Diuretic Activity of Compatible Triterpene Components of Alismatis rhizoma
Abstract
:1. Introduction
2. Results
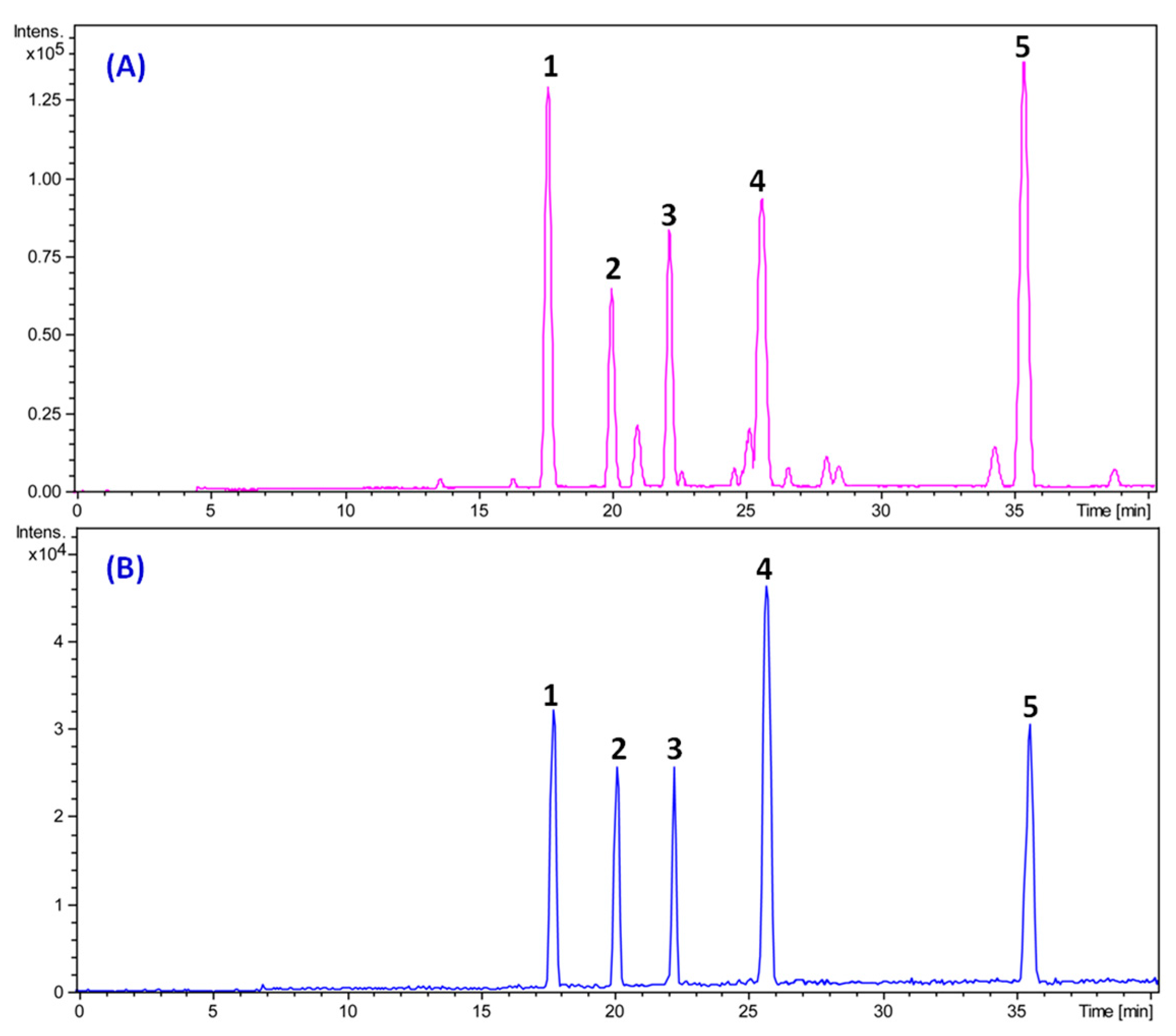
2.1. Determination of Triterpenes in Total Triterpene Extract (TTE)
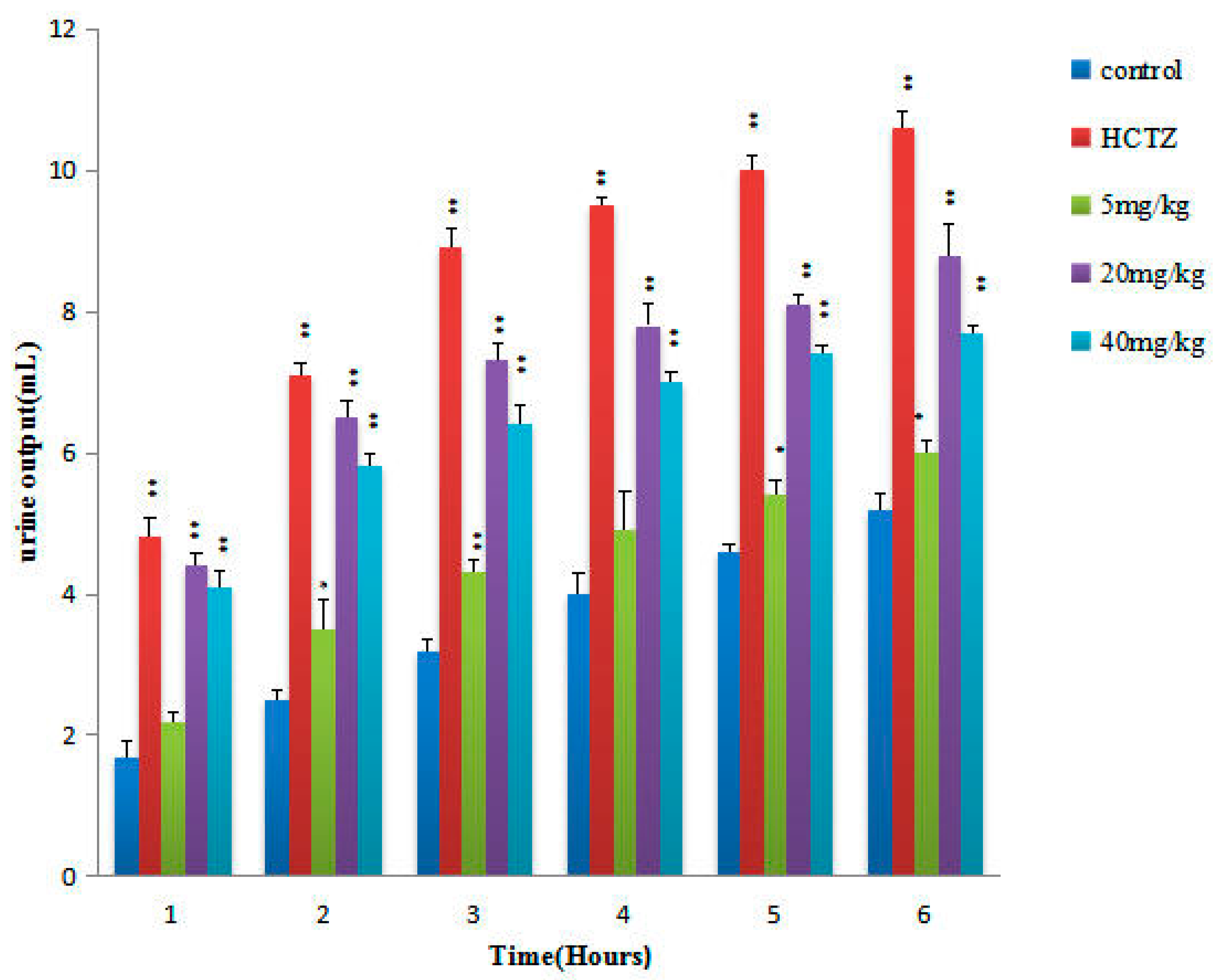
2.2. Urinary Volume and Electrolyte Excretion of the Diuretic Activity of TTE
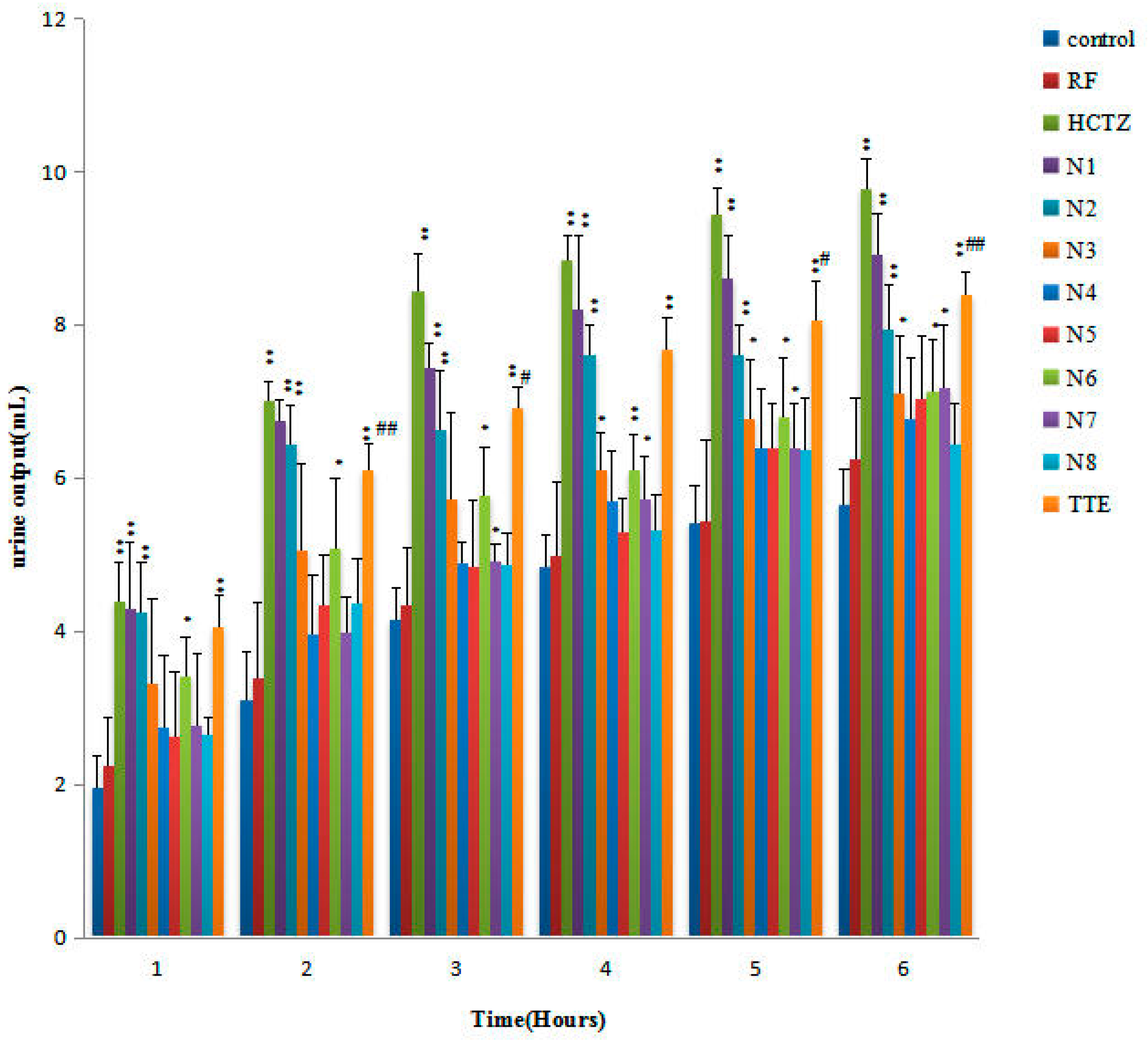
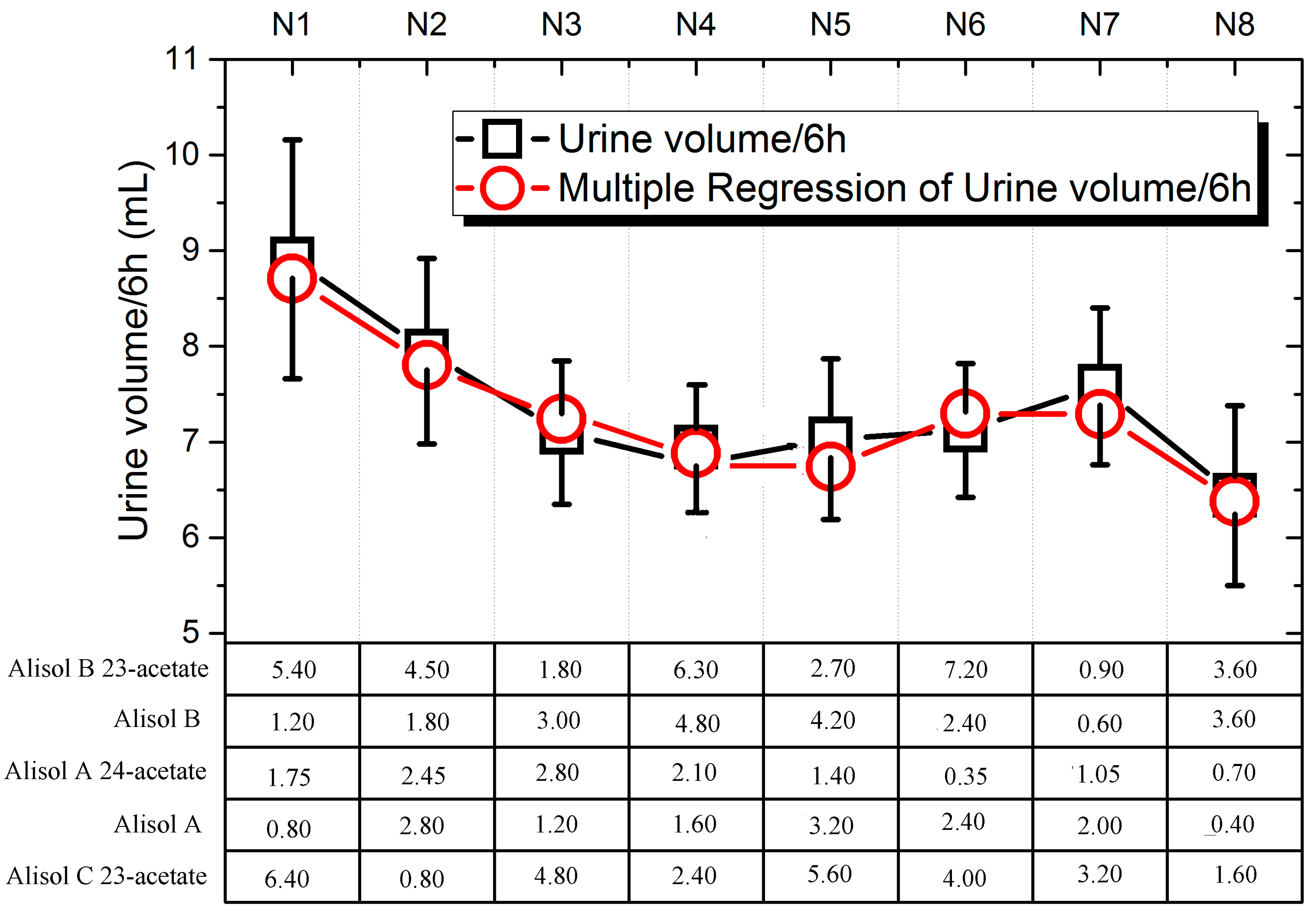
2.3. Urinary Volume and Electrolyte Excretion of the Diuretic Activity of Triterpene Component Compatibility (TCC)
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of TTE and Quantification
4.3. Preparation of TCC
4.4. Reference Drug
4.5. Experimental Animals
4.6. Diuretic Activity of TTE
4.7. Diuretic Activity of TCC
4.8. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, C.; Feng, L.; Ma, L.; Chen, H.; Tan, X.; Hou, X.; Song, J.; Cui, L.; Liu, D.; Chen, J.; et al. Alisol a 24-acetate and alisol b 23-acetate induced autophagy mediates apoptosis and nephrotoxicity in human renal proximal tubular cells. Front. Pharmacol. 2017, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.L.; Chen, H.; Tian, T.; Chen, D.Q.; Zhao, Y.Y.; Lin, R.C. Diuretic and anti-diuretic activities of the ethanol and aqueous extracts of Alismatis rhizoma. J. Ethnopharmacol. 2014, 154, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Han, C.W.; Kwun, M.J.; Kim, K.H.; Choi, J.Y.; Oh, S.R.; Ahn, K.S.; Lee, J.H.; Joo, M. Ethanol extract of Alismatis Rhizoma reduces acute lung inflammation by suppressing NF-kappaB and activating Nrf2. J. Ethnopharmacol. 2013, 146, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Xu, W.; Xu, Y.L.; Chen, X.; Huang, M.; Lu, J.J. Therapeutic potential of rhizoma alismatis: A review on ethnomedicinal application, phytochemistry, pharmacology, and toxicology. Ann. N.Y. Acad. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Fan, M.; Xue, Y.; Peng, L.Y.; Wu, X.D.; Liu, D.; Li, R.T.; Zhao, Q.S. Guaiane-type sesquiterpenoids from alismatis rhizoma and their anti-inflammatory activity. Chem. & Pharm. Bull. 2017, 65, 403–407. [Google Scholar]
- Li, S.; Jin, S.; Song, C.; Chen, C.; Zhang, Y.; Xiang, Y.; Xu, Y.; Feng, Y.; Wan, Q.; Jiang, H. The metabolic change of serum lysophosphatidylcholines involved in the lipid lowering effect of triterpenes from Alismatis rhizoma on high-fat diet induced hyperlipidemia mice. J. Ethnopharmacol. 2016, 177, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, D.M.; Liu, J.H.; Hu, L.S.; Xue, Q.C.; Ding, X.Q.; Kong, L.D. Wuling san protects kidney dysfunction by inhibiting renal TLR4/MyD88 signaling and NLRP3 inflammasome activation in high fructose-induced hyperuricemic mice. J. Ethnopharmacol. 2015, 169, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, S.L.; Zhou, Y.; Song, J.Z.; Zheng, Y.F.; Peng, G.P.; Xu, H.X. Characterization of protostane triterpenoids in alisma orientalis by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom.: RCM 2010, 24, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Q.; Feng, Y.L.; Tian, T.; Chen, H.; Yin, L.; Zhao, Y.Y.; Lin, R.C. Diuretic and anti-diuretic activities of fractions of Alismatis rhizoma. J. Ethnopharmacol. 2014, 157, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, T.; Qiu, J.F.; Wu, S.S.; Huang, M.Q.; Lin, L.G.; Zhang, Q.W.; Chen, X.P.; Lu, J.J. Anti-proliferative activities of terpenoids isolated from alisma orientalis and their structure-activity relationships. Anti-Cancer Agents Med. Chem. 2015, 15, 228–235. [Google Scholar] [CrossRef]
