Triarylborane-Based Materials for OLED Applications
Abstract
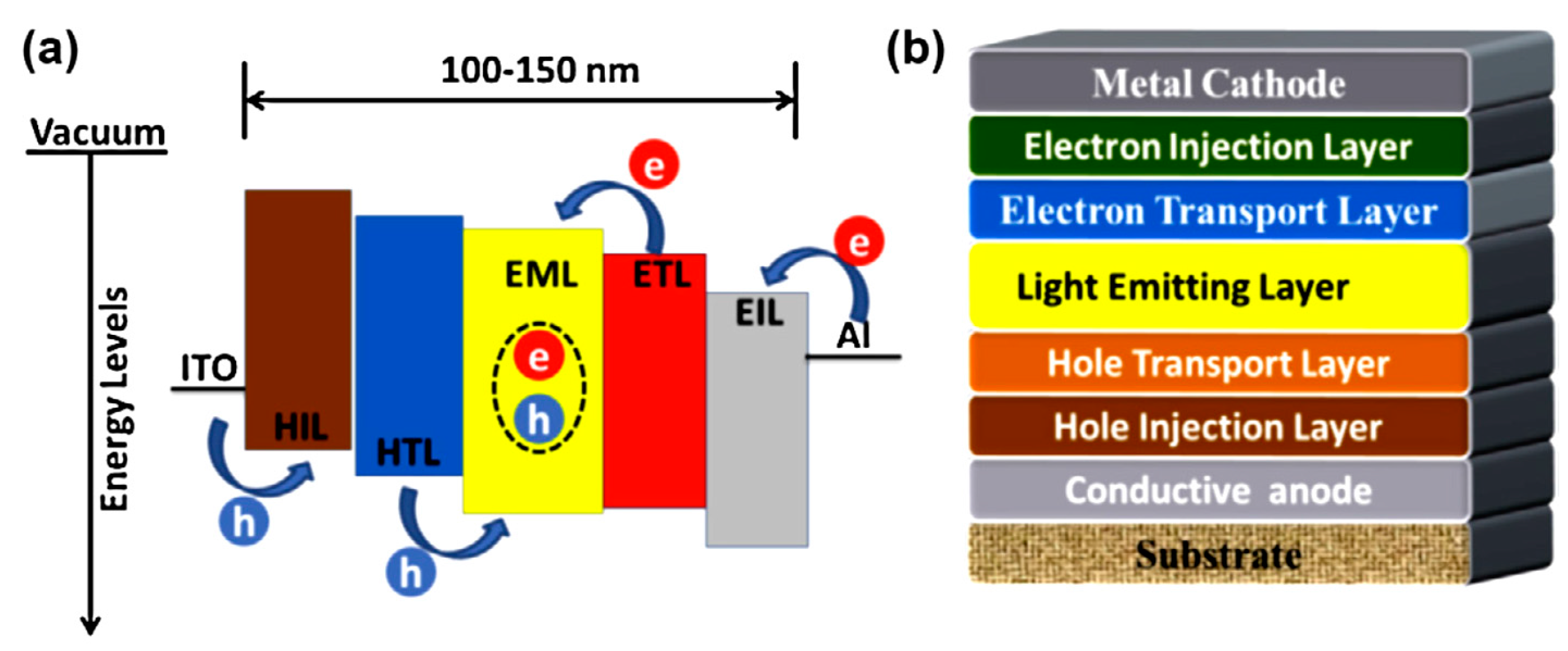
:1. Introduction
2. Triarylborane-Based Small Molecules/Oligomers
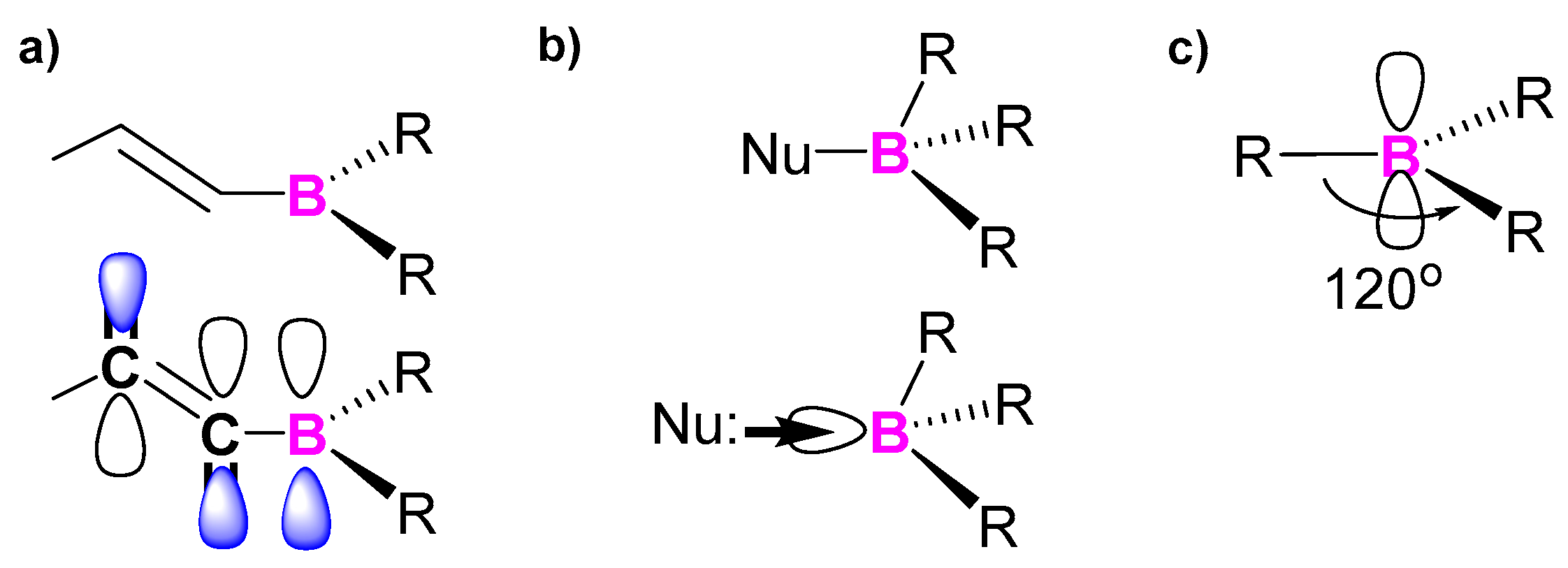
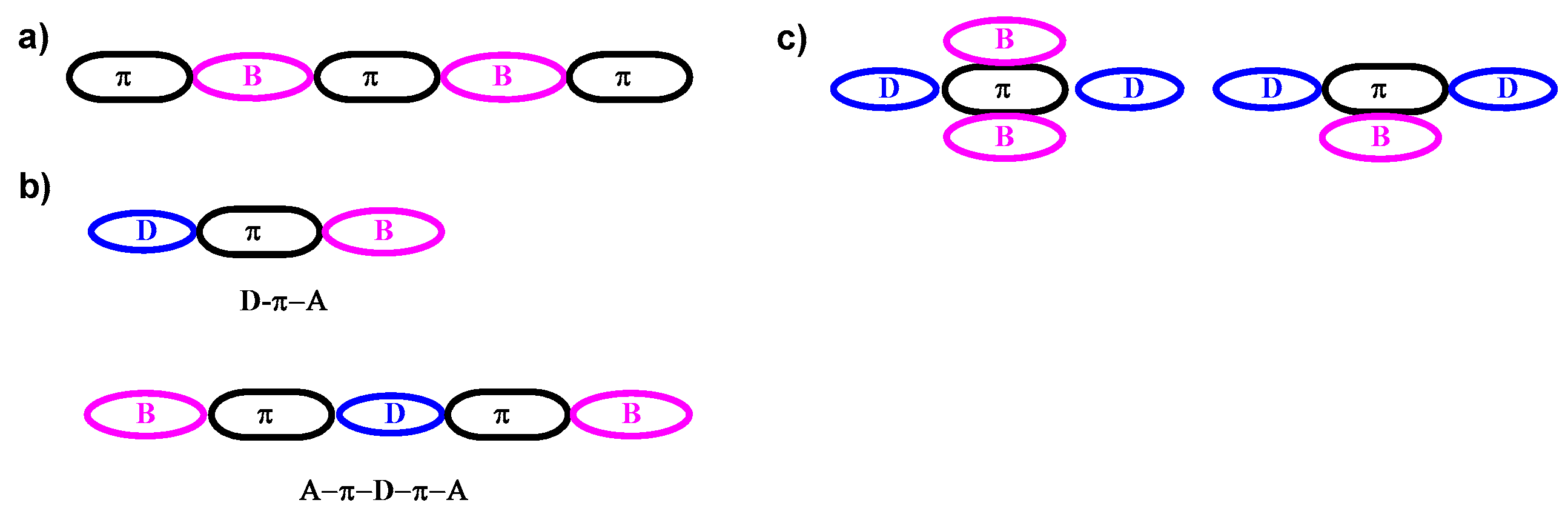
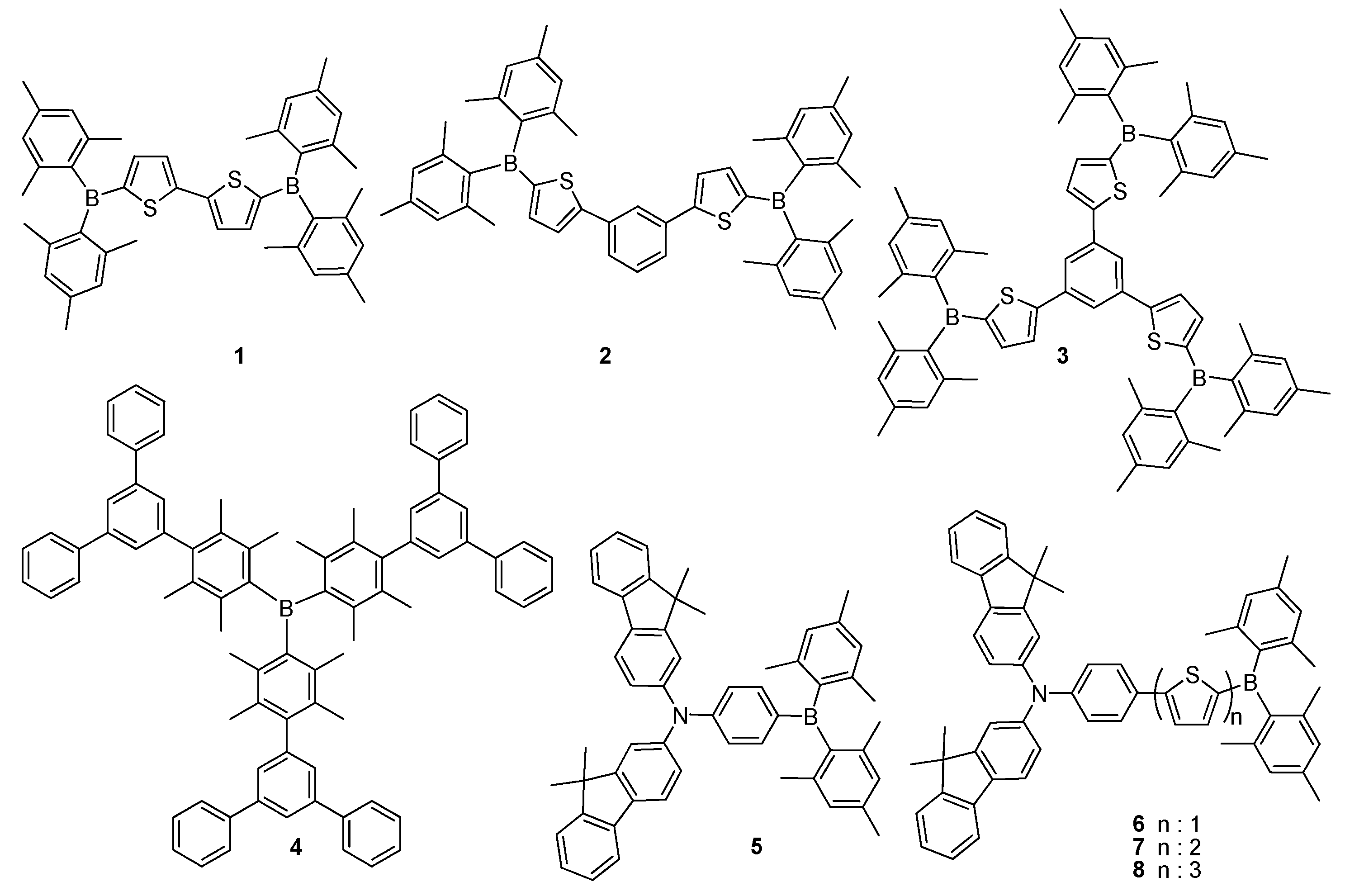
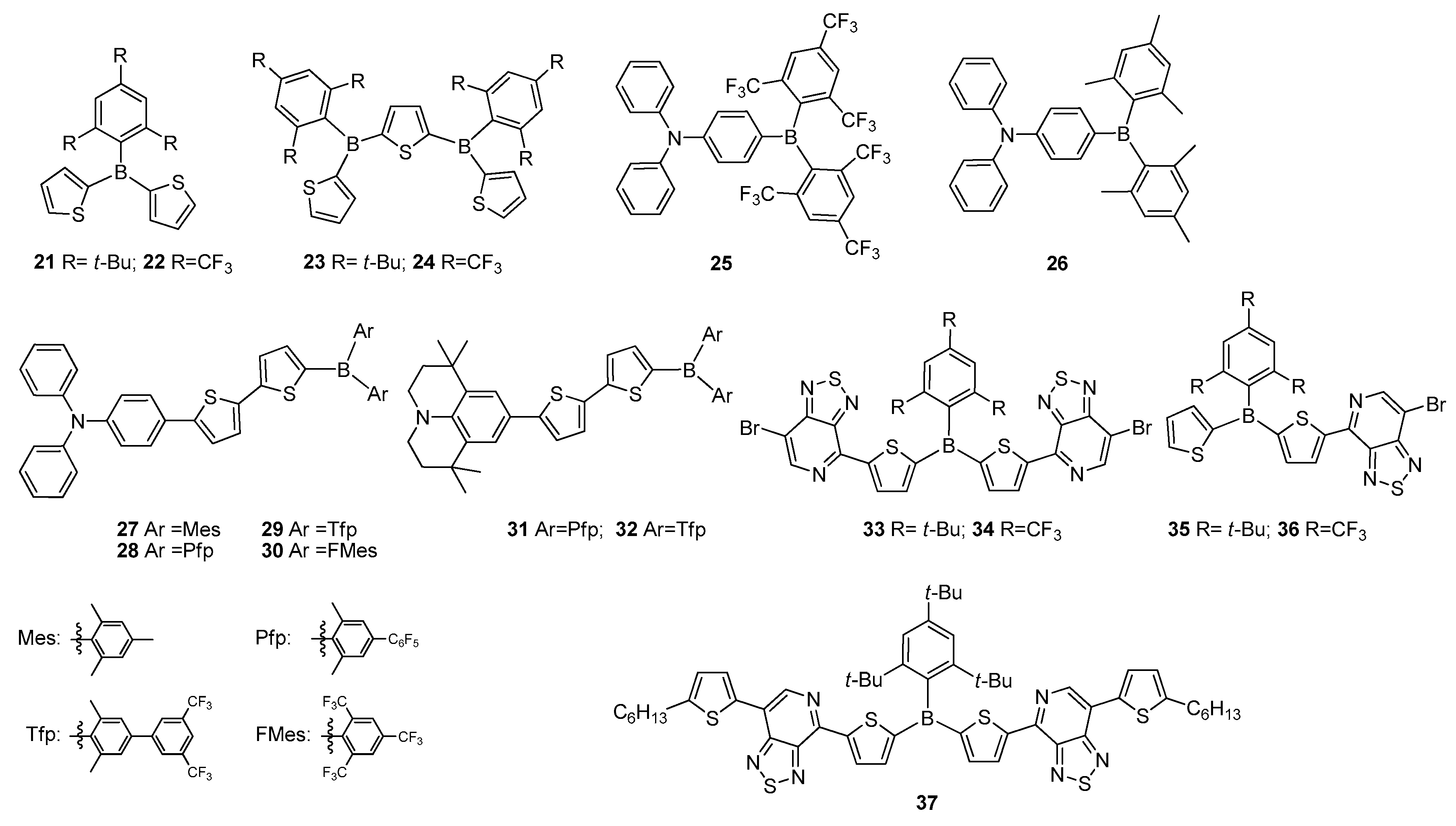
2.1. Triarylborane-Based Conjugated Systems Having Boron in the Main Chain or as A Terminator
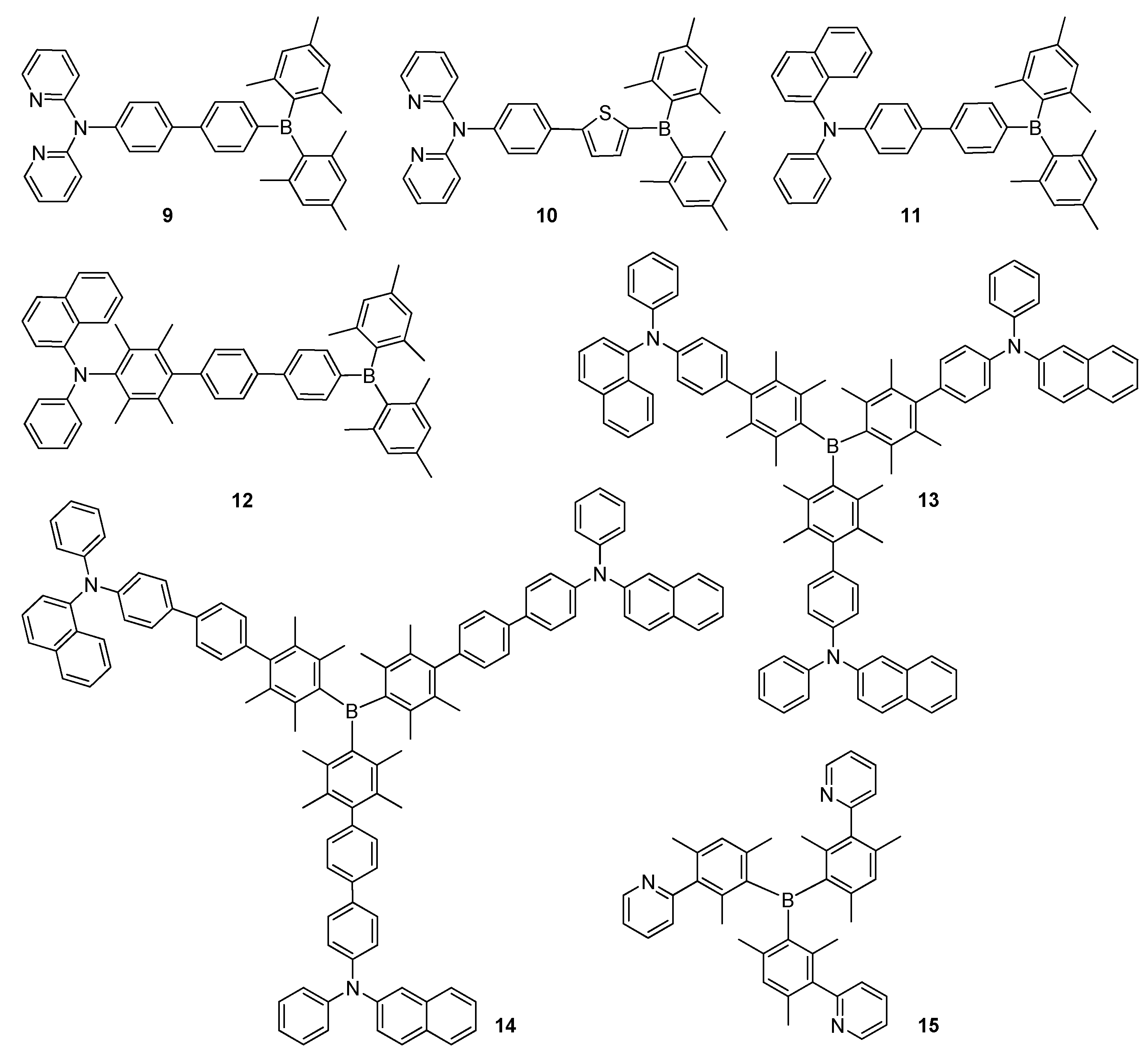
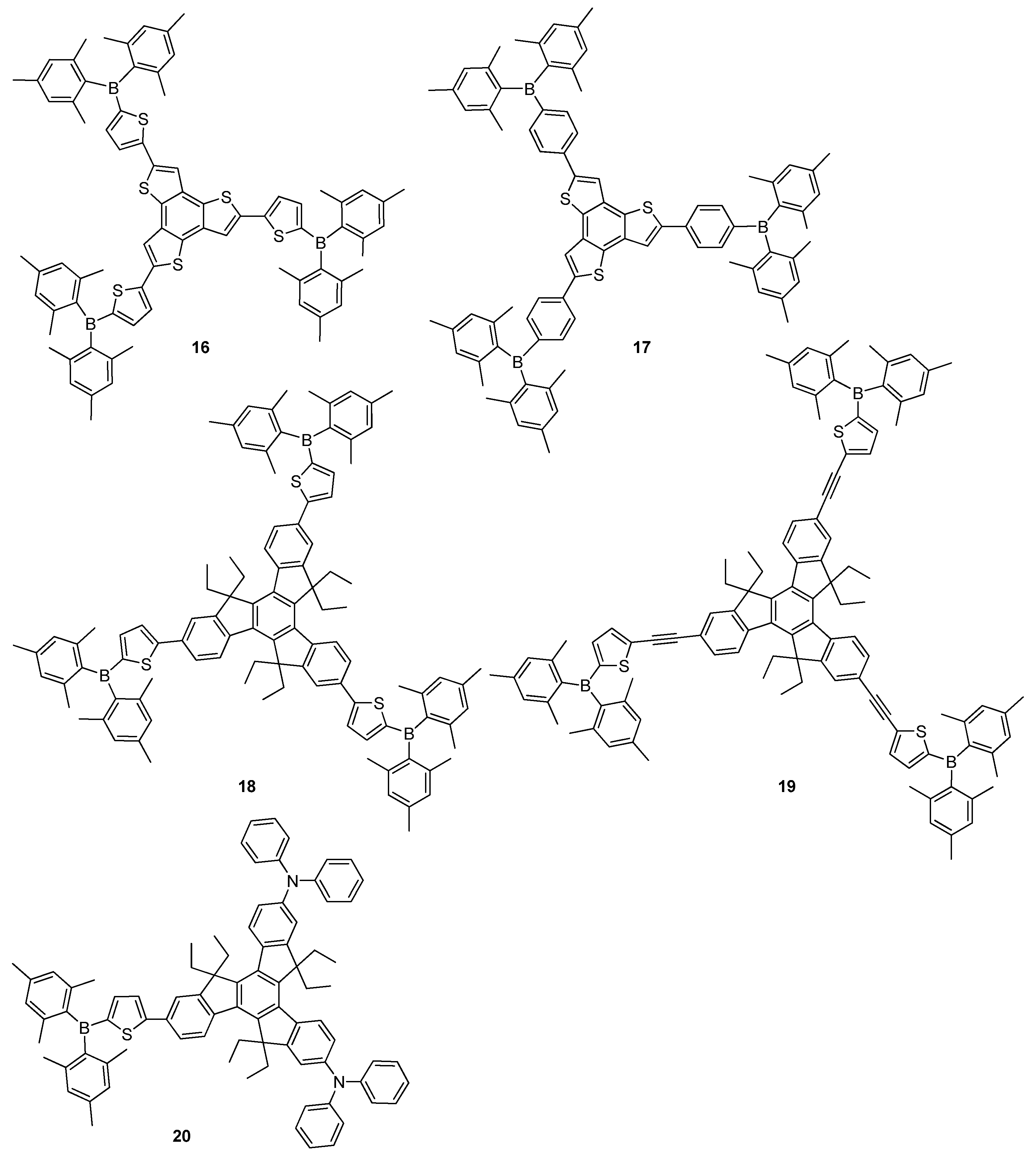
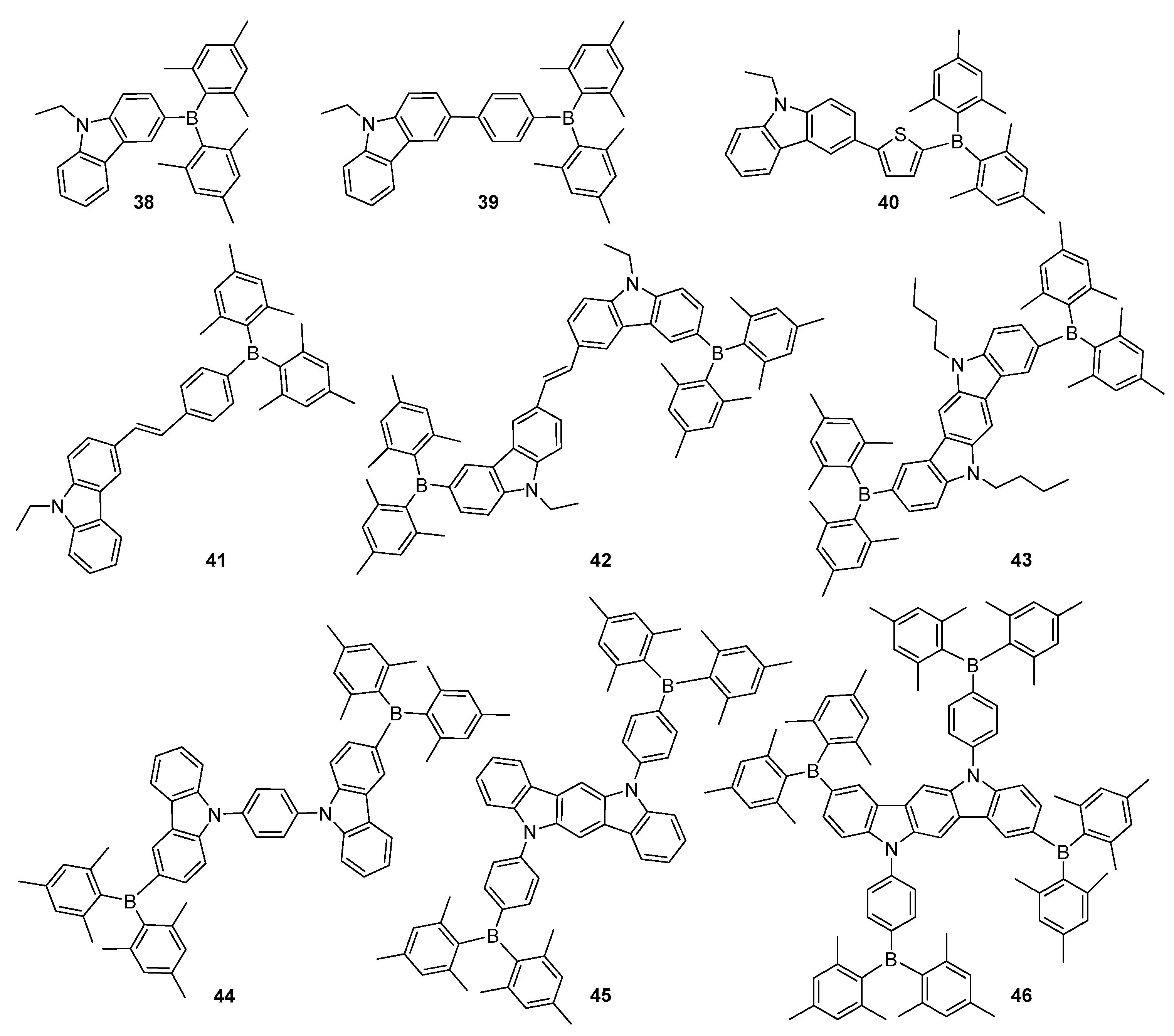
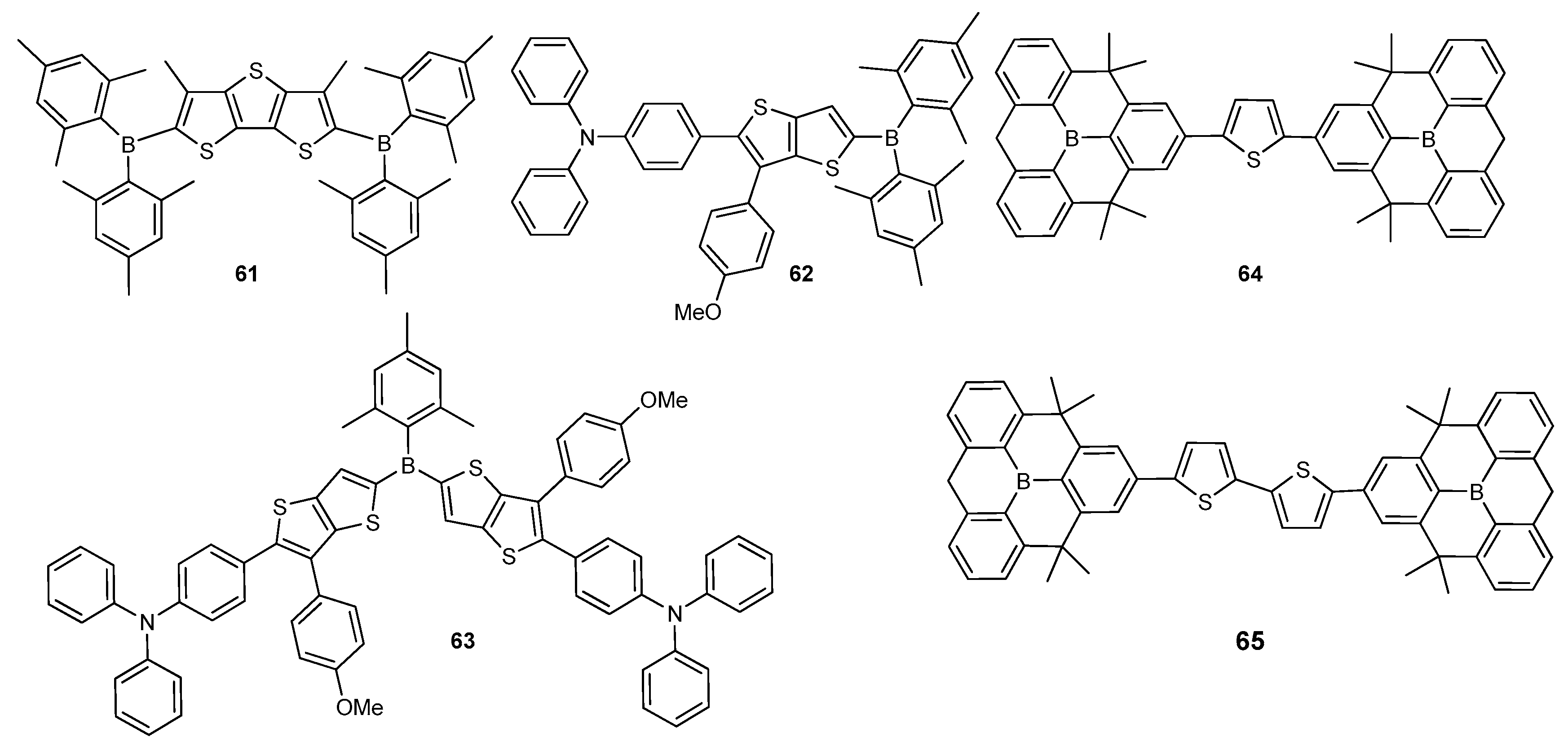
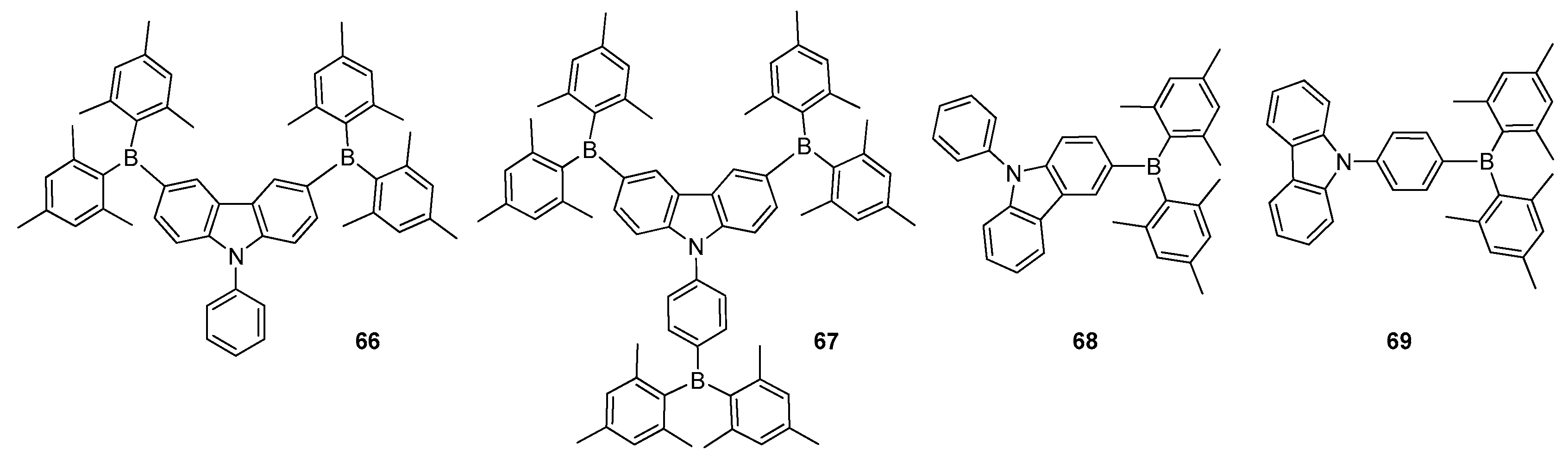
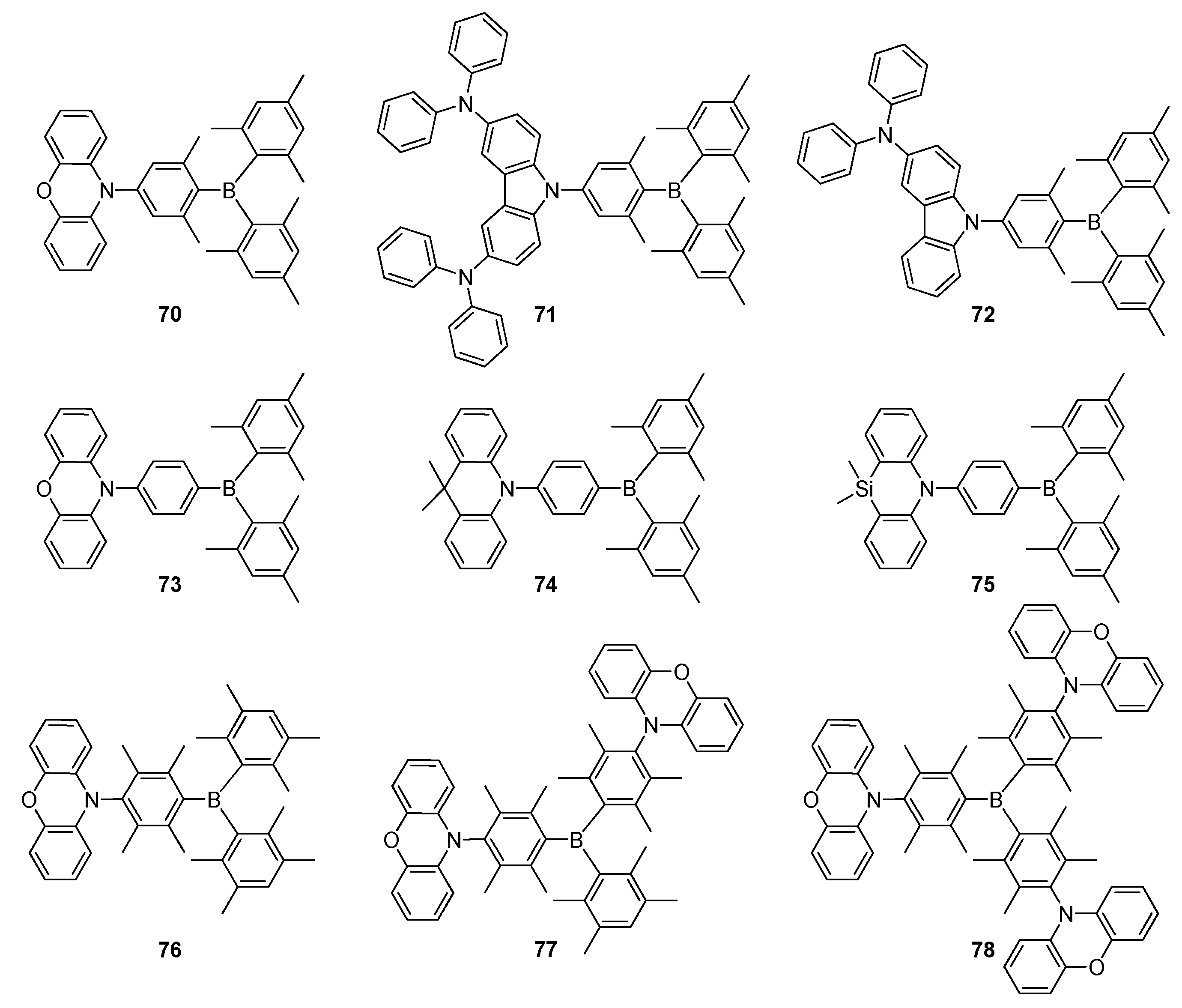
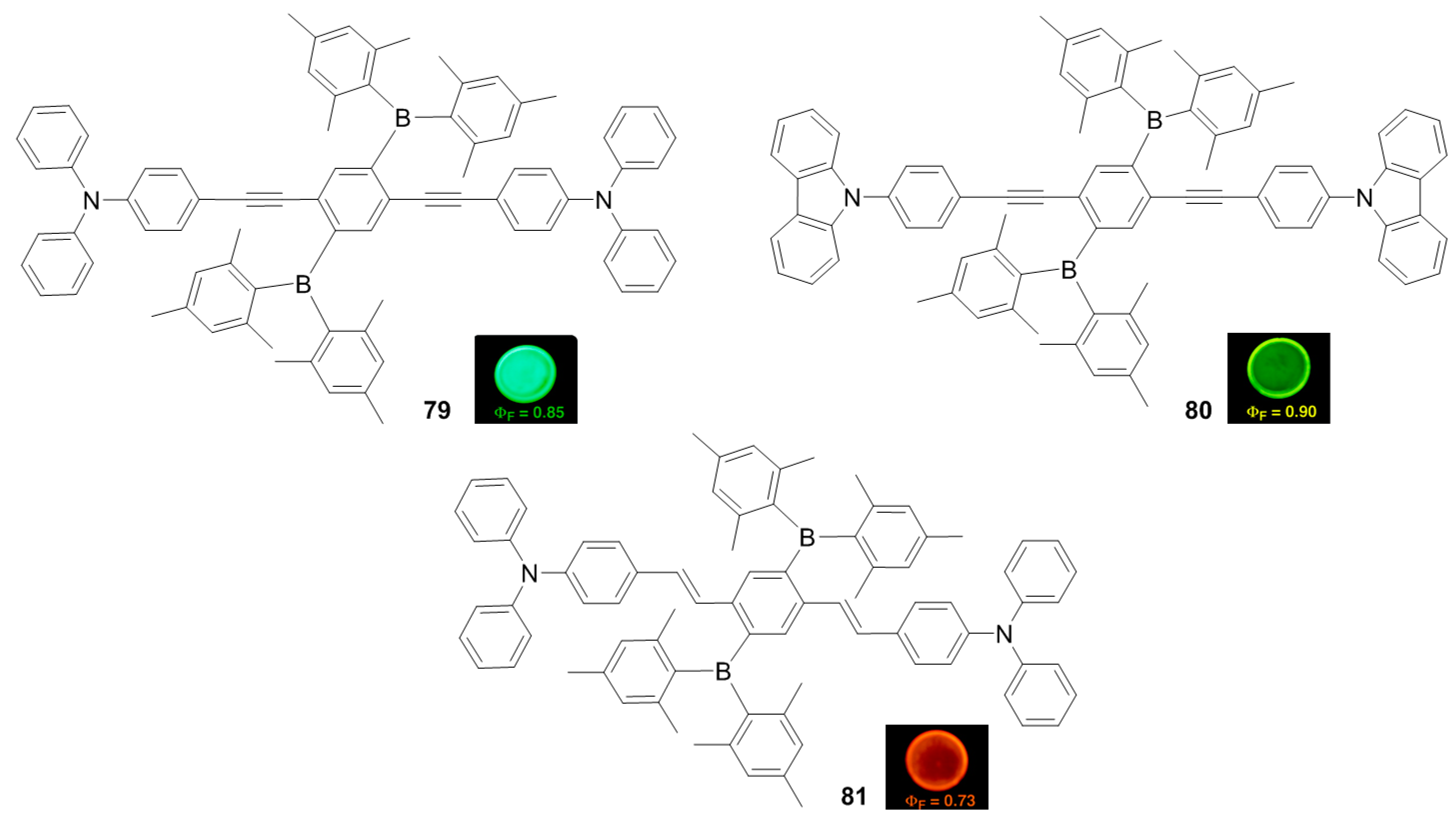
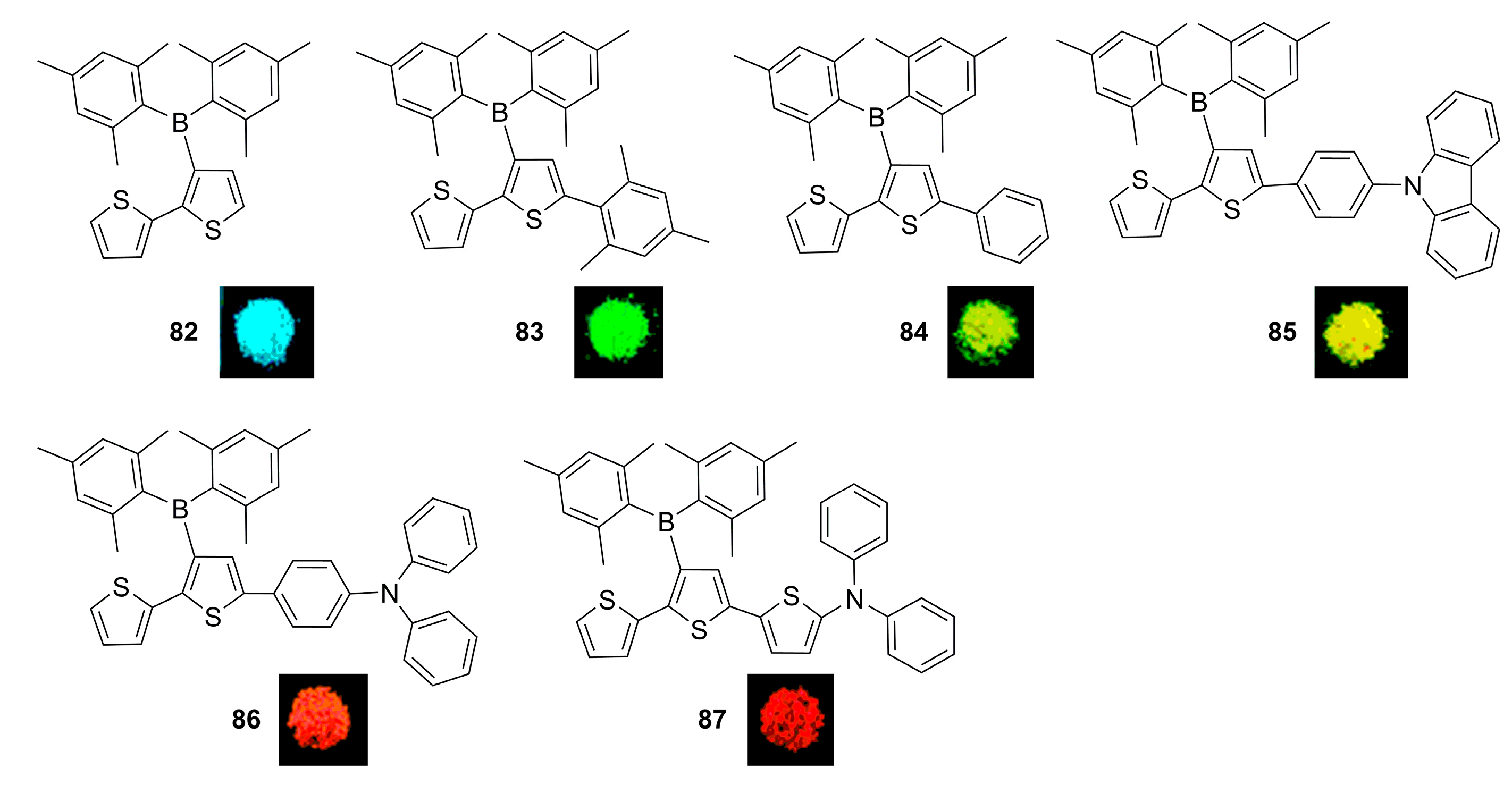
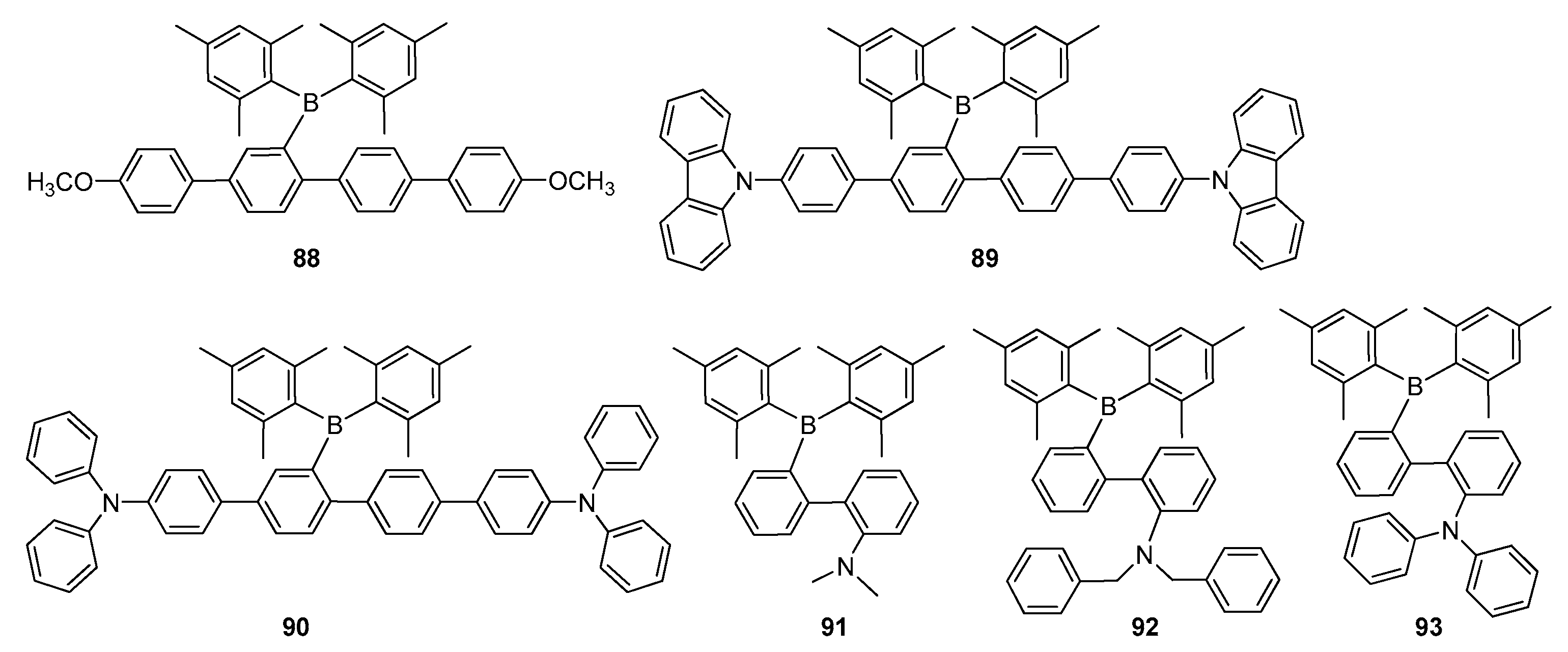
2.2. Triarylborane-Based Conjugated Systems Possessing Boron Pendant in the Side Chain
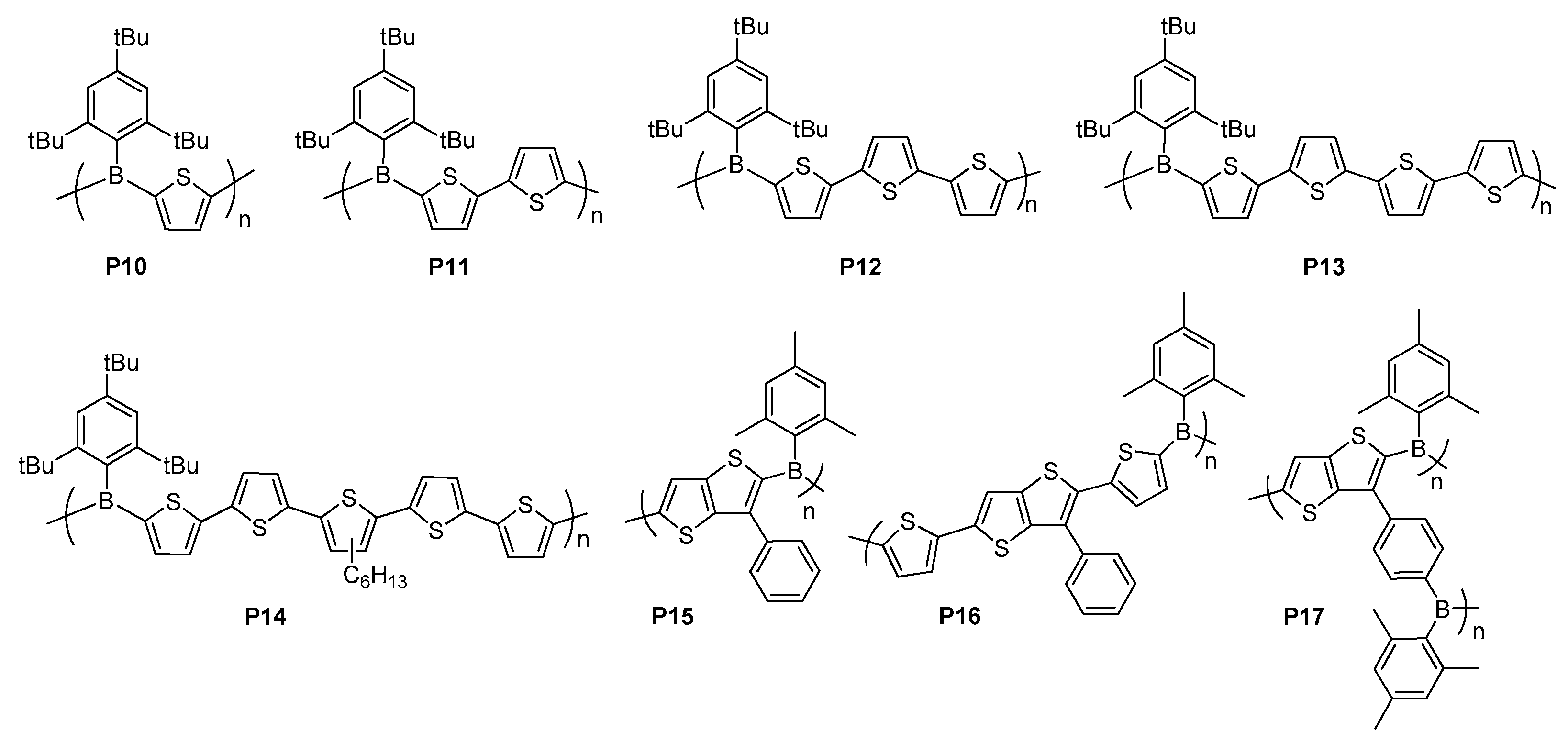
3. Triarylborane Based Polymers
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Liu, J.-Y.; Zhao, Y.-D.; Cao, Y.-C. Recent advancements of high efficient donor—Acceptor type blue small molecule applied for OLEDs. Mater. Today 2017, 20, 258–266. [Google Scholar] [CrossRef]
- Geffroy, B.; Roy, P.L.; Prat, C. Organic light-emitting diode (OLED) technology: Materials, devices and display technologies. Polym. Int. 2006, 55, 572–582. [Google Scholar] [CrossRef]
- Noda, T.; Shirota, Y. 5,5′-Bis. (dimesitylboryl)-2,2′-bithiophene and 5,5′′-bis(dimesitylboryl)-2,2′:5′,2′′-terthiophene as a novel family of electron-transporting amorphous molecular materials. J. Am. Chem. Soc. 1998, 120, 9714–9715. [Google Scholar] [CrossRef]
- Kinoshita, M.; Shirota, Y. 1,3-Bis[5-(dimesitylboryl)thiophen-2-yl]benzene and 1,3,5-tris[5-(dimesitylboryl)thiophen-2-yl]benzene as a novel family of electron-transporting hole blockers for organic electroluminescent devices. Chem. Lett. 2001, 30, 614–615. [Google Scholar] [CrossRef]
- Shirota, Y.; Kinoshita, M.; Noda, T.; Okumoto, K.; Ohara, T. A novel class of emitting amorphous molecular materials as bipolar radical formants: 2-{4-[Bis(4-methylphenyl)amino]phenyl}-5-(dimesitylboryl)thiophene and 2-{4-[Bis(9,9-dimethylfluorenyl)amino]phenyl}-5-(dimesitylboryl)thiophene. J. Am. Chem. Soc. 2000, 122, 11021–11022. [Google Scholar] [CrossRef]
