Statistical Methodologies for the Optimization of Lipase and Biosurfactant by Ochrobactrum intermedium Strain MZV101 in an Identical Medium for Detergent Applications
Abstract
:1. Introduction
2. Results
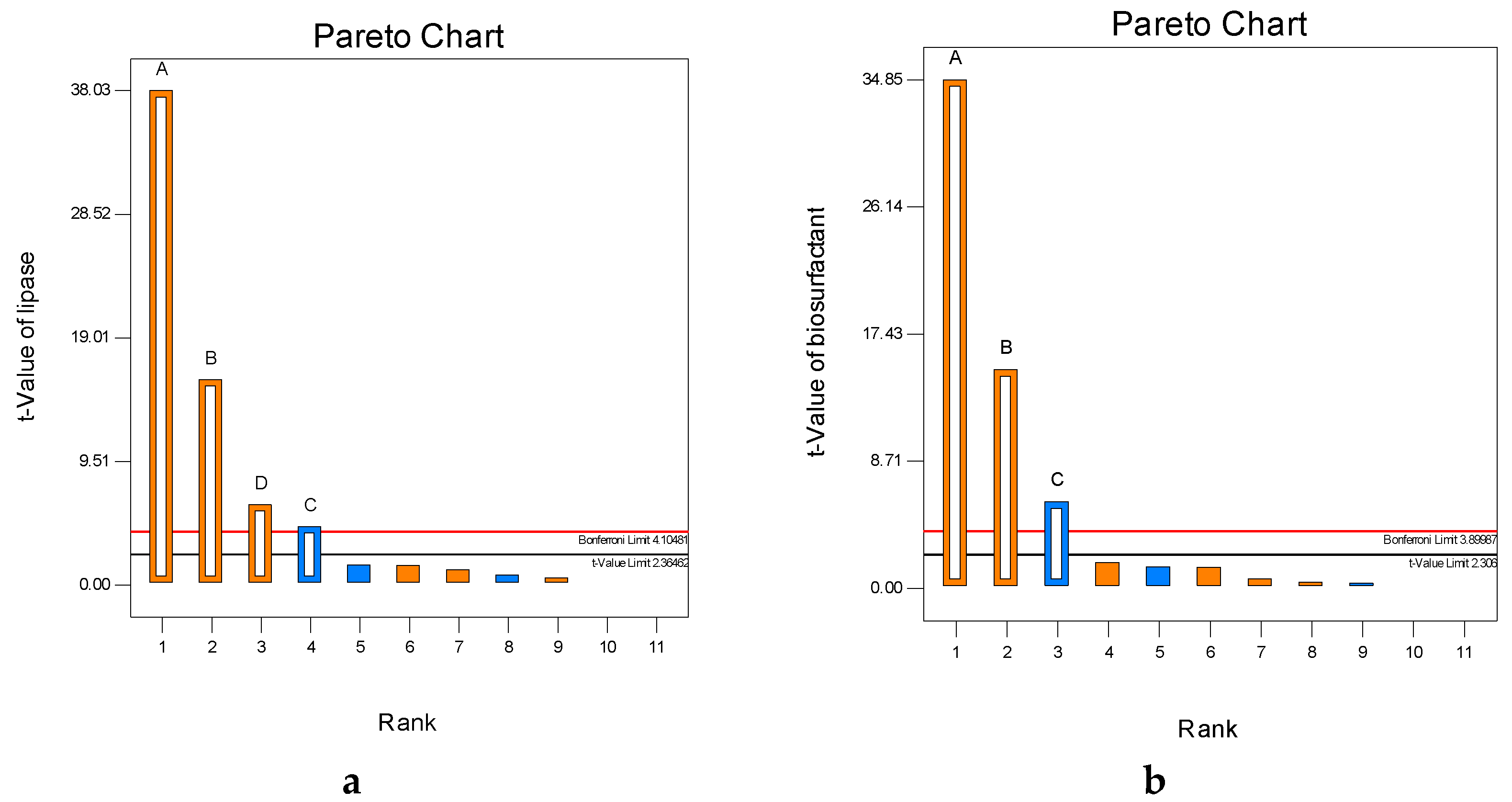
2.1. Plackett–Burman Design
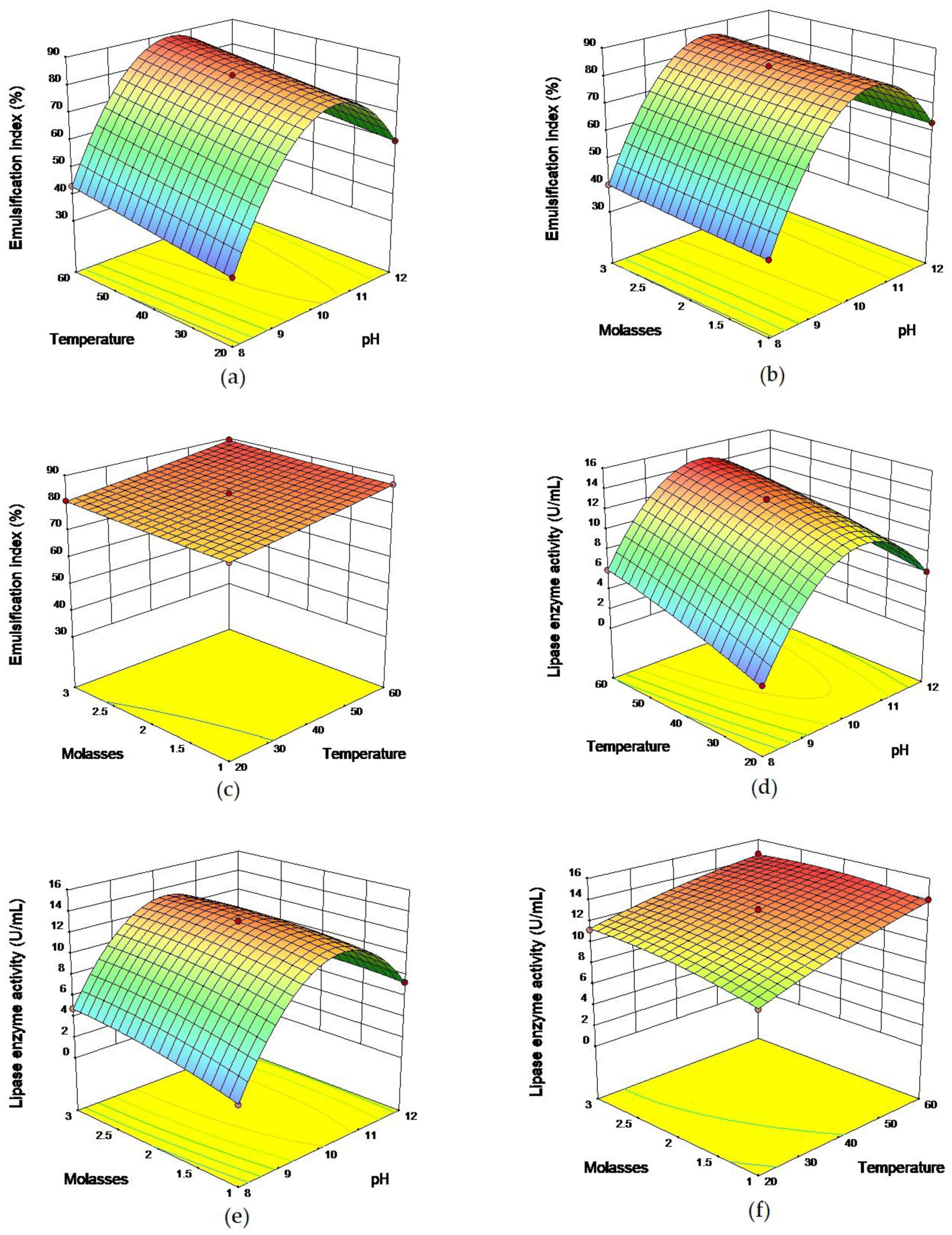
2.2 Response Surface Methodology
2.3 Validation of the Model
3. Materials and Methods
3.1. Materials
3.2. Microorganism and Growth Medium
3.3. Lipase Activity Assay (U/mL)
3.4. Emulsification Index (%)
3.5. Statistical Methodology
3.5.1. Plackett–Burman Design
3.5.2. Response Surface Methodology
3.5.3. Validation of the Model
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lang, S. Biological amphiphiles (microbial biosurfactants). Curr. Opin. Coll. Interface Sci. 2002, 7, 12–20. [Google Scholar]
- Colla, L.M.; Rizzardi, J.; Pinto, M.H.; Reinehr, C.O.; Bertolin, T.E.; Costa, J.A.V. Simultaneous production of lipases and biosurfactants by submerged and solid-state bioprocesses. Bioresour. Technol. 2010, 101, 8308–8314. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.G.; Sujitha, P.; Mamidyala, S.K.; Usharani, P.; Das, B.; Reddy, C.R. Ochrosin, a new biosurfactant produced by Halophilic ochrobactrum sp. Strain bs-206 (mtcc 5720): Purification, characterization and its biological evaluation. Process Biochem. 2014, 49, 1708–1717. [Google Scholar] [CrossRef]
- Kosaric, N. Biosurfactants in industry. In Pure and Applied Chemistry; Retrieved 2; Walter de Gruyter GmbH: Berlin/Boston, Germany, 1992; Volume 64, p. 1731. [Google Scholar]
- Joshi, S.; Bharucha, C.; Jha, S.; Yadav, S.; Nerurkar, A.; Desai, A.J. Biosurfactant production using molasses and whey under thermophilic conditions. Bioresour. Technol. 2008, 99, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Meylheuc, T.; Van Oss, C.J.; Bellon-Fontaine, M.N. Adsorption of biosurfactant on solid surfaces and consequences regarding the bioadhesion of Listeria monocytogenes lo28. J. Appl. Microbiol. 2001, 91, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Bhange, K.; Chaturvedi, V.; Bhatt, R. Simultaneous production of detergent stable keratinolytic protease, amylase and biosurfactant by Bacillus subtilis pf1 using agro industrial waste. Biotechnol. Repor. 