The Variation of Oncidium Rosy Sunset Flower Volatiles with Daily Rhythm, Flowering Period, and Flower Parts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of the Volatiles in Onc. Rosy Sunset Flowers
2.2. Changes of Volatile Components with Daily Rhythm
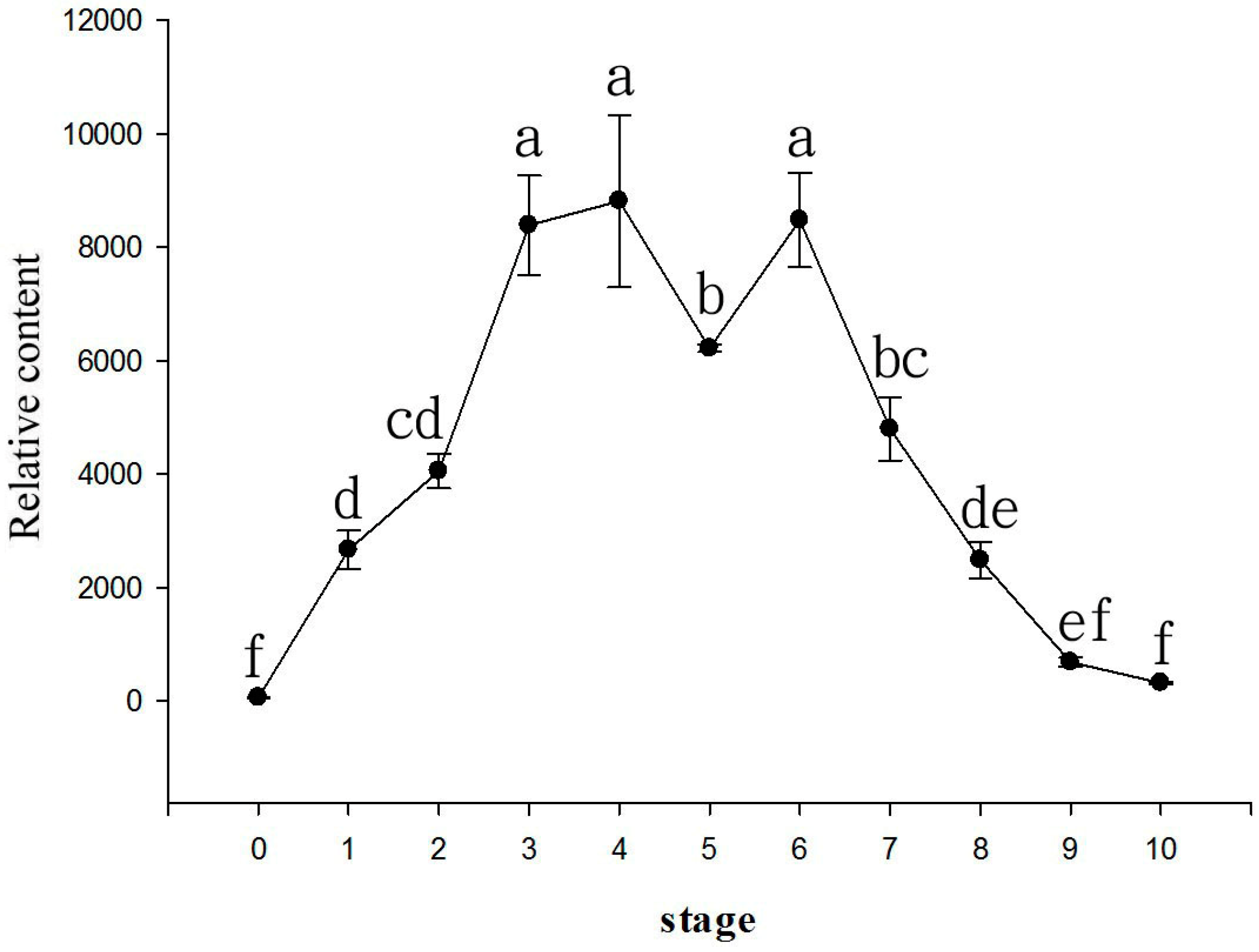
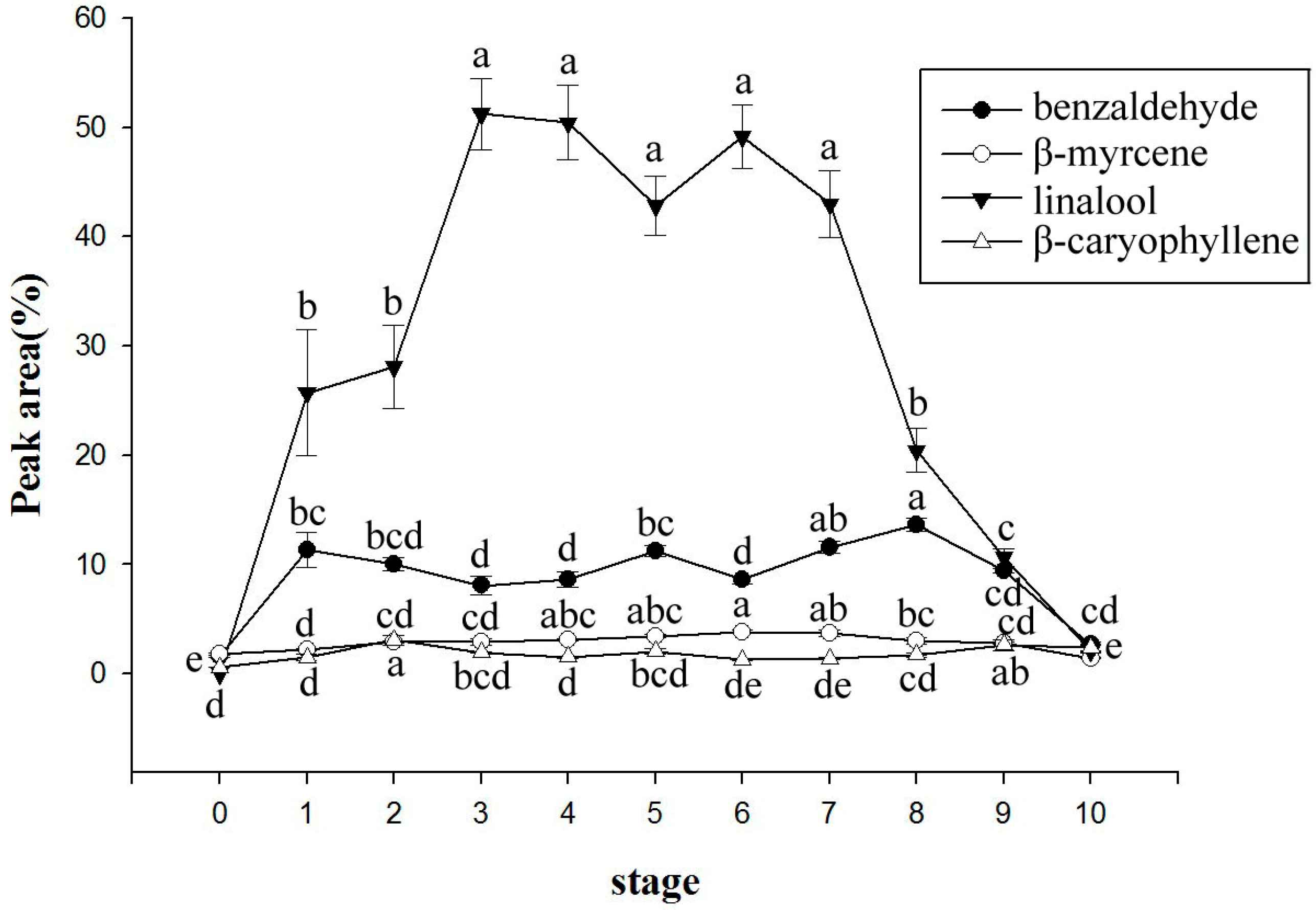
2.3. Changes of Volatile Components with the Flowering Period
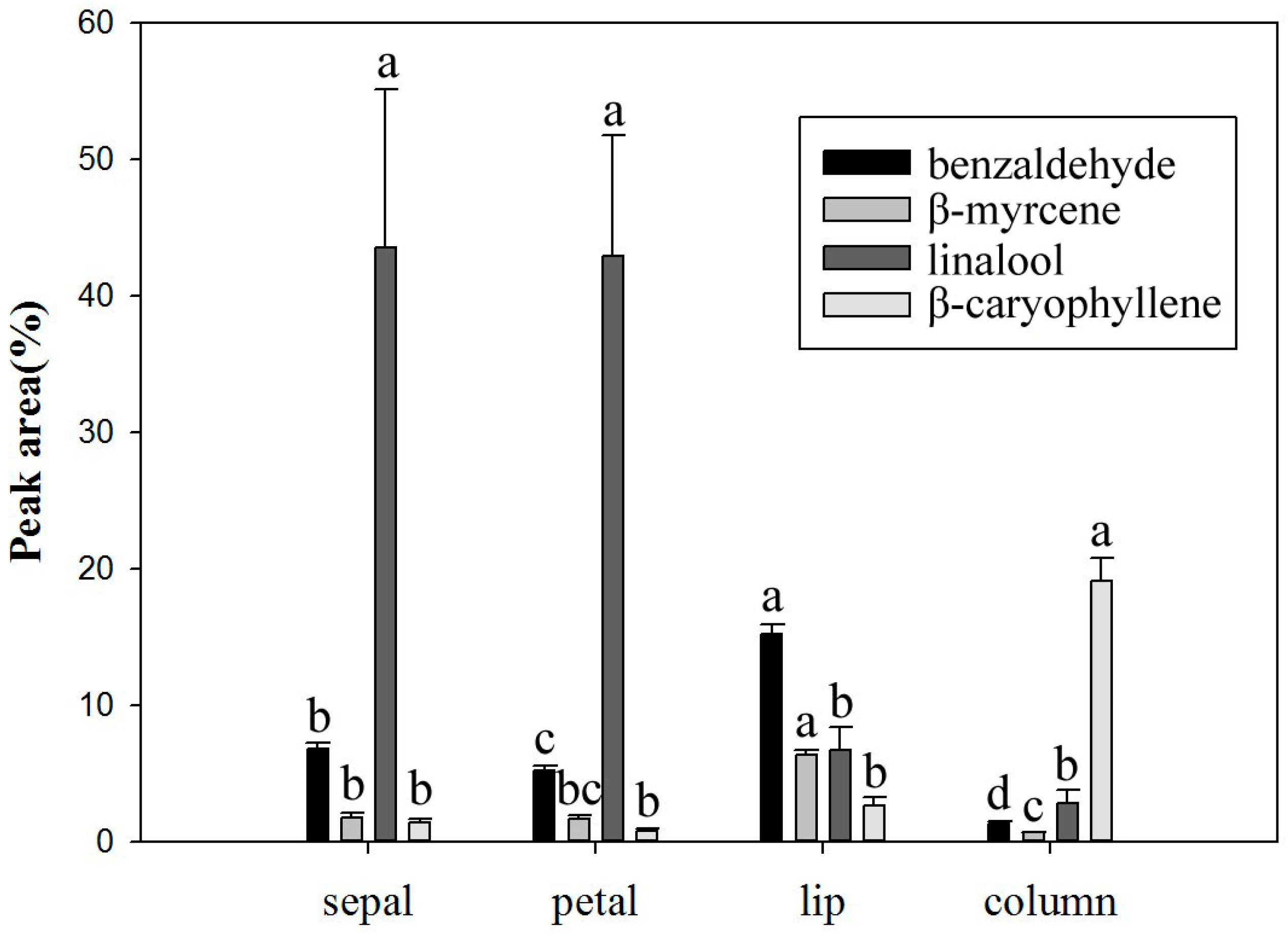
2.4. Volatile Components in Different Parts of Onc. Rosy Sunset Flowers
3. Materials and Methods
3.1. Plant Materials
3.2. Volatile Components of Onc. Rosy Sunset
3.3. Changes of Volatile Components with Daily Rhythm
3.4. Volatile Component Changes during the Flowering Period
3.5. Volatile Components in Different Parts of the Onc. Rosy Sunset Flowers
3.6. GC and GC-MS
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baker, M.L.; Baker, C.O. Orchid Species Culture: Oncidium/Odontoglossum Alliance; Timber Press: Portland, OR, USA, 2006; pp. 19–928. [Google Scholar]
- Chen, J.T.; Chang, W.C. Plant regeneration via embryo and shoot bud formation from flower-stalk explants of Oncidium Sweet Sugar. Plant Cell Tissue Organ Cult. 2000, 62, 95–100. [Google Scholar] [CrossRef]
- Kurt, M.N.; Whitten, W.M.; Williams, N.H.; Blanco, M.A.; Endara, L.; Burleigh, J.G.; Vera, K.S.; Cushman, J.C.; Chase, M.W. Generic recircumscriptions of Oncidiinae (Orchidaceae: Cymbidieae) based on maximum likelihood analysis of combined DNA datasets. Bot. J. Linn. Soc. 2012, 168, 117–146. [Google Scholar]
- Awano, K.; Honda, T.; Ogawa, T.; Suzuki, S.; Matsunaga, Y. Volatile components of Phalaenopsis schilleriana Rehb. f. Flavour Fragr. J. 1997, 12, 341–344. [Google Scholar] [CrossRef]
- Hsiao, Y.Y.; Tsai, W.C.; Kuoh, C.S.; Huang, T.H.; Wang, H.C.; Wu, T.S.; Leu, Y.L.; Chen, W.H.; Chen, H.H. Comparison of transcripts in Phalaenopsis bellina and Phalaenopsis equestris (Orchidaceae) flowers to deduce monoterpene biosynthesis pathway. BMC Plant Biol. 2006, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.Y.; Jeng, M.F.; Tsai, W.C.; Chuang, Y.C.; Li, C.Y.; Wu, T.S.; Kuoh, C.S.; Chen, W.H.; Chen, H.H. A novel homodimeric geranyl diphosphate synthase from the orchid Phalaenopsis bellina lacking a DD(X)2–4D motif. Plant J. 2008, 55, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R. The Scent of Orchids-Olfactory and Chemical Investigations; Elsevier: Amsterdam, The Netherlands, 1993; pp. 239–240. [Google Scholar]