- Doi, H.; Kinoshita, M.; Okumoto, K.; Shirota, Y. A novel class of emitting amorphous molecular materials with bipolar character for electroluminescence. Chem. Mater. 2003, 15, 1080–1089. [Google Scholar] [CrossRef]
- Mutaguchi, D.; Okumoto, K.; Ohsedo, Y.; Moriwaki, K.; Shirota, Y. Development of a new class of hole-transporting and emitting vinyl polymers and their application in organic electroluminescent devices. Org. Electron. 2003, 4, 49–59. [Google Scholar] [CrossRef]
- Yang, Z.; Mao, Z.; Xie, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Xu, J.; Chi, Z.; Aldred, M.P. Recent advances in organic thermally activated delayed fluorescence materials. Chem. Soc. Rev. 2017, 46, 915–1016. [Google Scholar] [CrossRef] [PubMed]
- Numata, M.; Yasuda, T.; Adachi, C. High efficiency pure blue thermally activated delayed fluorescence molecules having 10H-phenoxaborin and acridan units. Chem. Commun. 2015, 51, 9443–9446. [Google Scholar] [CrossRef]
- Suzuki, K.; Kubo, S.; Shizu, K.; Fukushima, T.; Wakamiya, A.; Murata, Y.; Adachi, C.; Kaji, H. Triarylboron-based fluorescent organic light-emitting diodes with external quantum efficiencies exceeding 20%. Angew. Chem. Int. Ed. 2015, 54, 15231–15235. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, T.; Shiren, K.; Nakajima, K.; Nomura, S.; Nakatsuka, S.; Kinoshita, K.; Ni, J.; Ono, Y.; Ikuta, T. Ultrapure blue thermally activated delayed fluorescence molecules: Efficient HOMO–LUMO separation by the multiple resonance effect. Adv. Mater. 2016, 28, 2777–2781. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Park, S.; Oh, J.; Shin, J.W.; Jung, J.; Yoo, S.; Lee, M.H. Rigidity-induced delayed fluorescence by ortho donor-appended triarylboron compounds: Record-high efficiency in pure blue fluorescent organic light-emitting diodes. ACS Appl. Mater. Interfaces 2017, 9, 24035–24042. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.C.; Dodson, V.H. Studies in stereochemistry. XXII. The preparation and reactions of trimesitylborane. Evidence for the non-localized nature of the odd electron in triarylborane radical ions and related free radicals1. J. Am. Chem. Soc. 1957, 79, 2302–2306. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Wakamiya, A. Boron as a key component for new π-electron materials. Pure Appl. Chem. 2006, 78, 1413–1424. [Google Scholar] [CrossRef]