2016, 10, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Donio, M.B.S.; Ronica, S.F.A.; Viji, V.T.; Velmurugan, S.; Jenifer, J.A.; Michaelbabu, M.; Citarasu, T. Isolation and characterization of Halophilic bacillus sp. Bs3 able to produce pharmacologically important biosurfactants. Asian Pac. J. Trop. Med. 2013, 6, 876–883. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Rangarajan, V.; Sen, R.; Rodrigues, L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013, 34, 667–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, A.K.; Adhikari, H.; Rai, S.K. Production of alkaline protease by a thermophilic bacillus subtilis under solid-state fermentation (ssf) condition using imperata cylindrica grass and potato peel as low-cost medium: Characterization and application of enzyme in detergent formulation. Biochem. Eng. J. 2008, 39, 353–361. [Google Scholar] [CrossRef]
- Rahman, P.K.S.M.; Gakpe, E. Production, characterisation and applications of biosurfactants-review. Biotechnology 2008, 7, 360–370. [Google Scholar] [CrossRef]
- Tanyildizi, M.S.; Özer, D.; Elibol, M. Optimization of α-amylase production by Bacillus sp. Using response surface methodology. Process Biochem. 2005, 40, 2291–2296. [Google Scholar] [CrossRef]
- Plackett, R.L.; Burman, J.P. The design of optimum multifactorial experiments. Biometrika 1946, 33, 305–325. [Google Scholar] [CrossRef]
- Korayem, A.S.; Abdelhafez, A.A.; Zaki, M.M.; Saleh, E.A. Optimization of biosurfactant production by Streptomyces isolated from egyptian arid soil using plackett-burman design. Ann. Agric. Sci. 2015, 60, 209–217. [Google Scholar] [CrossRef]
- Parkhey, P.; Gupta, P.; Eswari, J.S. Optimization of cellulase production from isolated cellulolytic bacterium: Comparison between genetic algorithms, simulated annealing, and response surface methodology. Chem. Eng. Commun. 2016, 204, 28–38. [Google Scholar] [CrossRef]
- Elibol, M. Optimization of medium composition for actinorhodin production by Streptomyces coelicolor a3(2) with response surface methodology. Process Biochem. 2004, 39, 1057–1062. [Google Scholar] [CrossRef]
- Holmes, B.; Popoff, M.; Kiredjian, M.; Kersters, K. Ochrobactrum anthropi gen. Nov., sp. Nov. From human clinical specimens and previously known as group vd. Intern. J. Syst. Bacteriol. 1988, 38, 406–416. [Google Scholar] [CrossRef]
