18 a | 96.6 ± 0.52 a | 95.7 ± 0.18 a | 95.0 ± 0.23 a | |
| ECGt | NI | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| DI | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| FI | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| Ce | NI | 1.0 ± 0.04 a | 1.1 ± 0.05 a | 1.0 ± 0.04 a | 1.1 ± 0.09 a | 1.1 ± 0.07 a | 6.8 ± 0.04 b | 14.1 ± 0.05 a | 8.3 ± 0.04 b | 1.3 ± 0.09 |
| DI | 1.0 ± 0.03 a | 1.1 ± 0.04 a | 1.3 ± 0.15 a | 1.2 ± 0.10 a | 1.3 ± 0.03 a | 5.6 ± 0.03 c | 12.6 ± 0.04 b | 8.9 ± 0.15 b | nd | |
| FI | 1.0 ± 0.09 a | 1.2 ± 0.05 a | 1.0 ± 0.03 a | 1.1 ± 0.04 a | 1.0 ± 0.11 a | 8.4 ± 0.09 a | 14.8 ± 0.05 a | 12.6 ± 0.03 a | nd | |
| ECe | NI | 56.7 ± 0.61 a | 55.6 ± 0.60 a | 57.7 ± 1.34 a | 62.7 ± 1.73 a | 64.5 ± 1.44 a | 51.8 ± 0.61 b | 42.6 ± 0.60 b | 72.6 ± 1.34 a | 81.5 ± 1.73 a |
| DI | 54.5 ± 0.86 a | 53.8 ± 0.49 a | 52.3 ± 1.05b | 54.3 ± 1.20 b | 59.2 ± 0.75 b | 58.7 ± 0.86 a | 51.5 ± 0.49 a | 66.2 ± 1.05 b | 73.6 ± 1.20 b | |
| FI | 51.4 ± 1.42 b | 50.5 ± 1.47 a | 52.2 ± 1.31 b | 53.9 ± 1.64 b | 58.5 ± 0.53 b | 48.4 ± 1.42 c | 48.0 ± 1.46 a | 60.7 ± 1.31 c | 71.7 ± 1.64 b | |
| ECGe | NI | 0.2 ± 0.01 a | 0.3 ± 0.03 a | 0.4 ± 0.01 a | 0.2 ± 0.03 b | 0.3 ± 0.00 a | 0.4 ± 0.01 b | 0.4 ± 0.02 a | 0.4 ± 0.01 a | 0.3 ± 0.02 a |
| DI | 0.3 ± 0.01 a | 0.2 ± 0.03 a | 0.2 ± 0.03 b | 0.2 ± 0.00 b | 0.2 ± 0.02 a | 0.6 ± 0.01 a | 0.4 ± 0.02 a | 0.3 ± 0.02 a | 0.4 ± 0.01 a | |
| FI | 0.3 ± 0.02 a | 0.2 ± 0.02 a | 0.1 ± 0.00 b | 0.4 ± 0.03 a | 0.3 ± 0.01 a | 0.3 ± 0.01 b | 0.4 ± 0.02 a | 0.4 ± 0.02 a | 0.2 ± 0.02 b | |
| EGCe | NI | 42.0 ± 0.60 b | 42.9 ± 0.49 b | 40.8 ± 1.21 b | 36.0 ± 1.66 b | 34.2 ± 1.49 b | 41.0 ± 1.29 a | 58.5 ± 1.03 a | 18.7 ± 2.13 c | 16.9 ± 0.44 b |
| DI | 44.3 ± 0.87 a | 44.8 ± 0.41 b | 46.2 ± 1.13 a | 44.3 ± 1.06 a | 39.3 ± 0.73 a | 35.1 ± 0.51 b | 35.5 ± 2.56 b | 24.6 ± 0.89 b | 26.0 ± 1.02 a | |
| FI | 47.3 ± 1.20 a | 48.0 ± 1.12 a | 46.7 ± 1.27 a | 44.6 ± 1.47 a | 40.2 ± 0.42 a | 42.9 ± 0.89 a | 36.8 ± 1.61 b | 36.3 ± 0.76 a | 28.1 ± 2.33 a | |
| Day of 2011 | Day of 2012 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 217 | 224 | 231 | 237 | 244 | 210 | 217 | 224 | 236 | ||