- Elbing, M.; Bazan, G.C. A new design strategy for organic optoelectronic materials by lateral boryl substitution. Angew. Chem. Int. Ed. 2008, 47, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Wakamiya, A.; Mori, K.; Yamaguchi, S. 3-Boryl-2,2′-bithiophene as a versatile core skeleton for full-color highly emissive organic solids. Angew. Chem. Int. Ed. 2007, 46, 4273–4276. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sundararaman, A.; Venkatasubbaiah, K.; Jäkle, F. Organoborane acceptor-substituted polythiophene via side-group borylation. J. Am. Chem. Soc. 2007, 129, 5792–5793. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Kim, T.; Huh, J.O.; Do, Y.; Lee, M.H. Luminescent polyethylene with side-chain triarylboranes: Synthesis and fluoride sensing properties. Polymer 2011, 52, 1510–1514. [Google Scholar] [CrossRef]
- Chen, P.; Lalancette, R.A.; Jäkle, F. Applying the oligomer approach to luminescent conjugated organoboranes. J. Am. Chem. Soc. 2011, 133, 8802–8805. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.G.; Dwyer, A.D.; Liu, Z.; Steffen, A.; Beeby, A.; Pålsson, L.-O.; Tozer, D.J.; Marder, T.B. Experimental and theoretical studies of the photophysical properties of 2- and 2,7-functionalized pyrene derivatives. J. Am. Chem. Soc. 2011, 133, 13349–13362. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, K.M.; An, J.; Shin, M.S.; Kim, H.; Lee, J.H.; Hwang, H.; Lee, J.; Kim, M.; Park, M.H.; et al. Selective Synthesis of homoleptic and heteroleptic triarylboranes and their novel colour tunable properties. Chem. Sel. 2016, 1, 1239–1242. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Chen, S.; Lam, J.W.Y.; Deng, C.; Lu, P.; Sung, H.H.Y.; Williams, I.D.; Kwok, H.S.; Zhang, Y.; Tang, B.Z. Towards high efficiency solid emitters with aggregation-induced emission and electron-transport characteristics. Chem. Commun. 2011, 47, 11216–11218. [Google Scholar] [CrossRef] [PubMed]
