Silver Oxide Coatings with High Silver-Ion Elution Rates and Characterization of Bactericidal Activity
Abstract
:1. Introduction
2. Results
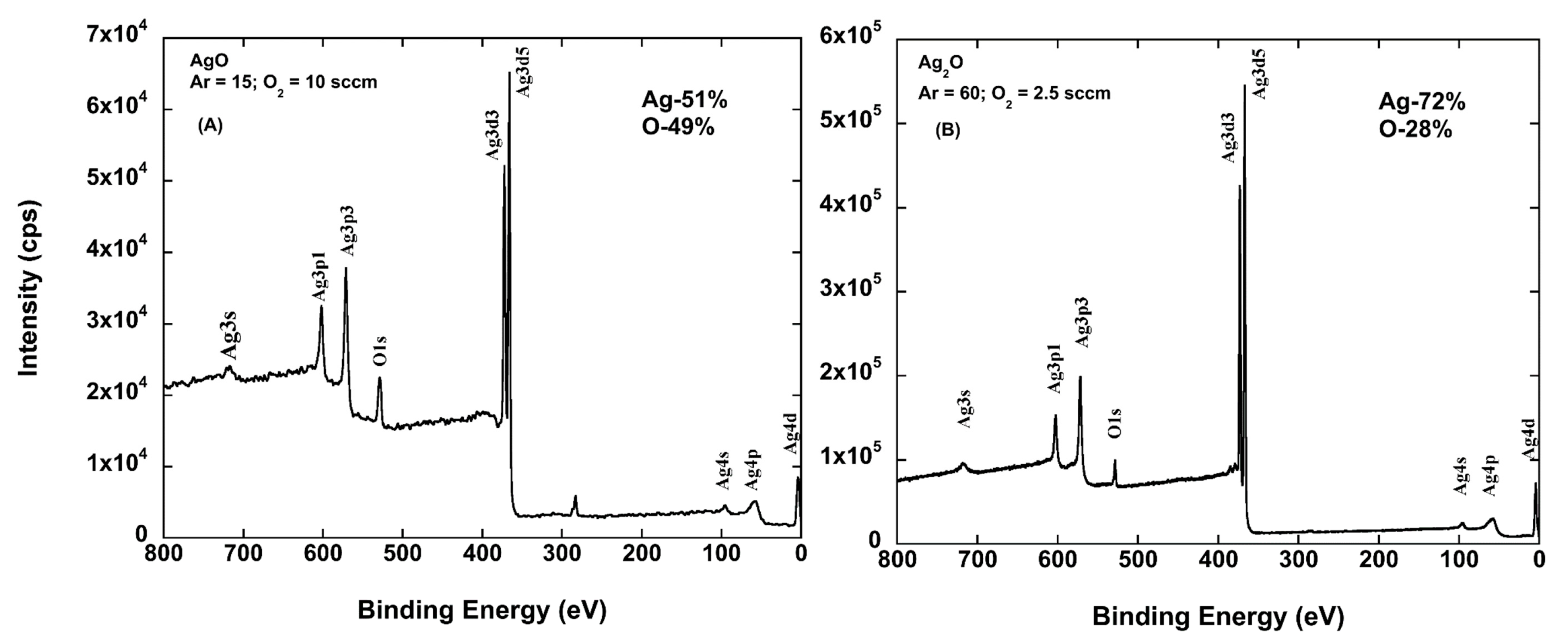
2.1. Oxygen Flow Dependence of Oxide Formed
2.2. Adhesion of AgxO Films
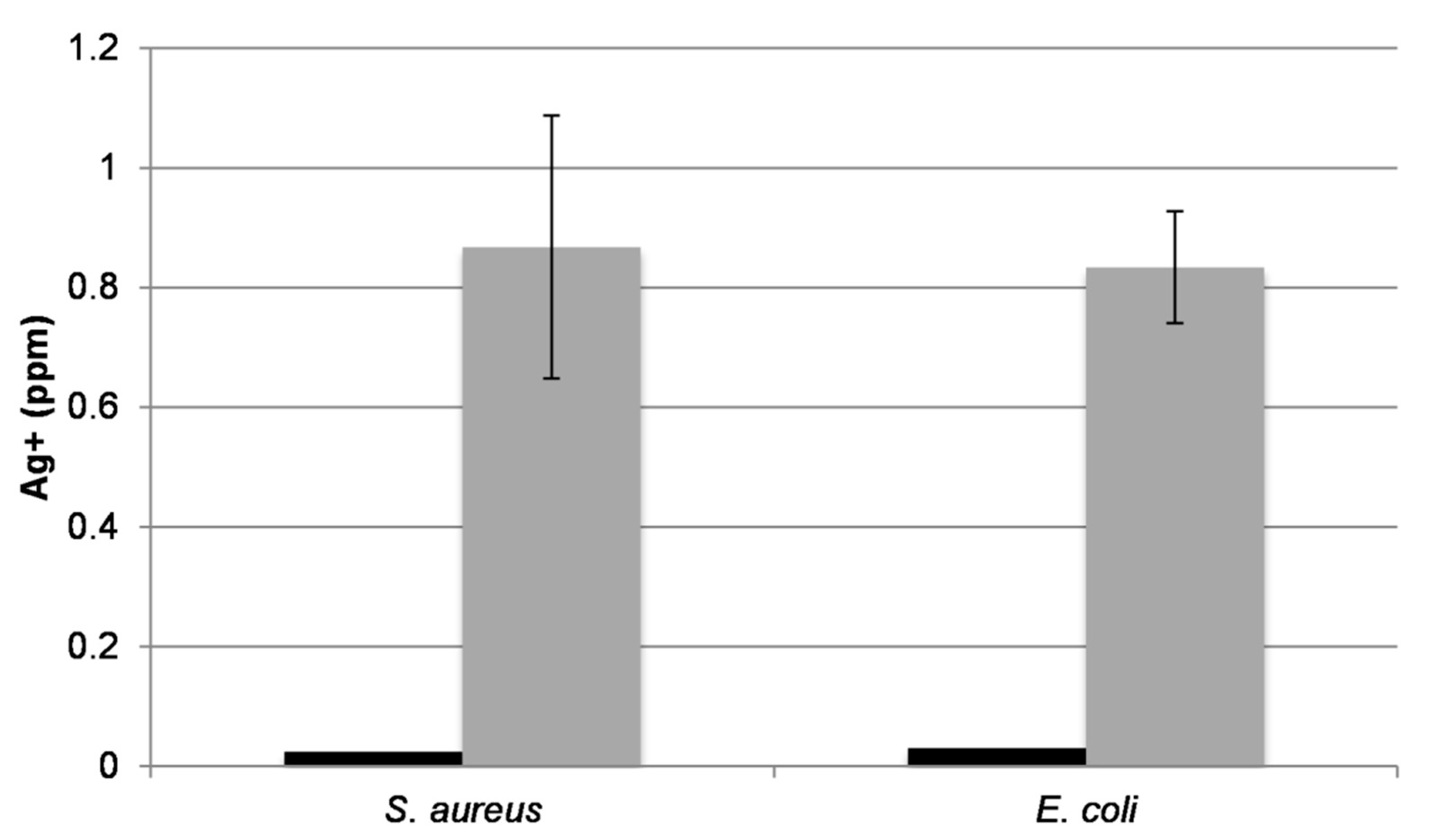
2.3. Ag Elution from Silver Oxide Films
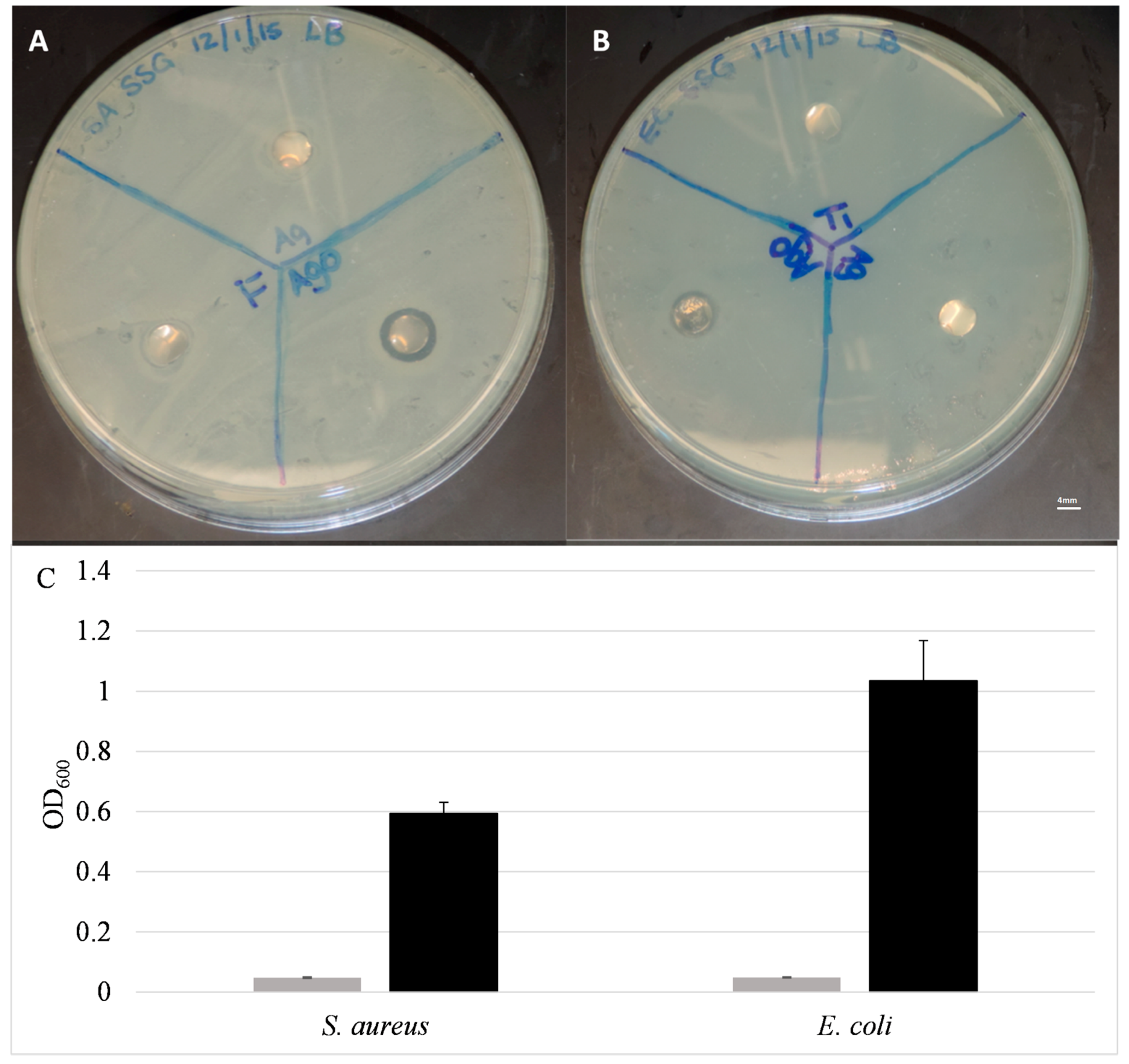
2.4. Antibacterial Activity
2.4.1. Static Testing
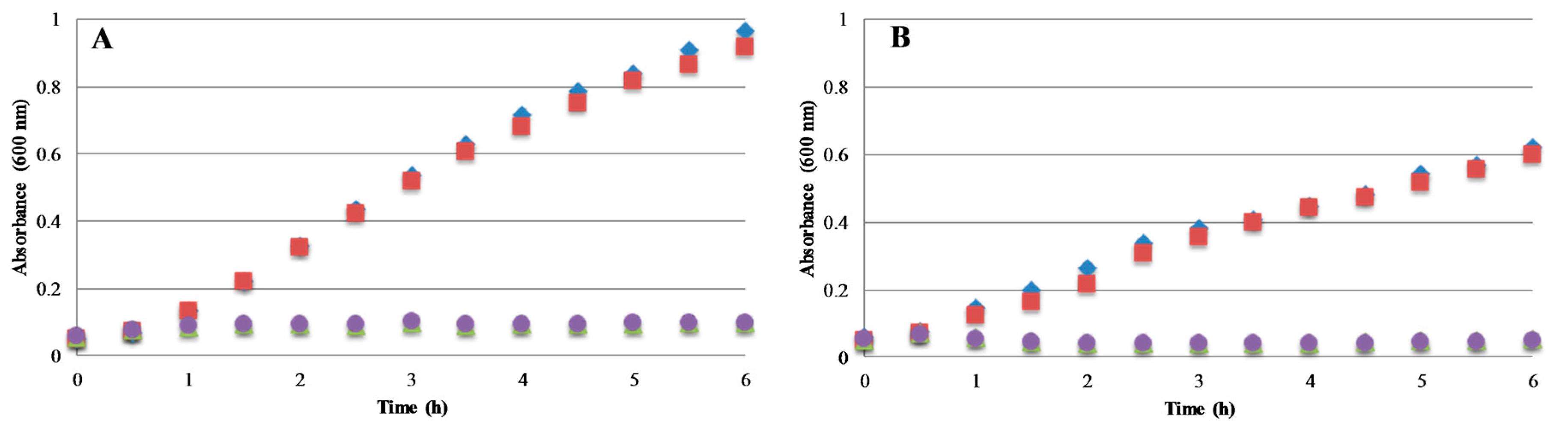
2.4.2. Bacterial Growth Kinetics
2.4.3. Bacterial Uptake of Ag
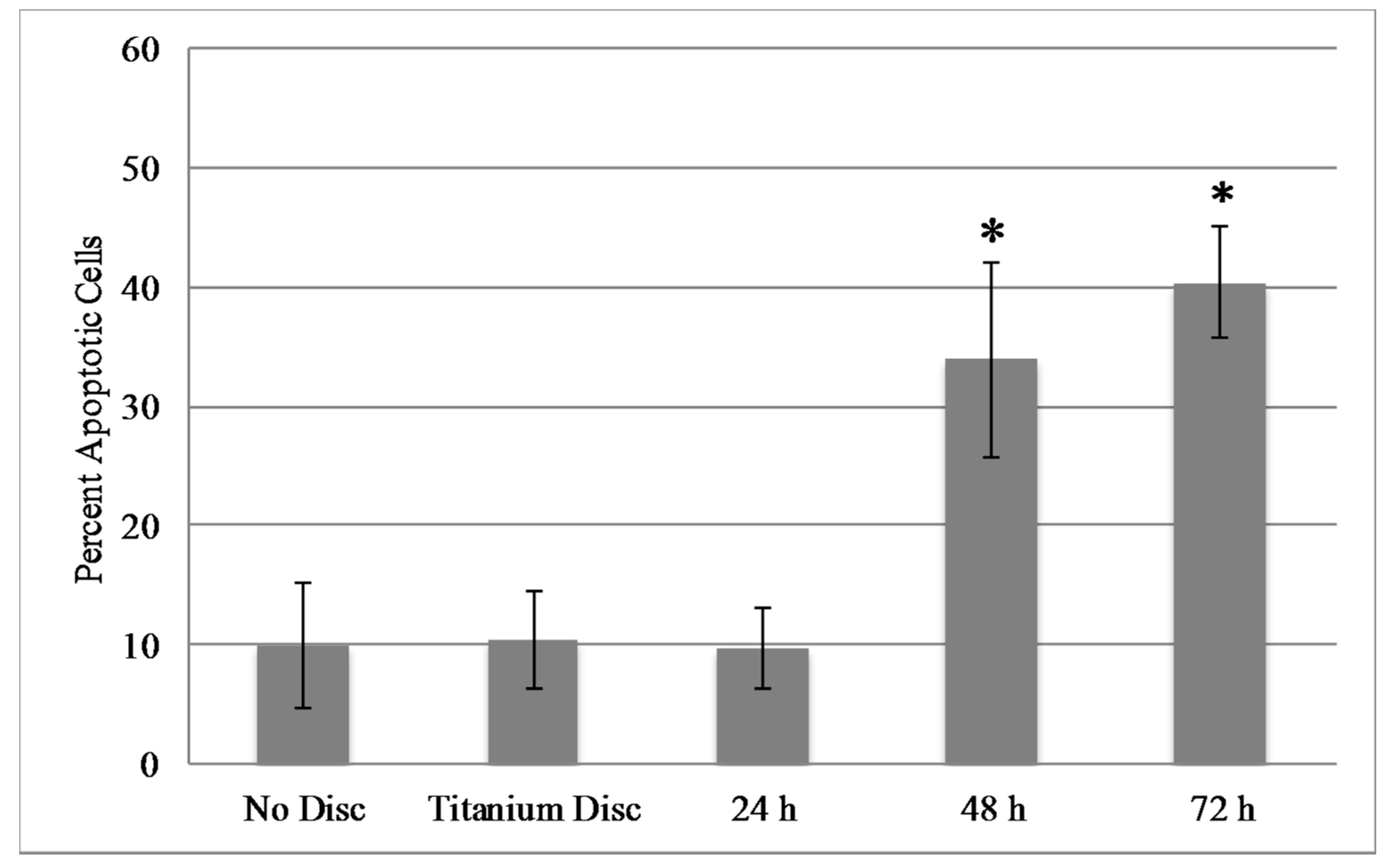
2.5. Mammalian Cell Viability
3. Discussion
3.1. Synthesis of and Analysis of AgO Containing Coatings
3.2. Antibacterial Activity
3.3. Broader Perspectives
4. Materials and Methods
4.1. Generation of Ag-Containing Films
4.2. Bacterial Culturing
4.3. Bacterial Growth Analysis
4.4. ICP-MS Analysis
4.5. Mammalian Cell Viability Assays
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| S. aureus | Staphylococcus aureus |
| E. coli | Escherichia coli |
| AgxO | Mixture of AgO and Ag2O |
| PI | Propidium Iodide |
| OD600 | Optical density at 600 nm |
| ZOI | Zone of inhibition |
References
- Yoneyama, H.; Katsumata, R. Antibiotic resistance in bacteria and its future for novel antibiotic development. Biosci. Biotechnol. Biochem. 2006, 70, 1060–1075. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- O’Neill, J. Resistance TRoA. In Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; HM Government: London, UK, 2014. [Google Scholar]