- Desai, J.D.; Banat, I.M. 97/02677 microbial production of surfactants and their commercial potential. Fuel Energy Abstr. 1997, 38, 221. [Google Scholar]
- Zinjarde, S.S.; Pant, A. Emulsifier from a tropical marine yeast, Yarrowia lipolytica ncim 3589. J. Basic Microbiol. 2002, 42, 67. [Google Scholar] [CrossRef]
- Bisht, D.; Yadav, S.K.; Darmwal, N.S. Computation of interactive effects and optimization of process parameters for alkaline lipase production by mutant strain of Pseudomonas aeruginosa using response surface methodology. Braz. J. Microbiol. 2013, 44, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Khoramnia, A.; Ebrahimpour, A.; Beh, B.K.; Lai, O.M. Production of a solvent, detergent, and thermotolerant lipase by a newly isolated Acinet obacter sp. In submerged and solid-state fermentations. J. Biomed. Biotechnol. 2011, 2011, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Singh, S.N. Microbial degradation of n-hexadecane in mineral salt medium as mediated by degradative enzymes. Bioresour. Technol. 2012, 111, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Rathi, P.; Saxena, R.K.; Gupta, R. A novel alkaline lipase from Burkholderia cepacia for detergent formulation. Process Biochem. 2001, 37, 187–192. [Google Scholar] [CrossRef]
- Noparat, P.; Maneerat, S.; Saimmai, A. Utilization of palm oil decanter cake as a novel substrate for biosurfactant production from a new and promising strain of Ochrobactrum anthropi 2/3. World J. Microbiol. Biotechnol. 2014, 30, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.K.; Mukherjee, A.K. Applications of a high maltose forming, thermo-stable α-amylase from an extremely alkalophilic Bacillus licheniformis strain as08e in food and laundry detergent industries. Biochem. Eng. J. 2013, 77, 220–230. [Google Scholar] [CrossRef]
- De Carvalho, C.C.C.R. Enzymatic and whole cell catalysis: Finding new strategies for old processes. Biotechnol. Adv. 2011, 29, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Grbavčić, S.; Bezbradica, D.; Izrael-Živković, L.; Avramović, N.; Milosavić, N.; Karadžić, I.; Knežević-Jugović, Z. Production of lipase and protease from an indigenous Pseudomonas aeruginosa strain and their evaluation as detergent additives: Compatibility study with detergent ingredients and washing performance. Bioresour. Technol. 