- Shizu, K.; Sato, T.; Tanaka, K.; Kaji, H. A boron-containing molecule as an efficient electron-transporting material with low-power consumption. Appl. Phys. Lett. 2010, 97, 142111. [Google Scholar] [CrossRef] [Green Version]
- Ji, L.; Fang, Q.; Yuan, M.-S.; Liu, Z.-Q.; Shen, Y.-X.; Chen, H.-F. Switching high two-photon efficiency: From 3,8,13-substituted triindole derivatives to their 2,7,12-isomers. Org. Lett. 2010, 12, 5192–5195. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Edkins, R.M.; Sewell, L.J.; Beeby, A.; Batsanov, A.S.; Fucke, K.; Drafz, M.; Howard, J.A.K.; Moutounet, O.; Ibersiene, F.; et al. Experimental and theoretical studies of quadrupolar oligothiophene-cored chromophores containing dimesitylboryl moieties as π-accepting end-groups: Syntheses, structures, fluorescence, and one- and two-photon absorption. Chem. Eur. J. 2014, 20, 13618–13635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Tian, K.; Hu, D.; Wang, S.; Li, S.; Zeng, Y.; Li, Y.; Yang, G. A triarylboron-based fluorescent thermometer: Sensitive over a wide temperature range. Angew. Chem. Int. Ed. 2011, 50, 8072–8076. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.C.; Reuter, L.G.; Hamacek, J.; Wenger, O.S. Multistage complexation of fluoride ions by a fluorescent triphenylamine bearing three dimesitylboryl groups: Controlling intramolecular charge transfer. J. Org. Chem. 2011, 76, 9081–9085. [Google Scholar] [CrossRef] [PubMed]
- Jäkle, F. Advances in the synthesis of organoborane polymers for optical, electronic, and sensory applications. Chem. Rev. 2010, 110, 3985–4022. [Google Scholar] [CrossRef] [PubMed]
- Hudson, Z.M.; Wang, S. Impact of donor—Acceptor geometry and metal chelation on photophysical properties and applications of triarylboranes. Acc. Chem. Res. 2009, 42, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Wade, C.R.; Broomsgrove, A.E.J.; Aldridge, S.; Gabbai, F.P. Fluoride ion complexation and sensing using organoboron compounds. Chem. Rev. 2010, 110, 3958–3984. [Google Scholar] [CrossRef] [PubMed]
- Sakuda, E.; Ando, Y.; Ito, A.; Kitamura, N. Extremely large dipole moment in the excited singlet state of tris{[p-(N,N-dimethylamino)phenylethynyl]duryl}borane. J. Phys. Chem. A 2010, 114, 9144–9150. [Google Scholar] [CrossRef] [PubMed]