- Berbari, E.F.; Hanssen, A.D.; Duffy, M.C.; Steckelberg, J.M.; Ilstrup, D.M.; Harmsen, W.S.; Dsmon, D.R. Risk factors for prosthetic joint infection: Case-control study. Clin. Infect. Dis. 1998, 27, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Lentino, J.R. Prosthetic joint infections: Bane of orthopedists, challenge for infectious disease specialists. Clin. Infect. Dis. 2003, 36, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.W. History of the medical use of silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.K.; Doktycz, M.J.; Wang, W.; Moon, J.W.; Gu, B.; Meyer, H.M., III; Hensley, D.K.; Allison, D.P.; Phelps, T.J.; Pelletier, D.A. Monodispersed biocompatible silver sulfide nanoparticles: Facile extracellular biosynthesis using the gamma-proteobacterium, Shewanella oneidensis. Acta Biomater. 2011, 7, 4253–4258. [Google Scholar] [CrossRef] [PubMed]
- Jain, J.; Arora, S.; Rajwade, J.M.; Omray, P.; Khandelwal, S.; Paknikar, K.M. Silver nanoparticles in therapeutics: Development of an antimicrobial gel formulation for topical use. Mol. Pharm. 2009, 6, 1388–1401. [Google Scholar] [CrossRef] [PubMed]
- Panacek, A.; Kolar, M.; Vecerova, R.; Prucek, R.; Soukupova, J.; Krystof, V.; Hamal, P.; Zboril, R.; Kvítek, L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2009, 30, 6333–6340. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.G.; Katayama, H.; Ohgaki, S. Inactivation of Legionella pneumophila and Pseudomonas aeruginosa: evaluation of the bactericidal ability of silver cations. Water Res. 2007, 41, 4097–4104. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D.; Hugo, W.B. Antimicrobial activity and action of silver. Prog. Med. Chem. 1994, 31, 351–370. [Google Scholar] [PubMed]
- Percival, S.L.; Slone, W.; Linton, S.; Okel, T.; Corum, L.; Thomas, J.G. The antimicrobial efficacy of a silver alginate dressing against a broad spectrum of clinically relevant wound isolates. Int. Wound J. 2011, 8, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Silver, S.; Phung le, T.; Silver, G. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J. Ind. Microbiol. Biotechnol. 2006, 33, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Jones, V. The benefits of silver in hygiene, personal care and healthcare. Lett. Appl. Microbiol. 2009, 49, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Chole, R.A.; Hubbell, R.N. Antimicrobial activity of silastic tympanostomy tubes impregnated with silver oxide. A double-blind randomized multicenter trial. Arch. Otolaryngol. Head Neck Surg. 1995, 121, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Fordham, W.R.; Redmond, S.; Westerland, A.; Cortes, E.G.; Walker, C.; Gallagher, C.; Medina, C.J.; Waecther, F.; Lunk, C.; Ostrum, R.F.; et al. Silver as a Bactericidal Coating for Biomedical Implants. Surf. Coat. Technol. 2014, 253, 52–57. [Google Scholar] [CrossRef]
- Schaeffer, A.J.; Story, K.O.; Johnson, S.M. Effect of silver oxide/trichloroisocyanuric acid antimicrobial urinary drainage system on catheter-associated bacteriuria. J. Urol. 1988, 139, 69–73. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan–silver oxide nanocomposite film: Preparation and antimicrobial activity. Bull. Mater. Sci. 2011, 34, 29–35. [Google Scholar] [CrossRef]
- Pierson, J.F.; Rousselot, C. Stability of reactively sputtered silver oxide films. Surf. Coat. Technol. 2005, 200, 276–279. [Google Scholar] [CrossRef]
- Ida, Y.; Watase, S. Tsutomu Shinagawa, Mitsuru Watanabe, Masaya Chigane, Minoru Inaba, Akimasa Tasaka, and Masanobu Izaki, “Direct Electrodeposition of 1.46 eV Bandgap Silver (I) Oxide Semiconductor Films by Electrogenerated Acid”. Chem. Mater. 2008, 20, 1254–1256. [Google Scholar] [CrossRef]