- Yeh, C.H.; Tsai, W.Y.; Chiang, H.M.; Wu, C.S.; Lee, Y.I.; Lin, L.Y.; Chen, H.C. Headspace solid-phase microextraction analysis of volatile components in Phalaenopsis Nobby’s Pacific Sunset. Molecules 2014, 19, 14080–14093. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fan, Y.P. Analysis of aroma components of Oncidium. Acta Agric. Univ. Jiangxiensis 2012, 34, 692–698. [Google Scholar]
- Zhang, Y.; Li, X.L.; Wang, Y.; Tian, M.; Fan, M.H. Changes of aroma components in Oncidium Sharry Baby in different florescence and flower parts. Sci. Agric. Sin. 2011, 44, 110–117. [Google Scholar]
- Zhang, Y.; Wang, Y.; Tian, M.; Zhou, W.W. Analysis of aroma components in different orchid varieties. J. Anal. Sci. 2012, 28, 502–506. [Google Scholar]
- Zhang, Y.; Tian, M.; Wang, C.X.; Chen, S. Component analysis and sensory evaluation of flower aroma of Oncidium Sharry Baby ‘Sweet Fragrance’ under different temperature conditions. J. Plant Resour. Environ. 2015, 24, 112–114. [Google Scholar]
- Pichersky, E.; Dudareva, N. Scent engineering: Toward the goal of controlling how flowers smell. Trends Biotechnol. 2007, 25, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, L.; Xu, Y.; Wang, L.; Wang, L. Identification of floral fragrances in tree peony cultivars by gas chromatography–mass spectrometry. Sci. Hortic. 2012, 142, 158–165. [Google Scholar] [CrossRef]
- Da Silva, U.F.; Borba, E.L.; Semir, J.; Marsaioli, A.J. A simple solid injection device for the analyses of Bulbophyllum (Orchidaceae) volatiles. Phytochemistry 1999, 50, 31–34. [Google Scholar] [CrossRef]
- Chen, H.C.; Chi, H.S.; Lin, L.Y. Headspace Solid-Phase Microextraction Analysis of Volatile Components in Narcissus tazetta var. chinensis Roem. Molecules 2013, 18, 13723–13734. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, G.; Wang, S.; Lin, Q.; Zhang, J.; Li, X. Analysis of the variation in scent components of Hosta flowers by HS-SPME and GC-MS. Sci. Hortic. 2014, 175, 57–67. [Google Scholar] [CrossRef]
- Xu, L.P.; Yu, F.Y. Corolla structure and fragrance components in Styrax tonkinensis. Trees 2015, 29, 1127–1134. [Google Scholar] [CrossRef]