- Wang, L.X.; Wu, Q.N.; Zhang, Q.; Peng, G.P.; Ding, A.W. Basic study of diuretic active compounds in Rhizoma Alismatis. West Chin. J. Pharm. Sci. 2008, 23, 670–672. [Google Scholar]
- Xu, W.; XU, M.T.; ZHAO, W.L.; Qiu, J.F.; Li, X.Y.; Luo, F.R.; Huang, M.Q.; Wu, S.S. Determination of triterpenoids in Rhizoma Alismatis extracts. Chin. J. Pharm. Anal. 2015, 35, 210–217. [Google Scholar]
- Zhao, W.; Huang, X.; Li, X.; Zhang, F.; Chen, S.; Ye, M.; Huang, M.; Xu, W.; Wu, S. Qualitative and quantitative analysis of major triterpenoids in Alismatis rhizoma by high performance liquid chromatography/diode-array detector/quadrupole-time-of-flight mass spectrometry and ultra-performance liquid chromatography/triple quadrupole mass spectrometry. Molecules 2015, 20, 13958–13981. [Google Scholar] [PubMed]
- Qiu, J.F.; Lin, J.; Xu, W.; Huang, M.Q.; Zhao, W.L.; Luo, F.R.; Li, X.Y.; Wu, S.S. HPLC-DAD-ELSD simultaneous of four determination terpenoids in rhizoma alismatis. Med. Plant 2014, 5, 22–26. [Google Scholar]
- Murata, T.; Shinohara, M.; Hirata, T.; Kamiya, K.; Nishikawa, M.; Miyamoto, M. New triterpenes of alisma plantago-aquatica l. Var. Orientale samuels. Tetrahedron Lett. 1968, 9, 103–108. [Google Scholar] [CrossRef]
- Tian, T.; Chen, H.; Zhao, Y.Y. Traditional uses, phytochemistry, pharmacology, toxicology and quality control of Alisma orientale (Sam.) Juzep: A review. J. Ethnopharmacol. 2014, 158 Pt A, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Pu, J.; Chen, L.; Zhang, Y.; Rahman, K.; Qin, L.; Zheng, C. Alisma orientale: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. Am. J. Chin. Med. 2016, 44, 227–251. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.M.; Choi, J.W.; Park, J.C. Effects of methanol extract of alisma orientale rhizome and its major component, alisol b 23-acetate, on hepatic drug metabolizing enzymes in rats treated with bromobenzene. Archives Pharm. Res. 2007, 30, 1543–1549. [Google Scholar] [CrossRef]
- Toma, C.C.; Olah, N.K.; Vlase, L.; Mogoșan, C.; Mocan, A. Comparative Studies on Polyphenolic Composition, Antioxidant and Diuretic Effects of Nigella sativa L. (Black Cumin) and Nigella damascena L. (Lady-in-a-Mist) Seeds. Molecules 2015, 20, 9560–9574. [Google Scholar] [CrossRef] [PubMed]
- Păltinean, R.; Mocan, A.; Vlase, L.; Gheldiu, A.M.; Crișan, G.; Ielciu, I.; Voștinaru, O.; Crișan, O. Evaluation of Polyphenolic Content, Antioxidant and Diuretic Activities of Six Fumaria Species. Molecules 2017, 22, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Ntchapda, F.; Bonabe, C.; Kemeta Azambou, D.R.; Talla, E.; Dimo, T. Diuretic and antioxidant activities of the aqueous extract of leaves of Vepris heterophylla (Engl.) R. Let (Rutaceae) in rats. BMC Complement. Altern. Med. 2016, 13, 516–525. [Google Scholar] [CrossRef] [PubMed]
- De Santana Aquino, D.F.; Signor Tirloni, C.A.; Tolouei Menegati, S.E.; Lima Cardoso, C.A.; Heredia Vieira, S.C.; Carmo Vieira, M.D.; Simonet, A.M.; Macías, F.A.; Junior, A.G. Alibertia edulis (L.C. Rich.) A.C. Rich-A potent diuretic arising from Brazilian indigenous species. J. Ethnopharmacol. 2017, 196, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zeng, X.; Han, L.; Wei, J.A.; Huang, H. Diuretic activity and kidney medulla aqp1, aqp2, aqp3, v2r expression of the aqueous extract of sclerotia of polyporus umbellatus fries in normal rats. J. Ethnopharmacol. 