- Kent, R.D.; Vikesland, P.J. Controlled Evaluation of Silver Nanoparticle Dissolution Using Atomic Force Microscopy. Environ. Sci. Technol. 2012, 46, 6977–6984. [Google Scholar] [CrossRef] [PubMed]
- Ambale, F.; Wagner, S.; Stahl, F.; Khaydarov, R.R.; Scheper, T.; Bahnemann, D. Investigations of the Toxic Effect of Silver Nanoparticles on Mammalian Cell Lines. J. Nanomater. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Bockstael, K.; Aerschot, A.V. Antimicrobial Resistance in Bacteria. Cent. Eur. J. Med. 2008, 4, 141–155. [Google Scholar] [CrossRef]
- Duran, N.; Duran, M.; de Jesus, M.B.; Seabra, A.B.; Favaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.L.; Ayala-Núñez, N.V.; del Carmen Ixtepan Turrent, L.; Rodríguez Padilla, C. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621. [Google Scholar] [CrossRef]
- Markowska, K.; Grudniak, A.M.; Wolska, K.I. Silver nanoparticles as an alternative strategy against bacterial biofilms. Acta Biochim. Pol. 2013, 60, 523–530. [Google Scholar] [PubMed]
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic silver nanoparticles: Synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I. The increasing use of silver-based products as antimicrobial agents: A useful development or a cause for concern? J. Antimicrob. Chem. 2007, 59, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Ostrum, R.; Hettinger, J.D.; Krchnavek, R.R.; Caputo, G.A. Use of Silver-Containing Layers at Implant Surfaces. U.S. Patent No. 9.649.338, 2017. [Google Scholar]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Amin, Y.M.; Hawas, A.M.; El-Batal, A.I.; Hassan, S.H.M.; Elsayed, M.E. Evaluation of Acute and Subchronic Toxicity of Silver Nanoparticles in Normal and Irradiated Animals. Br. J. Pharmacol. Toxicol. 2015, 6, 22–38. [Google Scholar]
