Masticadienonic and 3α-OH Masticadienoic Acids Induce Apoptosis and Inhibit Cell Proliferation and Tumor Growth in Prostate Cancer Xenografts in Vivo
Abstract
:1. Introduction
2. Results
2.1. Acute Toxicity Test
2.2. Cytotoxicity In Vitro of MDA and 3α-OH MDA on PC-3 Cells
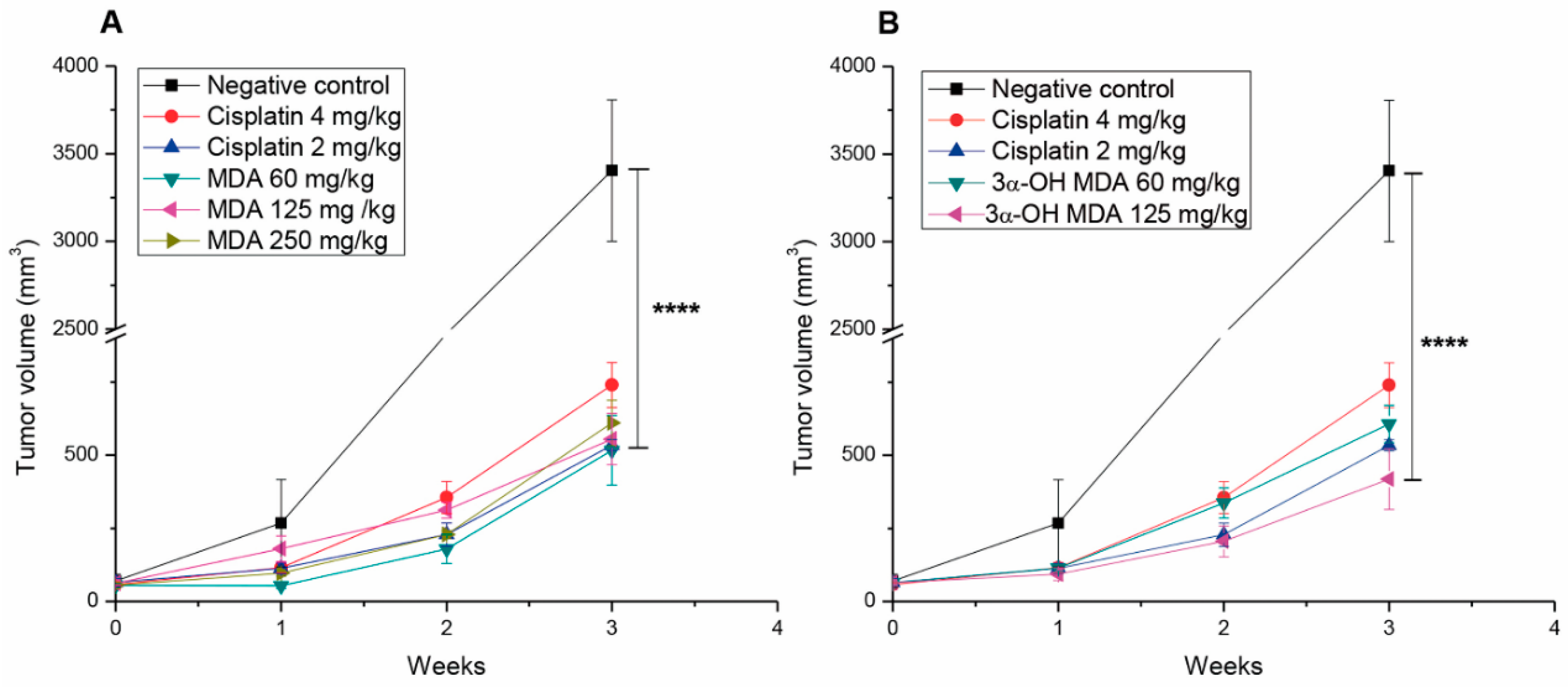
2.3. Masticadienonic and 3α-Hydroxy Masticadienoic Acids Inhibited the Growth of Prostate Cancer Xenografts
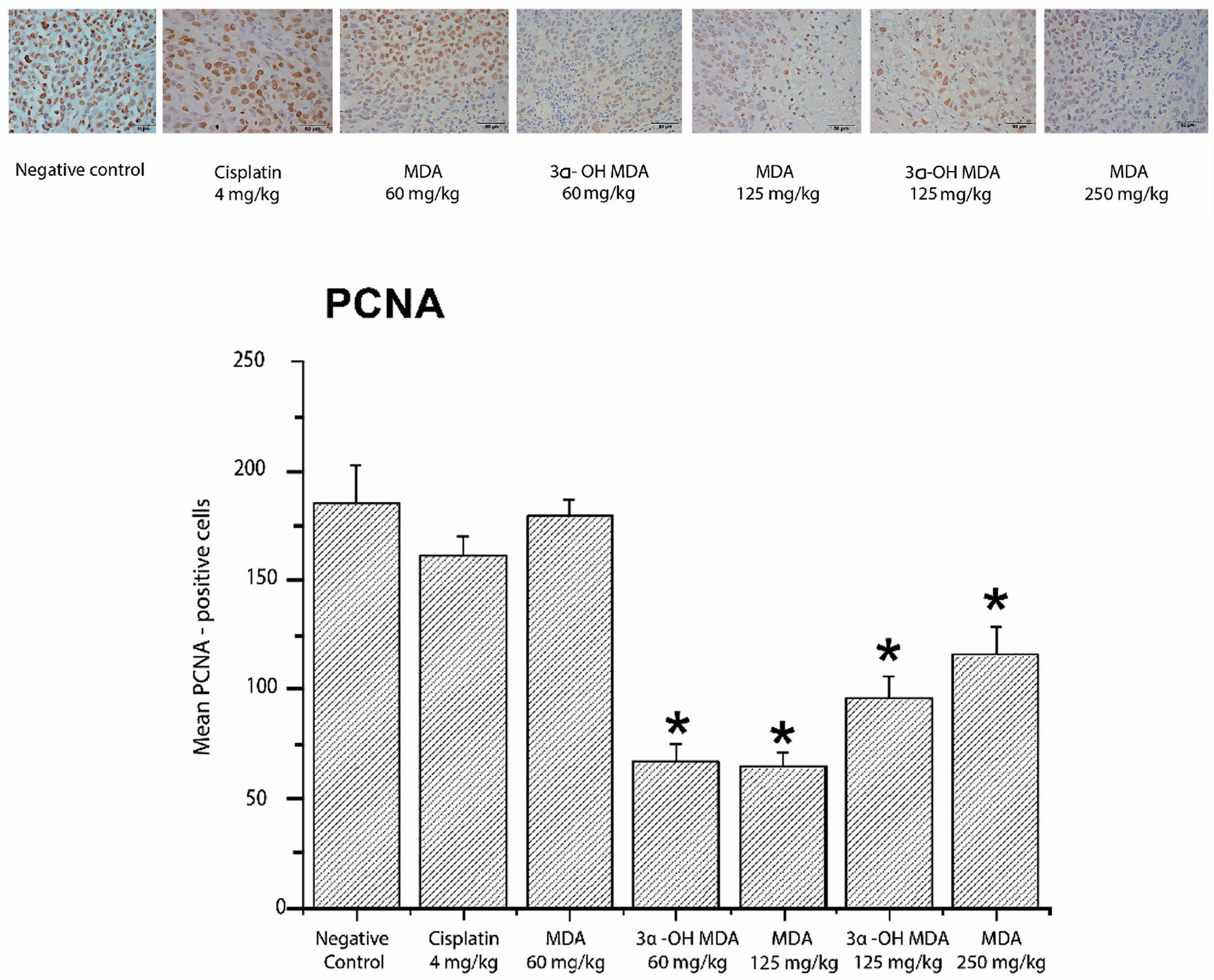
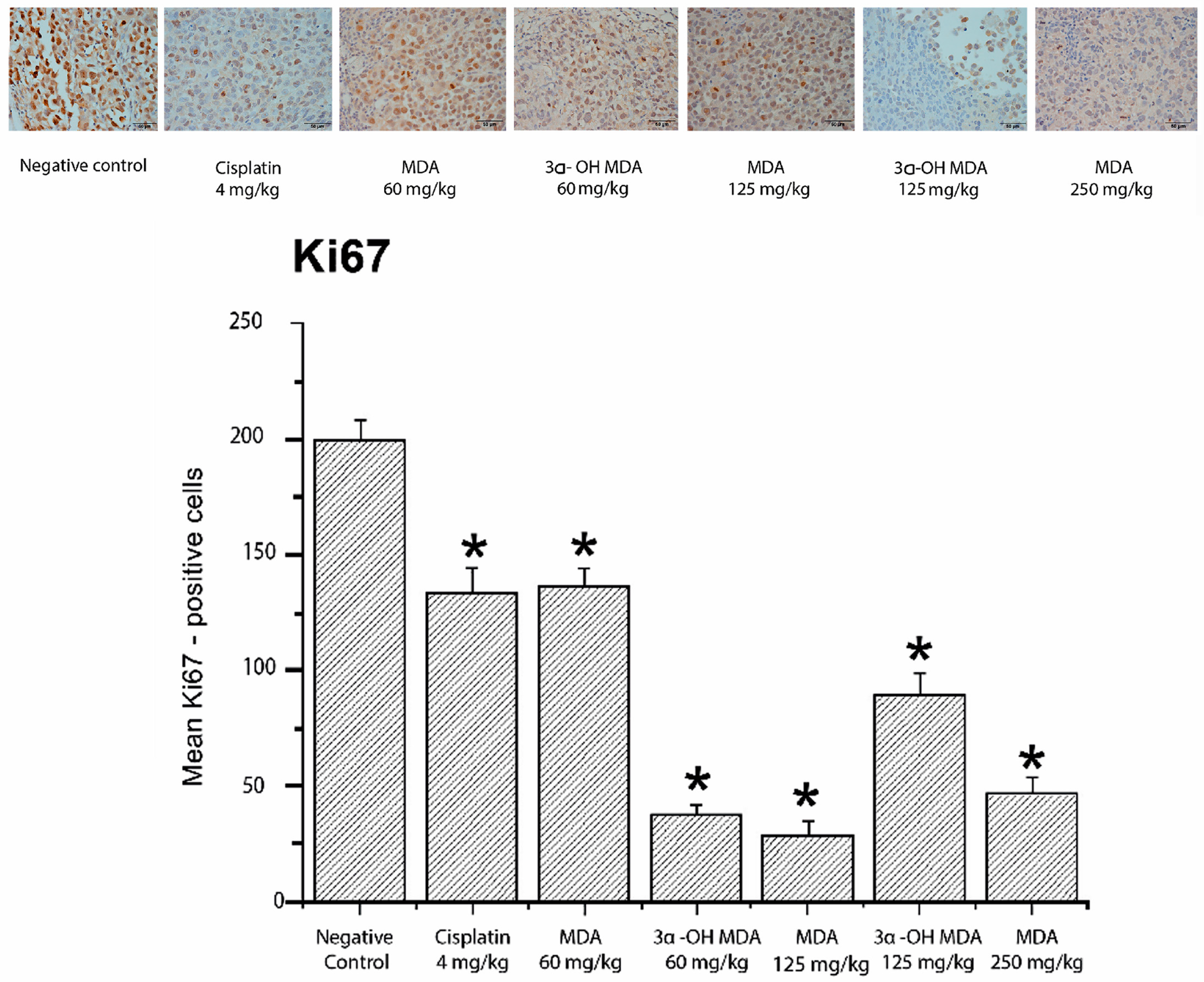
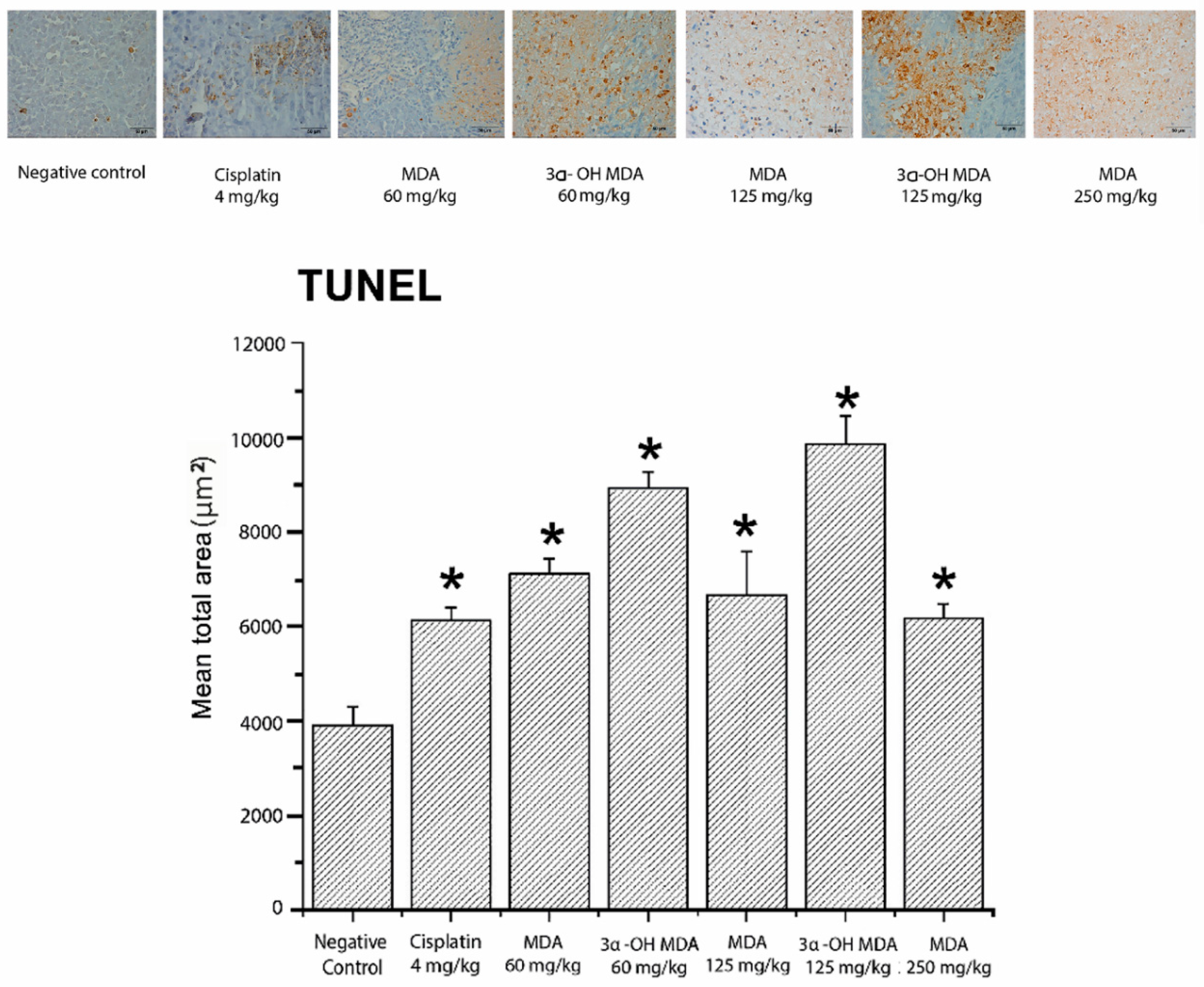
2.4. Histological and Immunohistochemical Analysis of Tumor Tissues
3. Discussion
4. Materials and Methods
4.1. Drugs and Reagents
4.1.1. Media
4.1.2. Dissolutions