| Oligomers | ||||||||||
| Ct | NI | 28.9 ±0.19 a 1 | 29.7 ± 0.10 b | 30.2 ± 0.35 a | 28.6 ± 0.14 b | 29.6 ± 0.35 a | 28.8 ± 0.15 a | 25.5 ± 0.21 a | 24.1 ± 0.21 a | 25.5 ± 0.65 a |
| DI | 28.8 ± 0.45 a | 31.4 ± 0.33 a | 30.9 ± 0.12 a | 32.3 ± 0.87 a | 27.3 ± 0.48 b | 28.0 ± 0.19 b | 24.7 ± 0.10 b | 24.9 ± 0.35 a | 23.4 ± 0.14 b | |
| FI | 27.9 ± 0.28 a | 27.6 ± 0.23 c | 27.2 ± 0.17 b | 27.5 ± 0.30 b | 26.9 ± 0.20 b | 27.7 ± 0.16 b | 21.4 ± 0.16 c | 24.1 ± 0.29 a | 22.9 ± 0.14 b | |
| ECt | NI | 42.5 ± 0.20 a | 44.4 ± 0.22 b | 47.3 ± 0.20 b | 47.8 ± 0.11 b | 51.5 ± 0.12 c | 53.0 ± 0.11 a | 58.6 ± 0.10 a | 61.4 ± 0.63 a | 61.6 ± 0.57 a |
| DI | 44.6 ± 0.93 a | 44.2 ± 0.52 b | 49.1 ± 0.39 a | 46.9 ± 0.84 b | 53.3 ± 0.95 b | 52.4 ± 0.45 ab | 59.0 ± 0.33 a | 60.2 ± 0.12 a | 62.5 ± 0.87 a | |
| FI | 44.7 ± 0.08 a | 48.1 ± 0.20 a | 49.8 ± 0.04 a | 53.6 ± 0.10 a | 55.2 ± 0.18 a | 51.4 ± 0.42 b | 60.0 ± 0.50 a | 62.5 ± 0.54 a | 62.2 ± 0.58 a | |
| ECGt | NI | 28.6 ± 0.19 a | 25.9 ± 0.16 a | 22.5 ± 0.29 a | 23.6 ± 0.14 a | 18.9 ± 0.21 a | 18.1 ± 0.23 b | 15.9 ± 0.09 b | 14.5 ± 0.18 a | 12.9 ± 0.38 b |
| DI | 26.6 ± 0.45 a | 24.4 ± 0.50 b | 20.0 ± 0.54 b | 20.8 ± 0.58 b | 19.4 ± 0.14 a | 19.6 ± 0.28 a | 16.3 ± 0.23 b | 14.8 ± 0.17 a | 14.1 ± 0.30 a | |
| FI | 27.4 ± 0.28 a | 24.3 ± 0.26 b | 23.0 ± 0.32 a | 18.9 ± 0.24 c | 18.0 ± 0.01 a | 20.9 ± 0.27 a | 18.6 ± 0.26 a | 13.4 ± 0.32 a | 14.9 ± 0.24 a | |
| Ce | NI | 9.3 ± 0.20 a | 9.8 ± 0.14 a | 9.3 ± 0.11 a | 9.1 ± 0.07 a | 9.5 ± 0.22 a | 4.1 ± 0.10 a | 4.7 ± 0.14 a | 4.2 ± 0.18 a | 4.2 ± 0.14 a |
| DI | 8.8 ± 0.93 b | 9.6 ± 0.78 a | 9.9 ± 0.60 a | 9.4 ± 0.36 a | 9.0 ± 0.25 b | 3.8 ± 0.20 a | 3.9 ± 0.22 b | 4.2 ± 0.20 a | 4.3 ± 0.11 a | |
| FI | 9.1 ± 0.08 a | 8.9 ± 0.09 b | 8.9 ± 0.03 b | 9.6 ± 0.07 a | 8.9 ± 0.05 b | 4.1 ± 0.18 a | 3.8 ± 0.14 b | 4.1 ± 0.11 a | 4.2 ± 0.07 a | |
| ECe | NI | 88.7 ± 0.15 a | 88.3 ± 0.21 a | 88.8 ± 0.21 a | 88.7 ± 0.65 a | 88.3 ± 0.12 a | 94.6 ± 0.40 a | 94.0 ± 0.24 a | 94.6 ± 0.71 a | 94.4 ± 0.73 a |
| DI | 89.3 ± 0.11 a | 88.7 ± 0.10 a | 88.5 ± 0.63 a | 88.6 ± 0.57 a | 88.9 ± 0.16 a | 94.9 ± 0.93 a | 94.9 ± 0.52 a | 94.5 ± 0.39 a | 94.3 ± 0.84 a | |