- Maneewattanapinyo, P.; Banlunara, W.; Thammacharoen, C.; Ekgasit, S.; Kaewamatawong, T. An evaluation of acute toxicity of colloidal silver nanoparticles. J. Vet. Med. Sci. 2011, 73, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Isaacs, F.J.; Carr, P.A.; Sun, Z.Z.; Xu, G.; Forest, C.R.; Church, G.M. Programming cells by multiplex genome engineering and accelerated evolution. Nature 2009, 460, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Retchkiman-Schabes, P.S.; Canizal, G.; Becerra-Herrera, R.; Zorrilla, C.; Liu, H.B.; Ascencio, J.A. Biosynthesis and characterization of Ti/Ni bimetallic nanoparticles. Opt. Mater. 2006, 29, 95–99. [Google Scholar] [CrossRef]
- Gu, H.; Ho, P.L.; Tong, E.; Wang, L.; Hu, B. Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett. 2003, 3, 1261–1263. [Google Scholar] [CrossRef]
- Ahmad, Z.; Pandey, R.; Sharma, S.; Khuller, G.K. Alginate nanoparticles as antituberculosis drug carriers: Formulation development, pharmacokinetics and therapeutic potential. Indian J. Chest. Dis. Allied Sci. 2006, 48, 171–176. [Google Scholar] [PubMed]
- Suresh, A.K.; Pelletier, D.A.; Wang, W.; Moon, J.W.; Gu, B.; Mortensen, N.P.; Allison, D.P.; Joy, D.C.; Phelps, T.J.; Doktycz, M.J. Silver nanocrystallites: Biofabrication using Shewanella oneidensis, and an evaluation of their comparative toxicity on gram-negative and gram-positive bacteria. Environ. Sci. Technol. 2010, 44, 5210–5215. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Lam, H.R. Oral toxicity of silver ions, silver nanoparticles and colloidal silver—A review. Regul. Toxicol. Pharmacol. 2014, 68, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.S.; Cho, H.S.; Rha, D.S.; Kim, J.M.; Park, J.D.; Choi, B.S.; Lim, R.; Chang, H.K.; Chung, Y.H. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2008, 20, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Damm, C.; Münstedt, H. Kinetic aspects of the silver ion release from antimicrobial polyamide/silver nanocomposites. Appl. Phys. A 2008, 91, 479–486. [Google Scholar] [CrossRef]