2011, 102, 11226–11233. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Jiang, H.-J. Recovery and purification of the lipopeptide biosurfactant of Bacillus subtilis by ultrafiltration. Biotechnol. Tech. 1997, 11, 413–416. [Google Scholar] [CrossRef]
- Bora, L.; Kalita, M.C. Production of thermostable alkaline lipase on vegetable oils from a Thermophilic bacillus sp. Dh4, characterization and its potential applications as detergent additive. J. Chem. Technol. Biotechnol. 2008, 83, 688–693. [Google Scholar]
- Cherif, S.; Mnif, S.; Hadrich, F.; Abdelkafi, S.; Sayadi, S. A newly high alkaline lipase: An ideal choice for application in detergent formulations. Lipids Health Dis. 2011, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Hemachander, C.; Puvanakrishnan, R. Lipase from Ralstonia pickettii as an additive in laundry detergent formulations. Process Biochem. 2000, 35, 809–814. [Google Scholar] [CrossRef]
- Shavandi, M.; Mohebali, G.; Haddadi, A.; Shakarami, H.; Nuhi, A. Emulsification potential of a newly isolated biosurfactant-producing bacterium, Rhodococcus sp. Strain ta6. Coll. surf. B Biointerfaces 2011, 82, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Ferhat, S.; Mnif, S.; Badis, A.; Eddouaouda, K.; Alouaoui, R.; Boucherit, A.; Mhiri, N.; Moulai-Mostefa, N.; Sayadi, S. Screening and preliminary characterization of biosurfactants produced by Ochrobactrum sp. 1c and Brevibacterium sp. 7g isolated from hydrocarbon-contaminated soils. Int. Biodeterior. Biodegrad. 2011, 65, 1182–1188. [Google Scholar]
- Bhattacharya, M.; Biswas, D.; Sana, S.; Datta, S. Biodegradation of waste lubricants by a newly isolated Ochrobactrum sp. C1. 3 Biotech. 2015, 5, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Potumarthi, R.; Subhakar, C.; Vanajakshi, J.; Jetty, A. Effect of aeration and agitation regimes on lipase production by newly isolated Rhodotorula mucilaginosa mtcc 8737 in stirred tank reactor using molasses as sole production medium. Appl. Biochem. Biotechnol. 2008, 151, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A.; Macedo, G.P.; Rodrigues, D.S.; Giordano, R.L.C.; Gonçalves, L.R.B. Immobilization of Candida antarctica lipase b by covalent attachment on chitosan-based hydrogels using different support activation strategies. Biochem. Eng. J. 2012, 60, 16–24. [Google Scholar] [CrossRef]