- Li, F.H.; Jia, W.L.; Wang, S.; Zhao, Y.Q.; Lu, Z.H. Blue organic light-emitting diodes based on Mes2B [p-4,4′-biphenyl-NPh(1-naphthyl)]. J. Appl. Phys. 2008, 103, 034509. [Google Scholar] [CrossRef]
- Hoven, C.V.; Wang, H.; Elbing, M.; Garner, L.; Winkelhaus, D.; Bazan, G.C. Chemically fixed p–n heterojunctions for polymer electronics by means of covalent B–F bond formation. Nat. Mater. 2010, 9, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Hudson, Z.M.; Sun, C.; Helander, M.G.; Amarne, H.; Lu, Z.-H.; Wang, S. Enhancing phosphorescence and electrophosphorescence efficiency of cyclometalated Pt(II) compounds with triarylboron. Adv. Funct. Mater. 2010, 20, 3426–3439. [Google Scholar] [CrossRef]
- Zhou, G.J.; Ho, C.L.; Wong, W.Y.; Wang, Q.; Ma, D.G.; Wang, L.X.; Lin, Z.Y.; Marder, T.B.; Beeby, A. Manipulating charge-transfer character with electron-withdrawing main-group moieties for the color tuning of iridium electrophosphors. Adv. Funct. Mater. 2008, 18, 499–511. [Google Scholar] [CrossRef]
- Entwistle, C.D.; Marder, T.B. Applications of three-coordinate organoboron compounds and polymers in optoelectronics. Chem. Mater. 2004, 16, 4574–4585. [Google Scholar] [CrossRef]
- Ren, Y.; Jäkle, F. Merging thiophene with boron: New building blocks for conjugated materials. Dalton Trans. 2016, 45, 13996–14007. [Google Scholar] [CrossRef] [PubMed]
- Sundararaman, A.; Venkatasubbaiah, K.; Victor, M.; Zakharov, L.N.; Rheingold, A.L.; Jäkle, F. Electronic communication and negative binding cooperativity in diborylated bithiophenes. J. Am. Chem. Soc. 2006, 128, 16554–16565. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Akiyama, S.; Tamao, K. Tri-9-anthrylborane and its derivatives: New boron-containing π-electron systems with divergently extended π-conjugation through boron. J. Am. Chem. Soc. 2000, 122, 6335–6336. [Google Scholar] [CrossRef]
- Hudnall, T.W.; Gabbaï, F.P. Ammonium boranes for the selective complexation of cyanide or fluoride ions in water. J. Am. Chem. Soc. 2007, 129, 11978–11986. [Google Scholar] [CrossRef] [PubMed]
- Lorbach, A.; Hübner, A.; Wagner, M. Aryl(hydro)boranes: Versatile building blocks for boron-doped π-electron materials. Dalton Trans. 2012, 41, 6048–6063. [Google Scholar] [CrossRef] [PubMed]