- Hanneguelle, S.; Thibault, J.N.; Naulet, N.; Martin, G.J. Authentication of Essential Oils Containing Linalool and Linalyl Acetate by Isotopic Methods. J. Agric. Food Chem. 1992, 40, 81–87. [Google Scholar] [CrossRef]
- Minh Tu, N.T.; Onishi, Y.; Choi, H.S.; Kondo, Y.; Bassore, S.M.; Ukeda, H.; Sawamura, M. Characteristic Odor Components of Citrus sphaerocarpa Tanaka (Kabosu) Cold-Pressed Peel Oil. J. Agric. Food Chem. 2002, 50, 2908–2913. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.A.; Machado, S.R.; Guimaraes, E. Structural and ultrastructural characterization of the floral lip in Gongora bufonia (Orchidaceae): Understanding the slip-and-fall pollination mechanism. Botany 2015, 93, 759–768. [Google Scholar] [CrossRef]
- Dötterl, S.; Jahrei, K.; Jhumur, U.S.; Jürgens, A. Temporal variation of flower scent in Silene otitis (Caryophyllaceae): A species with a mixed pollination system. Bot. J. Linn. Soc. 2012, 169, 447–460. [Google Scholar]
- Nunes, C.E.P.; Gerlachb, G.; Bandeirac, K.D.O.; Gobbo-Netoc, L.; Pansarind, E.R.; Sazima, M. Two orchids, one scent? Floral volatiles of Catasetum cernuum and Gongora bufonia suggest convergent evolution to a unique pollination niche. Flora 2016, 232, 207–216. [Google Scholar] [CrossRef]
- Borg-Karlson, A.K.; Tengö, J.; Valterová, I.; Unelius, C.R.; Taghizadeh, T.; Tolasch, T.; Francke, W. (S)-(+)-linalool, a mate attractant pheromone component in the bee Colletes cunicularius. J. Chem. Ecol. 2003, 29, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Azuma, H.; Thien, L.B.; Toyota, M.; Asakawa, Y.; Kawano, S. Distribution and differential expression of (E)-4,8-dimethyl-l,3,7-nonatriene in leaf and floral volatiles of magnolia and liriodendron taxa. J. Chem. Ecol. 1997, 23, 2467–2478. [Google Scholar] [CrossRef]
- Dressler, R.L. Phylogeny and Classification of the Orchid Family; Dioscorides Press: Portland, OR, USA, 1993. [Google Scholar]
- Vogel, S. The Role of Scent Glands in Pollination: On the Structure and Function of Osmophores; Amerind Publishing: New Delhi, India, 1990. [Google Scholar]
- Pansarin, E.R.; Aguiar, J.M.R.B.V.; Pansarin, L.M. Floral biology and histochemical analysis of Vanilla edwallii Hoehne (Orchidaceae: Vanilloideae): An orchid pollinated by Epicharis (Apidae: Centridini). Plant Species Biol. 2014, 29, 242–252. [Google Scholar] [CrossRef]