- Banat, I.M. The isolation of a thermophilic biosurfactant producing Bacillus sp. Biotechnol. Lett. 1993, 15, 591–594. [Google Scholar] [CrossRef]
- Chen, S.-J.; Cheng, C.-Y.; Chen, T.-L. Production of an alkaline lipase by Acinetobacter radioresistens. J. Ferment. Bioeng. 1998, 86, 308–312. [Google Scholar] [CrossRef]
- Joshi, S.; Bharucha, C.; Desai, A.J. Production of biosurfactant and antifungal compound by fermented food isolate Bacillus subtilis 20b. Bioresour. Technol. 2008, 99, 4603–4608. [Google Scholar] [CrossRef] [PubMed]
- Gutarra, M.L.; Cavalcanti, E.D.; Castilho, L.R.; Freire, D.M.; Sant´Anna, G.L. Lipase production by solid-state fermentation: Cultivation conditions and operation of tray and packed-bed bioreactors. Appl Biochem. Biotechnol. 2005, 121, 105–116. [Google Scholar] [CrossRef]
- Açıkel, Ü.; Erşan, M.; Sağ Açıkel, Y. Optimization of critical medium components using response surface methodology for lipase production by Rhizopus delemar. Food Bioprod. Process. 2010, 88, 31–39. [Google Scholar] [CrossRef]
- Papagora, C.; Roukas, T.; Kotzekidou, P. Optimization of extracellular lipase production by Debaryomyces hansenii isolates from dry-salted olives using response surface methodology. Food Bioprod. Process. 2013, 91, 413–420. [Google Scholar] [CrossRef]
- Kai, W.; Peisheng, Y. Optimization of lipase production from a novel strain Thalassospira permensism 35-15 using response surface methodology. Bioengineered 2016, 7, 298–303. [Google Scholar] [CrossRef] [PubMed]
- García-Silvera, E.E.; Martínez-Morales, F.; Bertrand, B.; Morales-Guzmán, D.; Rosas-Galván, N.S.; León-Rodríguez, R.; Trejo-Hernández, M.R. Production and application of a thermostable lipase from Serratia marcescens in detergent formulation and biodiesel production. Biotechnol. Appl. Biochem. 2017. [Google Scholar] [CrossRef]
- Techaoei, S.; Lumyong, S.; Prathumpai, W.; Santiarwar, D.; Leelapornp, P. Screening characterization and stability of biosurfactant produced by Pseudomonas aeruginosa scmu106 isolated from soil in northern thailand. Asian J. Biol. Sci. 2011, 4, 340–351. [Google Scholar] [CrossRef]