| FI | 89.0 ± 0.23 a | 89.2 ± 0.09 a | 89.0 ± 0.18 a | 88.6 ± 0.38 a | 89.0 ± 0.13 a | 94.7 ± 0.97 a | 94.9 ± 0.78 a | 94.6 ± 0.60 a | 94.5 ± 0.36 a | |
| ECGe | NI | 2.0 ± 0.10 a | 1.8 ± 0.14 a | 1.9 ± 0.18 a | 2.2 ± 0.14 a | 2.2 ± 0.05 a | 1.3 ± 0.12 a | 1.3 ± 0.11 a | 1.3 ± 0.08 a | 1.4 ± 0.09 a |
| DI | 1.9 ± 0.40 a | 1.8 ± 0.24 a | 1.6 ± 0.71 b | 2.0 ± 0.73 a | 2.1 ± 0.32 a | 1.3 ± 0.08 a | 1.2 ± 0.20 a | 1.4 ± 0.04 a | 1.4 ± 0.10 a | |
| FI | 1.9 ± 0.12 a | 1.9 ± 0.11 a | 2.0 ± 0.08 a | 1.8 ± 0.09 a | 2.1 ± 0.07 a | 1.3 ± 0.08 a | 1.3 ± 0.09 a | 1.3 ± 0.03 a | 1.3 ± 0.07 a | |
| Polymers | ||||||||||
| Ct | NI | 23.5 ± 0.12 a | 31.6 ± 0.29 a | 24.7 ± 0.33 c | 32.8 ± 0.36 a | 34.5 ± 0.10 a | 28.3 ± 0.12 a | 27.8 ± 0.29 a | 25.8 ± 0.33 a | 25.1 ± 0.36 a |
| DI | 23.8 ± 0.70 a | 28.8 ± 0.19 b | 29.9 ± 0.47 a | 28.3 ± 1.42 a | 32.7 ± 0.28 b | 26.1 ± 0.70 b | 25.8 ± 0.19 b | 25.2 ± 0.47 a | 24.6 ± 1.42 a | |
| FI | 23.8 ± 0.63 a | 24.8 ± 0.30 c | 27.3 ± 0.17 b | 33.4 ± 0.41 a | 31.4 ± 0.07 b | 27.0 ± 0.63 b | 26.5 ± 0.30 b | 26.7 ± 0.17 a | 23.5 ± 0.41 a | |
| ECt | NI | 44.6 ± 0.53 a | 41.5 ± 0.19 b | 54.6 ± 0.43 a | 40.9 ± 0.33 b | 41.5 ± 0.13 b | 52.7 ± 0.53 c | 52.6 ± 0.19 c | 56.8 ± 0.43 a | 58.0 ± 0.33 b |
| DI | 44.2 ± 0.31 a | 42.9 ± 0.18 b | 43.0 ± 0.54 b | 46.1 ± 0.75 a | 42.7 ± 0.22 b | 56.2 ± 0.31 b | 56.7 ± 0.18 b | 57.9 ± 0.54 a | 61.1 ± 0.75 a | |
| FI | 44.2 ± 0.32 a | 47.4 ± 0.30 a | 43.6 ± 0.21 b | 44.0 ± 0.77 a | 44.6 ± 0.23 a | 58.9 ± 0.32 a | 59.3 ± 0.30 a | 57.1 ± 0.21 a | 63.3 ± 0.77 a | |
| ECGt | NI | 31.9 ± 0.15 a | 26.9 ± 0.15 a | 20.7 ± 0.18 b | 26.3 ± 0.09 a | 24.0 ± 0.04 a | 19.0 ± 0.15 a | 19.5 ± 0.15 a | 17.4 ± 0.18 a | 16.9 ± 0.09 a |
| DI | 32.0 ± 0.37 a | 28.2 ± 0.19 a | 27.1 ± 0.41 a | 25.6 ± 0.06 a | 24.6 ± 0.17 a | 17.7 ± 0.37 b | 17.5 ± 0.19 b | 16.9 ± 0.41 ab | 14.3 ± 0.06 b | |
| FI | 32.0 ± 0.43 a | 27.7 ± 0.34 a | 29.0 ± 0.14 a | 22.6 ± 0.24 b | 24.0 ± 0.11 a | 14.1 ± 0.43 c | 14.2 ± 0.34 c | 16.2 ± 0.14 b | 13.2 ± 0.24 b | |
| Ce | NI | 4.8 ± 0.17 a | 4.9 ± 0.06 a | 5.3 ± 0.13 a | 5.1 ± 0.14 a | 5.4 ± 0.14 a | 6.7 ± 0.17 a | 6.9 ± 0.06 a | 6.7 ± 0.13 a | 6.7 ± 0.14 a |