- Ogunwande, I.A.; Olawore, N.O.; Adeleke, K.A.; Ekundayo, O.; Koening, W.A. Chemical composition of the leaf volatile oil of Pasidium guajava L. growing in Nigeria. Flavour Fragr. J. 2003, 18, 136–138. [Google Scholar] [CrossRef]
- Schomburg, G.; Dielmann, G. Identification by means of retention parameters. J. Chromatogr. Sci. 1973, 11, 151–159. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| Peak z | RI y | RI x | Compound | Content (%) | References |
|---|---|---|---|---|---|
| 1 | 718 | 713 | tiglaldehyde | 3.65 ± 0.70 w | |
| 2 | 754 | 761 | 2-methyl-2-buten-1-ol | 1.72 ± 0.31 | |
| 3 | 874 | 856 | o-xylene | 0.26 ± 0.06 | |
| 4 | 931 | 930 | benzaldehyde | 9.25 ± 0.45 | [8,17] |
| 5 | 968 | 963 | sabinene | 0.41 ± 0.02 | [17,18,21,24] |
| 6 | 975 | 971 | β-pinene | 0.37 ± 0.02 | [9,12,17,18,21,23,24] |
| 7 | 983 | 979 | β-myrcene | 4.15 ± 0.13 | [8,10,12,15,18,21,26] |
| 8 | 1011 | 995 | benzyl alcohol | 2.14 ± 0.14 | [17] |
| 9 | 1017 | 1009 | 2-ethyl-1-hexanol | 0.52 ± 0.07 | |
| 10 | 1022 | 1016 | limonene | 1.66 ± 0.02 | [8,9,15,17,18,24,26] |
| 11 | 1038 | 1031 | (E)-β-ocimene | 0.79 ± 0.03 | [10,12,15,17,18,23,24,26] |
| 12 | 1067 | 1064 | cis-linalool oxide | 1.47 ± 0.26 | [23] |
| 13 | 1085 | 1081 | linalool | 52.80 ± 2.75 | [8,9,12,17,18,23,26] |
| 14 | 1104 | 1097 | (E)-4,8-dimethyl-l,3,7-nonatriene | 0.14 ± 0.01 | [8,26] |
| 15 | 1116 | 1110 | butyl tiglate | 0.17 ± 0.02 | |
| 16 | 1117 | 1121 | alloocimene | 0.38 ± 0.02 | [9,18] |
| 17 | 1128 | 1124 | camphor | 0.20 ± 0.03 | |
| 18 | 1159 | 1144 | 2-ethylhexyl acetate | 0.24 ± 0.04 | |
| 19 | 1150 | 1153 | epoxylinalol | 0.10 ± 0.01 | |
| 20 | 1163 | 1157 | menthol | 0.48 ± 0.06 | |
| 21 | 1170 | 1167 | methyl salicylate | 0.82 ± 0.18 | |
| 22 | 1172 | 1176 | α-terpineol | 1.04 ± 0.14 | [17] |
| 23 | 1185 | 1183 | decanal | 0.10 ± 0.01 | |
| 24 | 1200 | 1200 | dodecane | <0.01 | [12,18] |
| 25 | 1201 | 1196 | benzenepropanol | 0.12 ± 0.01 | |
| 26 | 1215 | 1209 | 2-ethylhexyl acrylate | 0.24 ± 0.01 | |
| 27 | 1220 | 1216 | β-citral | 0.70 ± 0.05 | [10,15] |
| 28 | 1232 | 1236 | nerol | 0.19 ± 0.02 | [8,10,11,12,15,21] |
| 29 | 1237 | 1244 | geranial | 0.40 ± 0.05 | [8] |
| 30 | 1249 | 1253 | methyl hydrocinnamate | 0.08 ± 0.01 | |
| 31 | 1275 | 1279 | cinnamyl alcohol | <0.01 | |