- Fontes, G.C.; Fonseca Amaral, P.F.; Nele, M.; Zarur Coelho, M.A. Factorial design to optimize biosurfactant production byYarrowia lipolytica. J. Biomed. Biotechnol. 2010, 2010, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gagelidze, N.A.; Amiranashvili, L.L.; Varsimashvili, K.I.; Tinikashvili, L.M.; Tolordava, L.L.; Sadunishvili, T.A. Selection of effective biosurfactant producers among Bacillus strains isolated from soils of georgia. Ann. Agrar. Sci. 2016, 14, 72–75. [Google Scholar] [CrossRef]
- Sajna, K.V.; Sukumaran, R.K.; Jayamurthy, H.; Reddy, K.K.; Kanjilal, S.; Prasad, R.B.N.; Pandey, A. Studies on biosurfactants from Pseudozyma sp. Nii 08165 and their potential application as laundry detergent additives. Biochem. Eng. J. 2013, 78, 85–92. [Google Scholar] [CrossRef]
- Schlegel, H.G.; Kaltwasser, H.; Gottschalk, G. A submersion method for culture of hydrogen-oxidizing bacteria: Growth physiological studies. Arch. Mikrobiol. 1961, 38, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Schulte, G.; Bohne, L.; Winkler, U. Glycogen and various other polysaccharides stimulate the formation of exolipase by Pseudomonas aeruginosa. Can. J. Microbiol. 1982, 28, 636–642. [Google Scholar] [CrossRef]
- Willumsen, P.A.; Karlson, U. Screening of bacteria, isolated from pah-contaminated soils, for production of biosurfactants and bioemulsifiers. Biodegradation 1996, 7, 415–423. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

| Run | pH | Temp. (°C) | Molasses (g/L) | MgSO₄ (g/L) | Waste Engine Oil (g/L) | Waste Cocked Oil (g/L) | K₂HPO₄ (g/L) | Olive Oil (g/L) | CaCl2 (g/L) | Whey (g/L) | Yeast Extract (g/L) | Lipase Activity (U/mL) | Emulsification Index (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 20 | 1 | 0.1 | 10 | 10 | 0.8 | 10 | 0.8 | 1 | 1 | 0.571 | 28.23 |
| 2 | 12 | 20 | 1 | 0.5 | 30 | 30 | 0.8 | 10 | 0.8 | 5 | 5 | 3.768 | 40.54 |
| 3 | 8 | 60 | 1 | 0.1 | 30 | 30 | 1.2 | 10 | 1.2 | 5 | 1 | 1.775 | 33.31 |
| 4 | 8 | 60 | 5 | 0.1 | 30 | 10 | 0.8 | 30 | 0.8 | 5 | 5 | 1.341 | 32.09 |
| 5 | 8 | 20 | 5 | 0.5 | 10 | 30 | 0.8 | 30 | 1.2 | 5 | 1 | 0.715 | 26.48 |
| 6 | 12 | 60 | 5 | 0.1 | 10 | 30 | 0.8 | 10 | 1.2 | 1 | 5 | 4.146 | 43.12 |