4.1.3. Isolation of Masticadienonic (MDA) and 3α-OH Masticadienoic (3α-OH MDA) Acids
4.2. Acute Toxicity Test
4.3. Cell Culture
4.4. Determination of Cell Proliferation by Crystal Violet Staining
4.5. Human Prostate Tumor Xenografts
4.6. Immunohistochemical Analysis of PCNA and Ki-67
4.7. TUNEL Assay
4.8. Analysis of Photomicrographs
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, Y.; Hambuerger, A.W. EBP1 Inhibits Translation of Androgen Receptor mRNA in Castration Resistant Prostate Cancer Cells. Anticancer Res. 2011, 31, 3129–3135. [Google Scholar]
- Masters, G.A.; Krilov, L.; Bailey, H.H.; Brose, M.S.; Burstein, H.; Diller, L.R.; Dizon, D.S.; Fine, H.A.; Kalemkerian, G.P.; Moasser, M.; et al. Annual Report on Progress Against Cancer From the American Society of Clinical Oncology. J. Clin. Oncol. 2015, 33, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Aus, G.; Bolla, M.; Joniau, S.; Matveev, V.B.; Schmid, H.P.; Zattoni, F. EAU Guidelines on Prostate Cancer. Eur. Urol. 2008, 53, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.D.; Krilov, L.; Adams, S.; Aghajanian, C.; Basch, E.; Brose, M.S.; Carrol, W.L.; de Lima, M.; Gilbert, M.R.; Kris, M.G.; et al. Clinical Cancer Advances 2013: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology. J. Clin. Oncol. 2013, 31, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Rajendran, P.; Li, F.; Nema, T.