| 32 | 1300 | 1300 | tridecane | 0.16 ± 0.02 | [9,12,15] |
| 33 | 1337 | 1348 | butyl benzoate | <0.01 | |
| 34 | 1354 | 1362 | vanillin | 0.05 ± 0.01 | [17] |
| 35 | 1366 | 1367 | benzyl 3-methylbutanoate | 0.31 ± 0.02 | |
| 36 | 1373 | 1380 | copaene | 0.21 ± 0.01 | [11,12,21] |
| 37 | 1400 | 1400 | tetradecane | 0.06 ± 0.01 | [12,18] |
| 38 | 1429 | 1415 | isopentyl benzoate | 0.18 ± 0.02 | |
| 39 | 1432 | 1431 | β-caryophyllene | 1.41 ± 0.13 | [12,15,17,21,26] |
| 40 | 1444 | 1446 | (Z)-β-farnesene | 0.42 ± 0.03 | [8,21] |
| 41 | 1459 | 1455 | 2,6-di-tert-butylquinone | 0.05 ± 0.01 | |
| 42 | 1474 | 1477 | benzyl tiglate | 1.17 ± 0.15 | |
| 43 | 1500 | 1498 | β-bisabolene | 0.20 ± 0.02 | [17] |
| 44 | 1548 | 1547 | nerolidol | 2.11 ± 0.25 | [8,11,26] |
| 45 | 1591 | 1582 | 2-methylpropanoate | 0.17 ± 0.02 | |
| Total | 91.12 ± 1.21 |
| RI | Compound | 10:00 | 12:00 | 14:00 | 16:00 | 18:00 | 20:00 | 22:00 | 24:00 | 02:00 | 04:00 | 06:00 | 08:00 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total amount | 4555 ± 186 b Z | 5117 ± 387 a | 2838 ± 106 c | 1719 ± 174 de | 991 ± 126 fg | 640 ± 14 1g | 535 ± 133 g | 584 ± 114 g | 741 ± 115 fg | 934 ± 166 fg | 1215 ± 151 ef | 1917 ± 75 d | |
| relative proportion % | |||||||||||||
| 713 | tiglaldehyde | 9.18 ± 2.07 | 5.69 ± 0.99 | 9.01 ± 1.84 | 10.79 ± 1.57 | 9.31 ± 0.56 | 7.53 ± 0.70 | 6.84 ± 0.95 | 11.18 ± 3.58 | 12.83 ± 3.59 | 14.60 ± 3.46 | 14.86 ± 2.86 | 13.17 ± 2.66 |
| 761 | 2-methyl-2-buten-1-ol | 2.91 ± 0.63 | 2.58 ± 0.27 | 4.09 ± 0.71 | 4.62 ± 0.30 | 4.92 ±0.80 | 7.51 ± 1.17 | 6.98 ± 1.38 | 10.35 ± 0.23 | 7.68 ± 2.12 | 9.25 ± 3.12 | 9.13 ± 2.83 | 7.58 ± 2.40 |
| 856 | o-xylene | 0.78 ± 0.09 c | 1.57 ± 0.03 b | 1.67 ± 0.06 a | 0.74 ± 0.02 c | - | - | - | - | - | - | - | 0.54 ± 0.01 d |
| 930 | benzaldehyde | 6.64 ± 1.20 | 6.66 ± 1.19 | 8.38 ± 1.40 | 6.81 ± 0.58 | 6.44 ± 0.69 | 6.03 ± 0.42 | 5.74 ± 0.16 | 6.56 ± 0.77 | 5.66 ± 0.43 | 5.20 ± 0.41 | 5.94 ± 0.45 | 6.99 ± 0.79 |
| 963 | sabinene | 0.27 ± 0.01 c | 0.24 ± 0.02 d | 0.26 ± 0.01 cd | <0.01 | - | - | - | - | - | <0.01 | 0.62 ± 0.01 a | 0.43 ± 0.02 b |
| 971 | β-pinene | 0.37 ± 0.04 fg | 0.30 ± 0.02 g | 0.39 ± 0.02 efg | 0.51 ± 0.01 de | 0.79 ± 0.07 ab | 0.91 ± 0.04 a | <0.01 | <0.01 | <0.01 | 0.68 ± 0.05 bc | 0.60 ± 0.04 cd | 0.49 ± 0.04 def |