2010, 128, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Hl, K.; Sl, S.; Ps, V.; Vh, P.; Am, S.; Sibgatullah, M. Diuretic activity of ethanolic root extract of Mimosa pudica in albino rats. J. Clin. Diagn. Res. 2015, 9, 5–7. [Google Scholar]
- Alshahrani, S.; Rapoport, R.M.; Zahedi, K.; Jiang, M.; Nieman, M.; Barone, S.; Meredith, A.L.; Lorenz, J.N.; Rubinstein, J.; Soleimani, M. The non-diuretic hypotensive effects of thiazides are enhanced during volume depletion states. PLoS ONE 2017, 12, e0181376. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Q.; Xu, W.; Wu, S.S.; Lu, J.J.; Chen, X.P. A 90-day subchronic oral toxicity study of triterpene-enriched extract from Alismatis Rhizoma in rats. Food Chem. Toxicol. 2013, 58, 318–323. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| No. | tR | Molecular Ion | Error | Fragment Ions in Positive Mode | Molecular | Identity | Contents |
|---|---|---|---|---|---|---|---|
| (min) | MS1 | (ppm) | MS2 | Formula | (mg/g) | ||
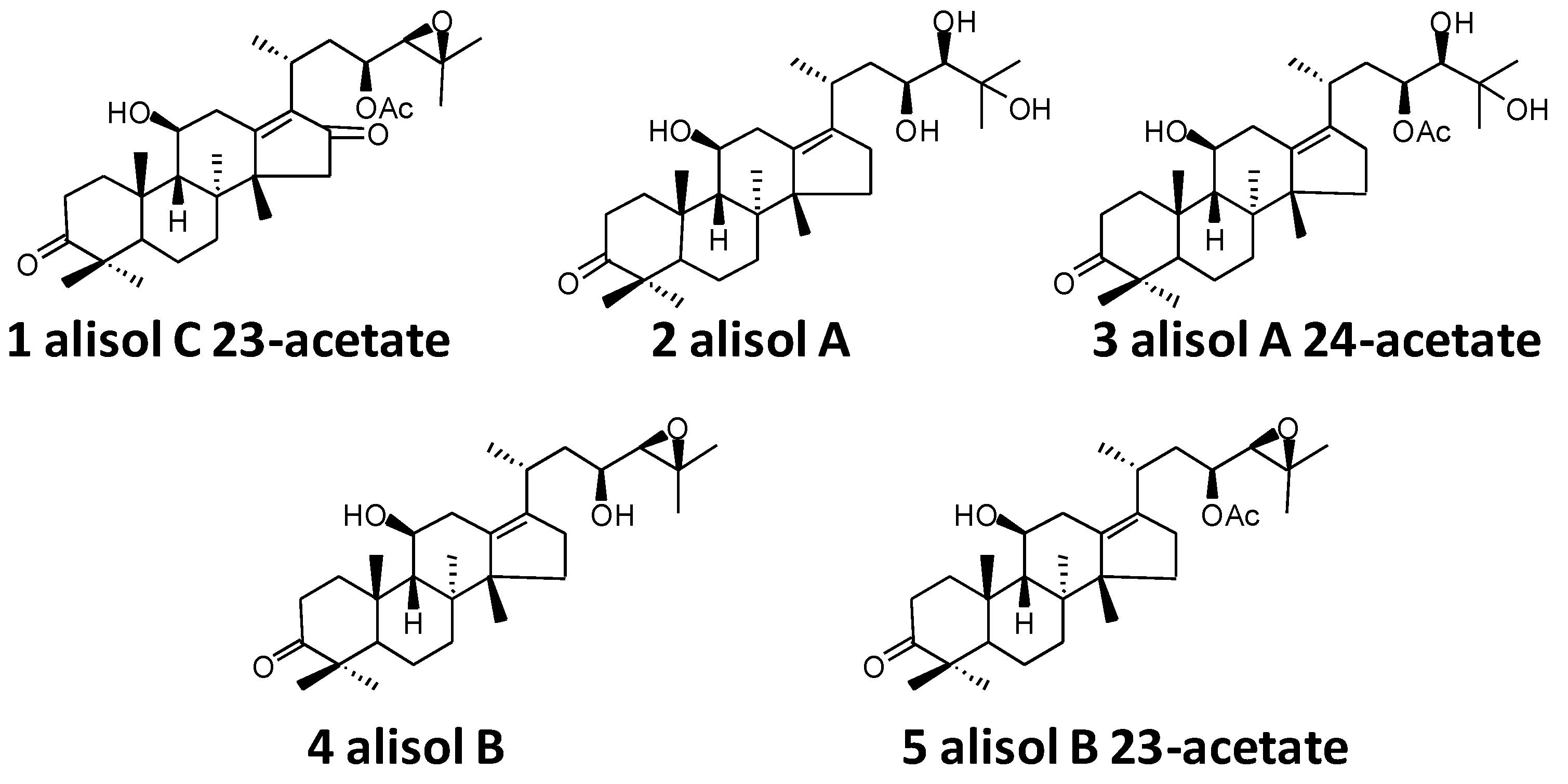
| 1 | 17.53 | 529.3527 [M + H]+ | 0.8 | 511 [M + H-H2O]+, 469 [M + H-HAc]+, 451 [M + H-HAc-H2O]+, 415 [M + H-C6H10O2]+, 397 [M + H- C6H10O2-H2O]+ | C32H48O6 | alisol C 23-acetate | 138.51 |
| 2 | 20.05 | 491.3139 [M + H]+ | 0.4 | 473 [M + H-H2O]+, 455 [M + H-2H2O]+, 437 [M + H-3H2O]+, 383 [M + H-H2O-C4H10O2]+ | C30H50O5 | alisol A | 81.93 |
| 3 | 22.23 | 533.3833 [M + H]+ | −0.6 | 515 [M + H-H2O]+, 497 [M + H-2H2O]+, 455 [M + H-HAc-H2O] +, 437 [M + H-HAc-2H2O]+, 383 [M + H- C6H12O3-H2O]+ | C32H52O6 | alisol A 24-acetate | 70.02 |
| 4 | 25.75 | 473.3631 [M + H]+ | 1.3 | 455 [M + H-H2O]+, 437 [M + H-2H2O]+, 383 [M + H-H2O-C4H8O]+ | C30H48O4 | alisol B | 52.95 |