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A.P.; Ho, P.C.; et al. Ursolic acid inhibits multiple cell survival pathways leading to suppression of growth of prostate cancer xenograft in nude mice. Mol. Med. 2011, 89, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Prasad, S.; Sung, B.; Gelovani, J.G.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Boswellic acid inhibits growth and metastasis of human colorectal cancer in orthotopic mouse model by downregulating inflammatory, proliferative, invasive and angiogenic biomarkers. Int. J. Can. 2012, 130, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Estrada, A.C.; Syrovets, T.; Pitterle, K.; Lunov, O.; Büchele, B.; Schimana-Pfeifer, J.; Schmidt, T.; Morad, S.A.F.; Simmet, T. Tirucallic Acids Are Novel Pleckstrin Homology Domain-Dependent Akt Inhibitors Inducing Apoptosis in Prostate Cancer Cells. Mol. Pharmacol. 2010, 77, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Ortega, O.A.G.; Hernández, M.; Martínez, V.M.; Salgado, T.T.; Arenas, S.F. Phytochemical study of cuachalalate (Amphiptherygium. adstringens, Schiede ex Schlecht). J. Ethnopharmacol. 1999, 68, 109–113. [Google Scholar] [CrossRef]
- Universidad Nacional Autónoma de México (UNAM). Atlas de la Medicina Tradicional Mexicana (2009). Biblioteca Digital de la Medicina Tradicional Mexicana. Available online: http://www.medicinatradicionalmexicana.unam.mx/index.php (accessed on 10 August 2017).