| 979 | β-myrcene | 2.72 ± 0.19 | 2.60 ± 0.31 | 2.16 ± 0.13 | 2.03 ± 0.23 | 2.89 ± 0.56 | 3.66 ± 0.80 | 3.59 ± 0.66 | 4.37 ± 1.16 | 3.59 ± 0.93 | 3.31 ± 0.83 | 3.12 ± 0.46 | 3.14 ± 0.22 |
| 995 | benzyl alcohol | 2.11 ± 0.68 a | 2.16 ± 0.56 a | 2.40 ± 0.50 a | 1.44 ± 0.19 ab | 0.75 ± 0.11 bc | - | - | - | - | 0.78 ± 0.01 bc | 1.04 ± 0.23 b | 1.61 ± 0.40 ab |
| 1009 | 2-ethylhexanol | 12.20 ± 11.47 | 11.08 ± 10.54 | 11.09 ± 10.61 | 12.39 ±11.76 | 12.73 ± 11.88 | 21.79 ± 16.71 | <0.01 | 23.04 ± 17.65 | 14.37 ± 12.97 | 13.35 ± 12.08 | 11.15 ± 10.13 | 8.48 ± 7.68 |
| 1016 | limonene | 1.26 ± 0.08 cd | 1.24 ± 0.08 cd | 1.34 ± 0.23 bcd | 1.14 ± 0.06 d | 1.66 ± 0.20 abcd | 1.96 ± 0.25 ab | 1.92 ± 0.16 ab | 2.20 ± 0.34 a | 1.82 ± 0.25 abc | 1.51 ± 0.11 bcd | 1.72 ± 0.31 abcd | 1.68 ± 0.25 abcd |
| 1031 | (E)-β-ocimene | 0.51 ± 0.05 a | 0.46 ± 0.06 a | 0.31 ± 0.01 b | <0.01 | - | - | - | - | - | - | - | 0.43 ± 0.04 a |
| 1064 | cis-linalool oxide | 0.85 ± 0.08 d | 1.75 ± 0.19 c | 2.59 ± 0.18 ab | 2.70 ± 0.05 a | 2.32 ± 0.07 b | 1.40 ± 0.19 c | 0.96 ± 0.19 d | 0.89 ± 0.06 d | 0.76 ± 0.04 d | 0.67 ± 0.06 d | 0.72 ± 0.10 d | 0.95 ± 0.12 d |
| 1081 | linalool | 35.8 ± 2.09 a | 37.09 ± 3.15 a | 21.50 ± 1.23 c | 10.17 ± 0.64 e | 7.74 ± 1.89 ef | 5.73 ± 1.20 ef | 4.72 ± 0.77 f | 6.41 ± 1.47 ef | 7.34 ± 1.48 ef | 8.13 ± 0.84 ef | 14.99 ± 1.51 d | 29.07 ± 1.49 b |
| 1097 | (E)-4,8-dimethyl-l,3,7-nonatriene | 0.22 ± 0.01 ef | 0.14 ± 0.01 f | 0.46 ± 0.07 cdef | 0.76 ± 0.14 bc | 1.31 ± 0.30 a | 1.31 ± 0.28 a | 0.91 ± 0.06 b | 0.81 ± 0.06 bc | 0.65 ± 0.05 bcd | 0.54 ± 0.07 bcde | 0.42 ± 0.0 6cdef | 0.32 ± 0.06 def |
| 1110 | butyl tiglate | 0.77 ± 0.21 a | 0.38 ± 0.11 b | 0.49 ± 0.01 b | 0.50 ± 0.12 b | - | - | - | - | - | - | - | 0.28 ± 0.01 b |
| 1121 | alloocimene | 0.26 ± 0.01 a | 0.25 ± 0.02 ab | 0.17 ± 0.01 c | - | - | - | - | - | - | - | - | 0.23 ± 0.02 b |
| 1124 | camphor | 0.18 ± 0.02 d | 0.17 ± 0.01 d | 0.27 ± 0.03 cd | 0.33 ± 0.03 c | 0.47 ± 0.05 b | 0.69 ± 0.04 a | 0.74 ± 0.01 a | 0.69 ± 0.05 a | 0.68 ± 0.01 a | 0.54 ± 0.06 b | 0.46 ± 0.02 b | 0.35 ± 0.04 c |
| 1144 | 2-ethylhexyl acetate | 1.83 ± 1.58 | 2.23 ± 2.03 | 2.21 ± 2.08 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| 1153 | epoxylinalol | <0.01 | 0.10 ± 0.01 c | 0.17 ± 0.02 b | 0.24 ± 0.04 a | <0.01 | - | - | - | - | - | - | - |