- Saidi, I.S.; Biedlingmaier, J.F.; Whelan, P. In vivo resistance to bacterial biofilm formation on tympanostomy tubes as a function of tube material. Otolaryngol. Head Neck Surg. 1999, 120, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Weis, T.L.; Schurr, M.J.; Faith, N.G.; Czuprynski, C.J.; McAnulty, J.F.; Murphy, C.J.; Abbott, N.L. Surfaces modified with nanometer-thick silver-impregnated polymeric films that kill bacteria but support growth of mammalian cells. Biomaterials 2010, 31, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Fielding, G.A.; Beyenal, H.; Bandyopadhyay, A.; Bose, S. Mechanical, in vitro antimicrobial, and biological properties of plasma-sprayed silver-doped hydroxyapatite coating. ACS Appl. Mater. Interfaces 2012, 4, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, X.; Cao, C.; Dong, Y.; Ding, C. Biological and antibacterial properties of plasma sprayed wollastonite/silver coatings. J. Biomed. Mater. Res. B 2009, 91, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Courtney, H.S.; Bettenga, M.; Agrawal, C.M.; Bumgardner, J.D.; Ong, J.L. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5517. [Google Scholar] [CrossRef] [PubMed]
- Eto, S.; Miyamoto, H.; Shobuike, T.; Noda, I.; Akiyama, T.; Tsukamoto, M.; Ueno, M.; Someya, S.; Kawano, S.; Sonohata, M.; et al. Silver oxide-containing hydroxyapatite coating supports osteoblast function and enhances implant anchorage strength in rat femur. J. Orthop. Res. 2015, 33, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Gholipourmalekabadi, M.; Nezafati, N.; Hajibaki, L.; Mozafari, M.; Moztarzadeh, F.; Hesaraki, S.; Samadikuchaksaraei, A. Detection and qualification of optimum antibacterial and cytotoxic activities of silver-doped bioactive glasses. IET Nanobiotechnol. 