- Oviedo, C.I.; Apan, R.T.; Soto, H.M.; Martínez, V.M. Principles of the bark of Amphipterygium adstringens (Julianaceae) with anti-inflammatory. Phytomedicine 2004, 11, 436–445. [Google Scholar]
- Oviedo, C.I.; Apan, R.T.; Martínez, V.M. Cytotoxic activity and effect on nitric oxide production of tirucallane-type triterpenes. J. Pharm. Pharmacol. 2005, 57, 1087–1091. [Google Scholar]
- Dalla, V.A.; Braga, A.; García, A.A.N.; Martínez, V.M.; Toninello, A. 3α-Hydroxymasticadienonic Acid As an Antiproliferative Agent That Impairs Mitochondrial Functions. J. Nat. Prod. 2012, 75, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Lorke, D.A. New Approach to Practical Acute Toxicity Testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Cheng, S.; Budhraja, A.; Gao, Z.; Chen, J.; Liu, E.H.; Huang, C.; Che, D.; Yang, Z.; Liu, O.; et al. Ursolic acid induces apoptosis in human leukaemia cells and exhibits anti-leukaemic activity in nude mice through the PKB pathway. Br. J. Pharmacol. 2012, 165, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Chintharlapalli, S.; Papineni, S.; Lei, P.; Pathi, S.; Safe, S. Betulinic acid inhibits colon cancer cell and tumor growth and induces proteasome dependent and -independent downregulation of specificity proteins (Sp) transcription factors. BMC Cancer 2011, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Swanson, K.; Eliaz, I.; McClintick, J.N.; Sandusky, G.E.; Sliva, D. Pachymic Acid Inhibits Growth and Induces Apoptosis of Pancreatic Cancer In Vitro and In Vivo by Targeting ER Stress. PLoS ONE 2015, 10, e0122270. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, J.M.R.; Rao, C.V. Triterpenoids for Cancer Prevention and Treatment: Current Status and Future Prospects. Curr. Pharm. Biotechnol. 2012, 13, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.A.J.; Villareal, M.L.; Salazar, O.L.A.; Gómez, S.M.; Domínguez, F.; García, C.A. Mexican medicinal plants used for cancer treatment: Pharmacological, phytochemical and ethnobotanical studies. J. Ethnopharmacol. 2011, 133, 945–972. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; Deng, L.; Fan, B.; Wager, M.J.; Hohmann, A.G. Optimization of a cisplatin model of chemotherapy induced peripheral neuropathy in mice: Use of vitamin C and sodium bicarbonate pretreatments to reduce nephrotoxicity and improve animal health status. Mol. Pain 2014, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Stoimenov, I.; Helleday, T. PCNA on the crossroad of cancer. Biochem. Soc. Trans. 2009, 37, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Bologna, M.R.; Mosqueda, T.A.; Molina, F.N.; Mori, E.D.; Sánchez, A.G. Comparison of the value of PCNA and Ki-67 as markers of cell proliferation in ameloblastic tumors. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e174–e179. [Google Scholar] [CrossRef]
- Gupta, S.C.; Prasad, S.; Sethumadhavan, D.R.; Nair, M.S.; Mo, Y.Y.; Aggarwal, B.B. Nimbolide, a Limonoid Triterpene, Inhibits Growth of Human Colorectal Cancer Xenografts by Suppressing the Proinflammatory Microenvironment. Clin. Cancer Res. 2013, 19, 4465–4476. [Google Scholar] [CrossRef] [PubMed]