- Wakamiya, A.; Mishima, K.; Ekawa, K.; Yamaguchi, S. Kinetically stabilized dibenzoborole as an electron-accepting building unit. Chem. Commun. 2008, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Agou, T.; Kojima, T.; Kobayashi, J.; Kawashima, T. Synthesis of π-conjugated dendrimers based on azaborines. Org. Lett. 2009, 11, 3534–3537. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-H.; Wakamiya, A.; Inukai, Y.; Yamaguchi, S. Highly emissive organic solids containing 2,5-diboryl-1,4-phenylene unit. J. Am. Chem. Soc. 2006, 128, 15934–15935. [Google Scholar] [CrossRef] [PubMed]
- Stahl, R.; Lambert, C.; Kaiser, C.; Wortmann, R.; Jakober, R. Electrochemistry and photophysics of donor-substituted triarylboranes: Symmetry breaking in ground and excited state. Chem. Eur. J. 2006, 12, 2358–2370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.H.; Sakuda, E.; Wakamiya, A.; Yamaguchi, S. Highly emissive diborylphenylene-containing bis(phenylethynyl)benzenes: Structure—Photophysical property correlations and fluoride ion sensing. Chem. Eur. J. 2009, 15, 10603–10612. [Google Scholar] [CrossRef] [PubMed]
- Mullen, K.; Scherf, U. Organic Light-Emitting Devices: Synthesis, Properties and Applications; Wiley-VCH: Weinheim, Germany, 2006; p. 426. ISBN 978-3-527-60723-5. [Google Scholar]
- Xin, M.; Shujuan, L.; Chunlei, D.; Tingchun, M.; Qiang, Z.; Qidan, L.; Wei, H. A class of fascinating optoelectronic materials: Triarylboron compounds. Sci. China Chem. 2010, 53, 1235–1245. [Google Scholar] [CrossRef]
- Noda, T.; Ogawa, H.; Shirota, Y. A blue-emitting organic electroluminescent device using a novel emitting amorphous molecular material, 5,5′-bis(dimesitylboryl)-2,2′- bithiophene. Adv. Mater. 1999, 11, 283–285. [Google Scholar] [CrossRef]
- Kinoshita, M.; Kita, H.; Shirota, Y. A novel family of boron-containing hole-blocking amorphous molecular materials for blue- and blue-violet-emitting organic electroluminescent devices. Adv. Funct. Mater. 2002, 12, 780–786. [Google Scholar] [CrossRef]
- Ma, Z.; Yu, J.; Li, L.; Jiang, Y. Non-doped white organic light-emitting diodes consisting of three primary colors based on a bipolar emitter. Displays 2012, 33, 42–45. [Google Scholar] [CrossRef]