| 1157 | menthol | 0.65 ± 0.01 ef | 0.47 ± 0.05 f | 0.90 ± 0.15 ef | 1.27 ± 0.22 def | 2.10 ± 0.35 cd | 3.66 ± 0.55 ab | 4.51 ± 0.62 a | 4.34 ± 0.54 a | 3.30 ± 0.29 b | 2.73 ± 0.07 bc | 2.06 ± 0.16 cd | 1.47 ± 0.10 de |
| 1167 | methyl salicylate | 0.91 ± 0.02 | 1.18 ± 0.08 | 1.83 ± 0.75 | 2.44 ± 0.92 | 3.22 ± 1.29 | 3.19 ± 1.26 | 2.33 ± 0.84 | 2.57 ± 0.98 | 2.24 ± 0.86 | 1.89 ± 0.73 | 2.40 ± 0.01 | 2.18 ± 0.13 |
| 1176 | α-terpineol | 0.99 ± 0.24 bc | 1.53 ± 0.37 bc | 2.84 ± 0.98 bc | 5.79 ± 2.22 a | 3.54 ± 0.96 ab | 1.18 ± 0.29 bc | 0.76 ± 0.18 c | 1.25 ± 0.43 bc | 2.30 ± 0.88 bc | 3.11 ± 1.14 abc | 2.97 ± 0.82 bc | 2.21 ± 0.49 bc |
| 1183 | decanal | 0.13 ± 0.02 d | 0.14 ± 0.02 d | 0.22 ± 0.02 c | 0.35 ± 0.05 b | 0.45 ± 0.01 a | <0.01 | <0.01 | - | - | - | - | <0.01 |
| 1196 | benzenepropanol | 0.13 ± 0.01 a | 0.14 ± 0.01 a | 0.14 ± 0.01 a | - | - | - | - | - | - | - | - | - |
| 1209 | 2-ethylhexyl acrylate | 0.52 ± 0.36 | 0.56 ± 0.39 | 0.84 ± 0.55 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| 1216 | β-citral | 0.91 ± 0.24 | 1.02 ± 0.28 | 1.58 ± 0.55 | 2.08 ± 0.78 | 1.10 ± 0.28 | 0.62 ± 0.02 | <0.01 | 1.30 ± 0.01 | 1.74 ± 0.65 | 2.19 ± 0.75 | 2.09 ± 0.69 | 1.84 ± 0.59 |
| 1236 | nerol | 0.12 ± 0.01 a | 0.11 ± 0.01 a | - | - | - | - | - | - | - | - | - | - |
| 1244 | geranial | 0.29 ± 0.04 b | 0.23 ± 0.06 c | 0.25 ± 0.03 c | <0.01 | - | - | - | - | - | 0.53 ± 0.03 a | 0.46 ± 0.05 a | 0.47 ± 0.04 a |
| 1300 | tridecane | 0.18 ± 0.03 e | 0.23 ± 0.05 e | 0.35 ± 0.07 de | 0.55 ± 0.12 bcde | 0.76 ± 0.17 abc | 0.99 ± 0.24 a | 0.83 ± 0.15 abc | 0.90 ± 0.18 ab | 0.81 ± 0.17 abc | 0.66 ± 0.11 abcd | 0.54 ± 0.12 bcde | 0.46 ± 0.09 cde |
| 1367 | benzyl 3-methylbutanoate | 0.22 ± 0.01 c | 0.26 ± 0.08 bc | 0.31 ± 0.03 ab | <0.01 | - | - | - | - | - | - | 0.28 ± 0.01 bc | 0.39 ± 0.02 a |
| 1380 | copaene | 0.33 ± 0.07 e | 0.39 ± 0.11 de | 0.60 ± 0.17 cde | 0.87 ± 0.25 abcd | 1.17 ± 0.38 ab | 1.38 ± 0.02 a | 1.11 ± 0.03 abc | 1.26 ± 0.04 ab | 0.81 ± 0.15 bcde | 0.66 ± 0.05 cde | 0.51 ± 0.05 de | 0.41 ± 0.04 de |
| 1415 | isopentyl benzoate | 0.12 ± 0.01 d | 0.18 ± 0.01 c | 0.26 ± 0.01 b | 0.30 ± 0.01 a | - | - | - | - | - | - | - | 0.19 ± 0.01 c |
| 1431 | β-caryophyllene | 1.23 ± 0.36 | 1.54 ± 0.44 | 1.98 ± 0.56 | 2.67 ± 0.85 | 4.05 ± 1.41 | 4.47 ± 1.53 | 3.33 ± 0.90 | 4.69 ± 1.48 | 4.24 ± 1.19 | 3.50 ± 0.69 | 2.89 ± 0.56 | 2.28 ± 0.47 |