- Medina, L.A.; Herrera, P.B.I.; Morales, C.M.A.; García, L.P.; Pérez, C.E.; Chanona, V.J.; Brandan, M.E. Use of an orthovoltage X-ray treatment unit as a radiation research system in a small-animal cancer model. J. Exp. Clin. Cancer Res. 2008, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stîngâ, A.C.; Mârgâritescou, O.; Stîngâ, S.A.; Pirici, D.; Ciurea, R.; Bunget, A.; Cruce, M. VEGFR1 and VEGFR2 immunohistochemical expression in oral squamous cell carcinoma: A morphometric study. Rom. J. Morphol. Embryol. 2011, 52, 1–11. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |


| Treatment | IC50 (µM) |
|---|---|
| MDA | 56.51 ± 2.31 |
| 3α-OH MDA | 54.52 ± 4.95 |
| Cisplatin | 15.56 ± 1.14 |
| Treatment | Weight (g) Initial | Weight (g) Final |
|---|---|---|
| Cisplatin 4 mg/kg | 19.61 ± 0.61 | 13.80 ± 0.82 ** |
| Cisplatin 2 mg/kg | 25.13 ± 0.68 | 21.73 ± 0.93 |
| MDA 60 mg/kg | 22.06 ± 0.98 | 20.96 ± 0.67 |
| MDA 125 mg/kg | 23.33 ± 1.01 | 22.25 ± 1.18 |
| MDA 250 mg/kg | 24.23 ± 0.88 | 24.31 ± 1.09 |
| 3α-OH MDA 60 mg/kg | 23.90 ± 1.16 | 21.96 ± 1.05 |
| 3α-OH MDA 125 mg/kg | 24.76 ± 1.76 | 22.46 ± 1.82 |
| Control without treatment | 22.02 ± 1.45 | 19.72 ± 1.48 |
| Healthy | Negative Control | Cisplatin 2 mg/kg | MDA 60 mg/kg | 3α-OH MDA 60 mg/kg | |
|---|---|---|---|---|---|
| Leucocytes (×109/L) | 4.46 ± 0.13 | 3.73 ± 1.73 | 1.93 ± 0.12 * | 4.56 ± 0.92 | 3.80 ± 0.35 |
| Lymphocytes (×109/L) | 2.83 ± 0.76 | 2.2 ± 1.17 | 0.75 ± 0.27 * | 2.06 ± 1.28 | 1.46 ± 0.33 |
| Erythrocytes (×1012 /L) | 7.66 ± 0.43 | 4.13 ± 1.27 | 7.16 ± 0.37 | 7.16 ± 1.0 | 8.53 ± 0.68 |
| Hemoglobin (g/L) | 149.0 ± 2.0 | 134 ± 6.08 | 120.66 ± 9.93 | 135.33 ± 13.24 | 153.66 ± 5.60 |
| Glucose (mmol/L) | 9.37 ± 0.64 | 11.25 ± 0.66 | 7.80 ± 0.86 | 6.94 ± 1.97 | 8.39 ± 1.92 |
| Urea (BUN) (mmol/L) | 7.93 ± 0.68 | 7.86 ± 0.78 | 9.03 ± 0.94 | 8.46 ± 0.03 | 8.56 ± 1.04 |
| Creatinine (mmol/L) | 37.33 ± 8.95 | 29.33 ± 1.33 | 84.0 ± 3.21 **** | 24.0 ± 6.02 | 26.66 ± 0.88 |
| Alanine transaminase ALT (U/L) | 52.66 ± 5.81 | 53 ± 5.50 | 56.66 ± 6.69 | 53.33 ± 17.48 | 51.33 ± 7.42 |
| Aspartate transaminase AST (U/L) | 155.33 ± 2.40 | 143.66 ± 9.35 | 285.66 ± 13.37 **** | 145.33 ± 18.66 | 164.66 ± 6.76 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Monroy, M.B.; Jacobo-Herrera, N.J.; Zentella-Dehesa, A.; Hernández-Téllez, B.; Martínez-Vázquez, M. Masticadienonic and 3α-OH Masticadienoic Acids Induce Apoptosis and Inhibit Cell Proliferation and Tumor Growth in Prostate Cancer Xenografts in Vivo. Molecules 2017, 22, 1479. https://doi.org/10.3390/molecules22091479
Sánchez-Monroy MB, Jacobo-Herrera NJ, Zentella-Dehesa A, Hernández-Téllez B, Martínez-Vázquez M. Masticadienonic and 3α-OH Masticadienoic Acids Induce Apoptosis and Inhibit Cell Proliferation and Tumor Growth in Prostate Cancer Xenografts in Vivo. Molecules. 2017; 22(9):1479. https://doi.org/10.3390/molecules22091479
Chicago/Turabian StyleSánchez-Monroy, Ma. Beatriz, Nadia J. Jacobo-Herrera, Alejandro Zentella-Dehesa, Beatriz Hernández-Téllez, and Mariano Martínez-Vázquez. 2017. "Masticadienonic and 3α-OH Masticadienoic Acids Induce Apoptosis and Inhibit Cell Proliferation and Tumor Growth in Prostate Cancer Xenografts in Vivo" Molecules 22, no. 9: 1479. https://doi.org/10.3390/molecules22091479