| 7 | 12 | 60 | 1 | 0.5 | 30 | 10 | 0.8 | 30 | 1.2 | 1 | 1 | 4.984 | 45.54 |
| 8 | 12 | 60 | 5 | 0.5 | 10 | 10 | 1.2 | 10 | 0.8 | 5 | 1 | 4.443 | 44.02 |
| 9 | 8 | 60 | 1 | 0.5 | 10 | 30 | 1.2 | 30 | 0.8 | 1 | 5 | 2.244 | 34.89 |
| 10 | 12 | 20 | 5 | 0.1 | 30 | 30 | 1.2 | 30 | 0.8 | 1 | 1 | 3.211 | 39.21 |
| 11 | 8 | 20 | 5 | 0.5 | 30 | 10 | 1.2 | 10 | 1.2 | 1 | 5 | 0.711 | 26.43 |
| 12 | 12 | 20 | 1 | 0.1 | 10 | 10 | 1.2 | 30 | 1.2 | 5 | 5 | 3.161 | 41.09 |
| Variable | Lipase Activity (U/mL) | Emulsification Index (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F-Value | p-value | Sum of Squares | df | Mean Square | F-Value | p-value | |||
| Model | 27.04 | 4 | 6.76 | 438.6 | <0.0001 | significant | 525.62 | 3 | 175.21 | 491.47 | <0.0001 | significant |
| A-pH | 22.29 | 1 | 22.29 | 1446.22 | <0.0001 | 433.08 | 1 | 433.08 | 1214.84 | <0.0001 | ||
| B-Temp | 3.85 | 1 | 3.85 | 249.68 | <0.0001 | 80.03 | 1 | 80.03 | 224.50 | <0.0001 | ||
| C-Molasses | 0.31 | 1 | 0.31 | 20.26 | 0.0028 | 12.51 | 1 | 12.51 | 35.08 | 0.0004 | ||
| D-MgSO | 0.59 | 1 | 0.59 | 38.25 | 0.0005 | - | - | - | - | - | ||
| Residual | 0.11 | 7 | 0.015 | 2.85 | 8 | 0.36 | ||||||
| Cor Total | 27.15 | 11 | 528.47 | 11 | ||||||||
| Run | pH | Temperature (°C) | Molasses (g/L) | Enzyme Activity (U/mL) | Emulsification Index (%) | ||
|---|---|---|---|---|---|---|---|
| Experimental | Predicted | Experimental | Predicted | ||||
| 1 | −1 | 0 | 1 | 4.80 | 4.81 | 40.17 | 40.24 |
| 2 | −1 | 1 | 0 | 5.99 | 6.04 | 43.09 | 43.04 |
| 3 | 0 | 0 | 0 | 13.01 | 13.10 | 81.55 | 83.17 |
| 4 | 0 | 1 | 1 | 14.53 | 14.46 | 89.30 | 89.08 |
| 5 | 0 | 0 | 0 | 13.15 | 13.10 | 83.59 | 83.17 |
| 6 | 1 | 1 | 0 | 8.60 | 8.77 | 68.23 | 68.25 |
| 7 | 0 | 1 | −1 | 14.02 | 13.88 | 86.74 | 86.72 |
| 8 | 0 | −1 | 1 | 11.22 | 11.38 | 80.84 | 80.73 |
| 9 | −1 | −1 | 0 | 1.38 | 1.22 | 34.21 | 34.06 |
| 10 | 1 | 0 | 1 | 7.33 | 7.24 | 65.24 | 65.24 |
| 11 | 1 | −1 | 0 | 6.11 | 6.06 | 59.95 | 59.86 |
| 12 | 0 | 0 | 0 | 13.14 | 13.10 | 83.54 | 83.17 |
| 13 | 1 | 0 | −1 | 7.35 | 7.33 | 63.24 | 63.04 |
| 14 | −1 | 0 | −1 | 2.10 | 2.19 | 37.17 | 37.04 |
| 15 | 0 | −1 | −1 | 9.36 | 9.43 | 77.62 | 77.70 |
| 16 | 0 | 0 | 0 | 13.04 | 13.09 | 83.59 | 83.31 |
| 17 | 1 | 0 | 1 | 7.33 | 7.23 | 65.24 | 65.38 |