| 5 | 35.46 | 515.3732 [M + H]+ | 0.2 | 497 [M + H-H2O]+, 479 [M + H-2H2O]+, 437 [M + H-H2O-HAc]+, 383 [M + H-H2O- C6H10O2]+ | C32H50O5 | alisol B 23-acetate | 153.67 |
| Group | Dose (mg/kg) | pH | Urine Electrolyte Concentration (mmoL∙L−1/6 h) | Saluretic Index | Na+/K+ | ||||
|---|---|---|---|---|---|---|---|---|---|
| K+ | Na+ | Cl− | K+ | Na+ | Cl− | ||||
| control | − | 6.85 ± 0.42 | 107.71 ± 20.40 | 110.77 ± 16.22 | 232.12 ± 20.15 | 1 | 1 | 1 | 1.03 ± 0.03 |
| HCTZ | 20 | 6.90 ± 0.72 | 120.11 ± 15.93 | 173.83 ± 22.90 * | 299.54 ± 11.10 ** | 1.15 | 1.57 | 1.29 | 1.61 ± 0.08 ** |
| TTE | 5 | 6.97 ± 0.53 | 117.52 ± 16.23 | 130.43 ± 10.46 * | 267.22 ± 11.09 * | 1.09 | 1.18 | 1.15 | 1.11 ± 0.06 * |
| 20 | 7.28 ± 0.42 * | 129.46 ± 18.13 * | 149.75 ± 17.38 ** | 287.88 ± 29.55 ** | 1.20 | 1.35 | 1.24 | 1.16 ± 0.04 ** | |
| 40 | 7.06 ± 0.58 | 120.77 ± 8.11 * | 146.28 ± 12.33 * | 272.56 ± 17.11 ** | 1.12 | 1.32 | 1.17 | 1.21 ± 0.05 ** | |
| Factor | Alisol B 23-acetate /mg·kg−1 | Alisol B/mg·kg−1 | Alisol A 24-acetate /mg·kg−1 | Alisol A/mg·kg−1 | Alisol C 23-acetate /mg·kg−1 |
|---|---|---|---|---|---|
| N1 | 5.40 | 1.20 | 1.75 | 0.80 | 6.40 |
| N2 | 4.50 | 1.80 | 2.45 | 2.80 | 0.80 |
| N3 | 1.80 | 3.00 | 2.80 | 1.20 | 4.80 |
| N4 | 6.30 | 4.80 | 2.10 | 1.60 | 2.40 |
| N5 | 2.70 | 4.20 | 1.40 | 3.20 | 5.60 |
| N6 | 7.20 | 2.40 | 0.35 | 2.40 | 4.00 |
| N7 | 0.90 | 0.60 | 1.05 | 2.00 | 3.20 |
| N8 | 3.60 | 3.60 | 0.70 | 0.40 | 1.60 |
| Urine Output/mL | ||||||
|---|---|---|---|---|---|---|
| Group | 1 h | 2 h | 3 h | 4 h | 5 h | 6 h |
| Control | 1.96 ± 1.01 | 3.11 ± 0.92 | 4.14 ± 0.93 | 4.84 ± 1.42 | 5.41 ± 0.80 | 5.65 ± 0.77 |