2015, 9, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Demidova, T.N.; Hamblin, M.R. Effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob. Agents Chem. 2005, 49, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Anderl, J.N.; Franklin, M.J.; Stewart, P.S. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chem. 2000, 44, 1818–1824. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Wahid, M.H.; Eroglu, E.; LaVars, S.M.; Newton, K.; Gibson, C.T.; Stroeher, U.H.; Chen, X.; Boulos, R.A.; Raston, C.L.; Harmer, S.L. Microencapsulation of bacterial strains in graphene oxide nano-sheets using vortex fluidics. RSC Adv. 2015, 5, 37424–37430. [Google Scholar] [CrossRef]
- Mensa, B.; Howell, G.L.; Scott, R.; DeGrado, W.F. Comparative Mechanistic Studies of Brilacidin, Daptomycin, and the Antimicrobial Peptide LL16. Antimicrob. Agents Chem. 2014, 58, 5136–5145. [Google Scholar] [CrossRef] [PubMed]
- Zoraghi, R.; See, R.H.; Axerio-Cilies, P.; Kumar, N.S.; Gong, H.; Moreau, A.; Hsing, M.; Kaur, S.; Swayze, R.D.; Worrall, L. Identification of Pyruvate Kinase in Methicillin-Resistant Staphylococcus aureus as a Novel Antimicrobial Drug Target. Antimicrob. Agents Chem. 2011, 55, 2042–2053. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Sun, D.-S.; Chu, W.-C.; Tseng, Y.-H.; Ho, H.-C.; Wang, J.-B.; Chung, P.-H.; Chen, J.-H.; Tsai, P.-J.; Lin, N.-T.; et al. The effects of the bacterial interaction with visible-light responsive titania photocatalyst on the bactericidal performance. J. Biomed. Sci. 2009, 16. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, Z.; Picciano, A.L.; Gosavi, P.M.; Moroz, Y.S.; Angevine, C.E.; Chavis, A.E.; Reiner, J.E.; Korendovych, I.V.; Caputo, G.A. Functional characterization of a melittin analog containing a non-natural tryptophan analog. Biopolymers 2015, 104, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Clinical Laboratory Standards Institute Performance standards for antimicrobial disk susceptibility tests. In Approved Standard, 9th ed.; CLSI document M2–A9, 26:1; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2012.
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [PubMed]
Sample Availability: Samples of the thin film coatings are not available from the authors. |


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goderecci, S.S.; Kaiser, E.; Yanakas, M.; Norris, Z.; Scaturro, J.; Oszust, R.; Medina, C.D.; Waechter, F.; Heon, M.; Krchnavek, R.R.; et al. Silver Oxide Coatings with High Silver-Ion Elution Rates and Characterization of Bactericidal Activity. Molecules 2017, 22, 1487. https://doi.org/10.3390/molecules22091487
Goderecci SS, Kaiser E, Yanakas M, Norris Z, Scaturro J, Oszust R, Medina CD, Waechter F, Heon M, Krchnavek RR, et al. Silver Oxide Coatings with High Silver-Ion Elution Rates and Characterization of Bactericidal Activity. Molecules. 2017; 22(9):1487. https://doi.org/10.3390/molecules22091487
Chicago/Turabian StyleGoderecci, Sarah S., Eric Kaiser, Michael Yanakas, Zachary Norris, Jeffrey Scaturro, Robert Oszust, Clarence D. Medina, Fallon Waechter, Min Heon, Robert R. Krchnavek, and et al. 2017. "Silver Oxide Coatings with High Silver-Ion Elution Rates and Characterization of Bactericidal Activity" Molecules 22, no. 9: 1487. https://doi.org/10.3390/molecules22091487