- Jia, W.-L.; Bai, D.-R.; McCormick, T.; Liu, Q.-D.; Motala, M.; Wang, R.-Y.; Seward, C.; Tao, Y.; Wang, S. Three-coordinate organoboron compounds BAr2R (Ar = mesityl, R = 7-azaindolyl- or 2,2′-dipyridylamino-functionalized aryl or thienyl) for electroluminescent devices and supramolecular assembly. Chem. Eur. J. 2004, 10, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.-L.; Feng, X.-D.; Bai, D.-R.; Lu, Z.-H.; Wang, S.; Vamvounis, G. Mes2B(p-4,4′-biphenyl-NPh(1-naphthyl)): A multifunctional molecule for electroluminescent devices. Chem. Mater. 2005, 17, 164–170. [Google Scholar] [CrossRef]
- Jia, W.-L.; Moran, M.J.; Yuan, Y.-Y.; Lu, Z.-H.; Wang, S. (1-Naphthyl)phenylamino functionalized three-coordinate organoboron compounds: Syntheses, structures, and applications in OLEDs. J. Mater. Chem. 2005, 15, 3326–3333. [Google Scholar] [CrossRef]
- Tanaka, D.; Takeda, T.; Chiba, T.; Watanabe, S.; Kido, J. Novel electron-transport material containing boron atom with a high triplet excited energy level. Chem. Lett. 2007, 36, 262–263. [Google Scholar] [CrossRef]
- Chopra, N.; Lee, J.; Zheng, Y.; Eom, S.-H.; Xue, J.; So, F. Effect of the charge balance on high-efficiency blue-phosphorescent organic light-emitting diodes. Appl. Mater. Interfaces 2009, 1, 1169–1172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fang, Q.; Wang, D.; Cao, D.; Xue, G.; Yu, W.; Lei, H. Trivalent boron as an acceptor in donor–π–acceptor-type compounds for single- and two-photon excited fluorescence. Chem. Eur. J. 2003, 9, 5074–5084. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fang, Q.; Cao, D.; Wang, D.; Xu, G. Triaryl boron-based a-π-a vs. triaryl nitrogen-based D–π–D quadrupolar compounds for single- and two-photon excited fluorescence. Org. Lett. 2004, 6, 2933–2936. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, G.-Q.; Wang, Y.-Y.; Yu, P.; Liua, Z.; Fang, Q. Octupolar organoboron chromophores derived from terthienobenzene. Synth. Met. 2012, 162, 291–295. [Google Scholar] [CrossRef]
- Yuan, M.-S.; Wang, D.-E.; Li, T.-B.; Xu, Y.; Wang, W.-J.; Tu, Q.; Zhang, Y.; Li, M.; Wang, J. Triarylborane-terminalized branched p-conjugative dyes: Synthesis, structure and optoelectronic properties. Dyes Pigments 2014, 107, 60–68. [Google Scholar] [CrossRef]