| TTE | 3.05 ± 0.59 * | 6.51 ± 0.65 ** | 7.31 ± 0.62 ** | 7.87 ± 0.80 ** | 8.15 ± 0.91 ** | 8.59 ± 1.02 ** |
| RF Group | 2.24 ± 0.64 | 3.39 ± 0.99 | 4.35 ± 0.73 | 4.99 ± 0.95 | 5.43 ± 1.08 | 6.26 ± 0.78 |
| HCTZ | 4.40 ± 1.09 ** | 6.02 ± 1.65 ** | 7.04 ± 1.58 ** | 7.44 ± 1.83 ** | 8.54 ± 1.34 ** | 10.19 ± 1.07 ** |
| Verify1 | 3.99 ± 0.67 ** | 5.85 ± 0.89 ** | 6.24 ± 0.91 ** | 7.40 ± 0.71 ** | 8.34 ± 1.08 ** | 9.49 ± 0.96 ** |
| Verify2 | 3.73 ± 1.05 ** | 6.60 ± 1.01 ** | 7.63 ± 0.78 ** | 7.81 ± 0.88 ** | 8.61 ± 1.08 ** | 9.81 ± 0.89 ** |
| Verify3 | 4.06 ± 0.69 ** | 5.71 ± 1.01 ** | 6.36 ± 0.78 ** | 7.87 ± 0.94 ** | 8.49 ± 0.77 ** | 9.63 ± 1.10 ** |
| Group | Diuretic index | pH | Urine Electrolyte Concentration(mmoL∙L−1/6h) | Saluretic Index | Na+/ K+ | ||||
|---|---|---|---|---|---|---|---|---|---|
| K+ | Na+ | Cl− | K+ | Na+ | Cl− | ||||
| Control | 1 | 7.45 ± 0.35 | 102.02 ± 1.23 | 124.81 ± 8.23 | 234.64 ± 3.28 | 1 | 1 | 1 | 1.22 ± 0.21 |
| TTE | 1.52 | 8.09 ± 0.31 ** | 130.44 ± 16.9 ** | 195.18 ± 7.28 ** | 348.61 ± 8.64 ** | 1.27 | 1.56 | 1.48 | 1.49 ± 0.18 |
| RF | 1.11 | 7.49 ± 0.38 | 108.08 ± 9.90 | 164.94 ± 17.53 | 276.83 ± 9.52 | 1.06 | 1.32 | 1.18 | 1.52 ± 0.08 ** |
| HCTZ | 1.83 | 7.48 ± 0.66 | 112.79 ± 8.84 | 195.95 ± 7.86 ** | 358.31 ± 9.99 ** | 1.11 | 1.57 | 1.53 | 1.74 ± 0.12 ** |
| N1 | 1.58 | 8.12 ± 0.25 * | 131.01 ± 11.16 ** | 211.83 ± 16.00 ** | 363.70 ± 12.75 ** | 1.28 | 1.69 | 1.55 | 1.62 ± 0.16 ** |
| N2 | 1.41 | 8.24 ± 0.18 ** | 141.45 ± 1.42** | 184.76 ± 12.43 ** | 359.05 ± 13.87 ** | 1.39 | 1.48 | 1.1 | 1.30 ± 0.06 |
| N3 | 1.26 | 7.80 ± 0.72 | 106.80 ± 31.11 | 166.92 ± 14.05 * | 300.34 ± 11.37 ** | 1.05 | 1.33 | 1.28 | 1.56 ± 0.09 ** |
| N4 | 1.20 | 7.54 ± 0.89 | 107.72 ± 13.76 | 152.05 ± 16.84 * | 297.06 ± 9.26 ** | 1.06 | 1.21 | 1.26 | 1.41 ± 0.11 * |