| 1446 | (Z)-β-farnesene | 0.94 ± 0.41 | 0.94 ± 0.38 | 1.30 ± 0.50 | 1.62 ± 0.65 | 1.86 ± 0.76 | 2.24 ± 0.05 | 1.66 ± 0.06 | 2.17 ± 0.09 | 1.79 ± 0.67 | 1.56 ± 0.51 | 1.35 ± 0.47 | 1.19 ± 0.41 |
| 1477 | benzyl tiglate | 0.97 ± 0.33 | 1.31 ± 0.36 | 1.82 ± 0.50 | 1.21 ± 0.22 | 0.53 ± 0.02 | <0.01 | 0.54 ± 0.04 | <0.01 | <0.01 | 0.57 ± 0.02 | 0.68 ± 0.05 | 0.97 ± 0.19 |
| 1498 | β-bisabolene | 0.38 ± 0.01 d | 0.38 ± 0.01 d | 0.52 ± 0.02 c | 0.74 ± 0.05 b | 0.89 ± 0.05 a | <0.01 | - | - | - | <0.01 | <0.01 | 0.46 ± 0.01 cd |
| 1547 | nerolidol | 5.69 ± 2.57 | 6.21 ± 2.57 | 7.61 ± 3.02 | 8.78 ± 3.68 | 9.57 ± 4.08 | 7.30 ± 3.01 | 4.85 ± 1.92 | 5.17 ± 2.09 | 5.41 ± 2.19 | 4.70 ± 1.74 | 4.96 ± 1.88 | 5.04 ± 1.90 |
| 1582 | 2-methylpropanoate | 0.26 ± 0.01 c | 0.20 ± 0.05 c | 0.26 ± 0.03 c | 0.50 ± 0.04 a | 0.52 ± 0.03 a | - | - | - | - | - | - | 0.34 ± 0.01 b |
| Part | Component | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldehydes | Alcohols | Esters | Hydrocarbons | Ketones | (Ep) Oxides | Total | ||||||||
| Sum | RC y | Sum | RC | Sum | RC | Sum | RC | Sum | RC | Sum | RC | Sum | RC | |
| sepal | 5 | 1964 a Z | 10 | 8675 a | 11 | 1026 a | 13 | 884 a | 2 | 36 a | 2 | 213 a | 43 | 12798 a |
| petal | 5 | 1981 a | 10 | 7337 a | 11 | 876 a | 13 | 690 a | 2 | 45 a | 2 | 191 a | 43 | 11120 a |
| lip | 2 | 280 b | 7 | 197 b | 4 | 18 b | 10 | 291 b | 2 | 21 a | 2 | 52 b | 27 | 859 b |
| column | 2 | 22 b | 5 | 155 b | 5 | 33 b | 9 | 409 b | 3 | 22 a | 1 | 75 b | 25 | 716 b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, Y.-T.; Chen, H.-C.; Chang, C. The Variation of Oncidium Rosy Sunset Flower Volatiles with Daily Rhythm, Flowering Period, and Flower Parts. Molecules 2017, 22, 1468. https://doi.org/10.3390/molecules22091468
Chiu Y-T, Chen H-C, Chang C. The Variation of Oncidium Rosy Sunset Flower Volatiles with Daily Rhythm, Flowering Period, and Flower Parts. Molecules. 2017; 22(9):1468. https://doi.org/10.3390/molecules22091468
Chicago/Turabian StyleChiu, Yi-Tien, Hsin-Chun Chen, and Chen Chang. 2017. "The Variation of Oncidium Rosy Sunset Flower Volatiles with Daily Rhythm, Flowering Period, and Flower Parts" Molecules 22, no. 9: 1468. https://doi.org/10.3390/molecules22091468