| 18 | 0 | 1 | −1 | 14.03 | 13.87 | 86.74 | 86.86 |
| 19 | −1 | 0 | 1 | 4.80 | 4.81 | 40.18 | 40.38 |
| 20 | 0 | 1 | 1 | 14.53 | 14.46 | 89.30 | 89.22 |
| 21 | 1 | 0 | −1 | 7.33 | 7.32 | 63.24 | 63.18 |
| 22 | 1 | 1 | 0 | 8.60 | 8.76 | 68.23 | 68.39 |
| 23 | −1 | 1 | 0 | 5.97 | 6.03 | 43.10 | 43.18 |
| 24 | 0 | −1 | −1 | 9.34 | 9.42 | 77.63 | 77.84 |
| 25 | 1 | −1 | 0 | 6.11 | 6.05 | 59.94 | 60.00 |
| 26 | −1 | 0 | −1 | 2.10 | 2.18 | 37.17 | 37.18 |
| 27 | −1 | −1 | 0 | 1.38 | 1.21 | 34.21 | 34.20 |
| 28 | 0 | 0 | 0 | 13.11 | 13.09 | 83.59 | 83.31 |
| 29 | 0 | −1 | 1 | 11.22 | 11.37 | 80.85 | 80.87 |
| 30 | 0 | 0 | 0 | 13.10 | 13.09 | 83.59 | 83.31 |
| Source | Lipase | Biosurfactant | |||
|---|---|---|---|---|---|
| F-value | p-value | F-value | p-value | ||
| Model | 5284.42 | <0.0001 | 6371.84 | <0.0001 | significant |
| pH | 15939.05 | <0.0001 | 14069.85 | <0.0001 | |
| Temperature | 4966.13 | <0.0001 | 1632.34 | <0.0001 | |
| Molasses | 285.005 | <0.0001 | 157.30 | <0.0001 | |
| AB | 209.27 | <0.0001 | 0.96 | 0.3405 | |
| AC | 1.428 | 0.9702 | 2.72 | 0.1157 | |
| BC | 2.285 | 0.9881 | 1.19 | 0.2885 | |
| A² | 26145.87 | <0.0001 | 40953.20 | <0.0001 | |
| B² | 9.10 | 0.0071 | 0.89 | 0.3577 | |
| C² | 13.58 | 0.0016 | 2.21 | 0.1532 | Not significant |
| Lack of fit | 3.22 | 0.1333 | 0.07899 | 0.9999 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahimipour, G.; Sadeghi, H.; Zarinviarsagh, M. Statistical Methodologies for the Optimization of Lipase and Biosurfactant by Ochrobactrum intermedium Strain MZV101 in an Identical Medium for Detergent Applications. Molecules 2017, 22, 1460. https://doi.org/10.3390/molecules22091460
Ebrahimipour G, Sadeghi H, Zarinviarsagh M. Statistical Methodologies for the Optimization of Lipase and Biosurfactant by Ochrobactrum intermedium Strain MZV101 in an Identical Medium for Detergent Applications. Molecules. 2017; 22(9):1460. https://doi.org/10.3390/molecules22091460
Chicago/Turabian StyleEbrahimipour, Gholamhossein, Hossein Sadeghi, and Mina Zarinviarsagh. 2017. "Statistical Methodologies for the Optimization of Lipase and Biosurfactant by Ochrobactrum intermedium Strain MZV101 in an Identical Medium for Detergent Applications" Molecules 22, no. 9: 1460. https://doi.org/10.3390/molecules22091460