| N5 | 1.24 | 7.48 ± 0.29 | 107.31 ± 18.53 | 161.88 ± 15.77 ** | 309.02 ± 24.57 * | 1.05 | 1.29 | 1.32 | 1.51 ± 0.03 ** |
| N6 | 1.28 | 7.50 ± 0.73 | 106.80 ± 11.12 | 140.12 ± 26.06 | 254.34 ± 11.22 ** | 1.05 | 1.12 | 1.08 | 1.31 ± 0.07 |
| N7 | 1.29 | 7.54 ± 0.90 | 107.72 ± 19.77 | 152.17 ± 9.85 ** | 268.16 ± 28.45 | 1.06 | 1.22 | 1.14 | 1.41 ± 0.05 * |
| N8 | 1.16 | 7.18 ± 0.80 | 107.31 ± 10.54 | 131.88 ± 6.18 | 248.02 ± 18.98 | 1.05 | 1.06 | 1.06 | 1.23 ± 0.15 |
| Verify1 | 1.56 | 8.29 ± 0.71 ** | 138.58 ± 11.16 ** | 201.06 ± 7.11 ** | 368.39 ± 9.07 ** | 1.36 | 1.62 | 1.57 | 1.45 ± 0.08 ** |
| Verify2 | 1.63 | 8.17 ± 0.37 ** | 140.42 ± 1.64 ** | 199.56 ± 28.71 ** | 381.32 ± 9.78 ** | 1.38 | 1.59 | 1.62 | 1.42 ± 0.13 ** |
| Verify3 | 1.65 | 8.42 ± 0.52 ** | 135.93 ± 6.62 ** | 210.56 ± 17.02 ** | 352.24 ± 6.34 ** | 1.33 | 1.68 | 1.50 | 1.56 ± 0.12 ** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, X.-Y.; Lin, N.; Zhao, W.-L.; Huang, X.-Q.; Chen, Y.; Huang, M.-Q.; Xu, W.; Wu, S.-S. Diuretic Activity of Compatible Triterpene Components of Alismatis rhizoma. Molecules 2017, 22, 1459. https://doi.org/10.3390/molecules22091459
Zhang X, Li X-Y, Lin N, Zhao W-L, Huang X-Q, Chen Y, Huang M-Q, Xu W, Wu S-S. Diuretic Activity of Compatible Triterpene Components of Alismatis rhizoma. Molecules. 2017; 22(9):1459. https://doi.org/10.3390/molecules22091459
Chicago/Turabian StyleZhang, Xue, Xiao-Yan Li, Na Lin, Wan-Li Zhao, Xiao-Qiang Huang, Ying Chen, Ming-Qing Huang, Wen Xu, and Shui-Sheng Wu. 2017. "Diuretic Activity of Compatible Triterpene Components of Alismatis rhizoma" Molecules 22, no. 9: 1459. https://doi.org/10.3390/molecules22091459