| DI | 4.6 ± 0.22 a | 5.1 ± 0.06 a | 4.5 ± 0.28 b | 5.1 ± 0.16 a | 4.8 ± 0.06 b | 6.5 ± 0.22 a | 6.7 ± 0.06 a | 6.7 ± 0.28 a | 6.8 ± 0.16 a | |
| FI | 4.6 ± 0.08 a | 4.1 ± 0.13 b | 4.4 ± 0.08 b | 5.0 ± 0.09 a | 4.9 ± 0.05 b | 6.4 ± 0.08 a | 6.4 ± 0.13 a | 6.5 ± 0.08 a | 6.4 ± 0.09 a | |
| ECe | NI | 93.0 ± 0.43 a | 93.0 ± 0.14 a | 92.7 ± 0.24 a | 92.6 ± 0.19 a | 92.2 ± 0.22 a | 92.2 ± 0.43 a | 91.9 ± 0.14 a | 92.1 ± 0.24 a | 92.1 ± 0.19 a |
| DI | 93.1 ± 0.49 a | 92.9 ± 0.15 a | 93.9 ± 1.03 a | 92.5 ± 0.71 a | 92.7 ± 0.25 a | 92.4 ± 0.49 a | 92.2 ± 0.15 a | 92.2 ± 1.03 a | 92.0 ± 0.71 a | |
| FI | 93.1 ± 0.51 a | 93.7 ± 0.16 a | 93.4 ± 0.29 a | 92.7 ± 0.56 a | 92.6 ± 0.31 a | 92.6 ± 0.51 a | 92.5 ± 0.16 a | 92.4 ± 0.29 a | 92.5 ± 0.56 a | |
| ECGe | NI | 2.2 ± 0.08 a | 2.1 ± 0.07 a | 2.0 ± 0.04 b | 2.4 ± 0.06 a | 2.4 ± 0.02 a | 1.1 ± 0.08 a | 1.2 ± 0.07 a | 1.1 ± 0.04 a | 1.2 ± 0.06 a |
| DI | 2.3 ± 0.11 a | 2.0 ± 0.10 a | 1.6 ± 0.04 c | 2.4 ± 0.10 a | 2.4 ± 0.08 a | 1.1 ± 0.11 a | 1.1 ± 0.10 a | 1.1 ± 0.04 a | 1.2 ± 0.10 a | |
| FI | 2.3 ± 0.13 a | 2.1 ± 0.05 a | 2.3 ± 0.10 a | 2.3 ± 0.07 a | 2.5 ± 0.07 a | 1.0 ± 0.13 a | 1.0 ± 0.05 a | 1.1 ± 0.10 a | 1.2 ± 0.07 a | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyraleou, M.; Kallithraka, S.; Theodorou, N.; Teissedre, P.-L.; Kotseridis, Y.; Koundouras, S. Changes in Tannin Composition of Syrah Grape Skins and Seeds during Fruit Ripening under Contrasting Water Conditions. Molecules 2017, 22, 1453. https://doi.org/10.3390/molecules22091453
Kyraleou M, Kallithraka S, Theodorou N, Teissedre P-L, Kotseridis Y, Koundouras S. Changes in Tannin Composition of Syrah Grape Skins and Seeds during Fruit Ripening under Contrasting Water Conditions. Molecules. 2017; 22(9):1453. https://doi.org/10.3390/molecules22091453
Chicago/Turabian StyleKyraleou, Maria, Stamatina Kallithraka, Nikolaos Theodorou, Pierre-Louis Teissedre, Yorgos Kotseridis, and Stefanos Koundouras. 2017. "Changes in Tannin Composition of Syrah Grape Skins and Seeds during Fruit Ripening under Contrasting Water Conditions" Molecules 22, no. 9: 1453. https://doi.org/10.3390/molecules22091453