- Kim, Y.; Zhao, H.; Gabbai, F.P. Sulfonium boranes for the selective capture of cyanide ions in water. Angew. Chem. Int. Ed. 2009, 48, 4957–4960. [Google Scholar] [CrossRef] [PubMed]
- Varlan, M.; Blight, B.A.; Wang, S. Selective activation of lanthanide luminescence with triarylboron-functionalized ligands and visual fluoride indicators. Chem. Commun. 2012, 48, 12059–12061. [Google Scholar] [CrossRef] [PubMed]
- Araneda, J.F.; Neue, B.; Piers, W.E.; Parvez, M. Photochemical synthesis of a ladder diborole: A new boron-containing conjugate material. Angew. Chem. Int. Ed. 2012, 51, 8546–8550. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.R.; Siegler, M.A.; Tovar, J.D. Thiophene-fused borepins as directly functionalizable boron-containing π-electron systems. J. Am. Chem. Soc. 2014, 136, 7132–7139. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Chen, J.; Lalancette, R.A.; Marder, T.B.; Jäkle, F. Highly electron-deficient and air-stable conjugated thienylboranes. Angew. Chem. Int. Ed. 2014, 53, 9761–9765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Edkins, R.M.; Nitsch, J.; Fucke, K.; Steffen, A.; Longobardi, L.E.; Stephan, D.W.; Lambert, C.; Marder, T.B. Optical and electronic properties of airstable organoboron compounds with strongly electron-accepting bis (fluoromesityl) boryl groups. Chem. Sci. 2015, 6, 308–321. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Edkins, R.M.; Nitsch, J.; Fucke, K.; Eichhorn, A.; Steffen, A.; Wang, Y.; Marder, T.B. D–π–A triarylboron compounds with tunable push–pull character achieved by modification of both the donor and acceptor moieties. Chem. Eur. J. 2014, 21, 177–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, X.; Liu, K.; Ren, Y.; Lalancette, R.A.; Loo, Y.-L.; Jäkle, F. Pyridalthiadiazole acceptor-functionalized triarylboranes with multi-responsive optoelectronic characteristics. Chem. Sci. 2017, 8, 5497–5505. [Google Scholar] [CrossRef]
- Huang, T.-H.; Lin, J.T.; Chen, L.-Y.; Lin, Y.-T.; Wu, C.-C. Dipolar dibenzothiophene S,S-dioxide derivatives containing diarylamine: Materials for single-layer organic light-emitting devices. Adv. Mater. 2006, 18, 602–606. [Google Scholar] [CrossRef]
- Duan, L.; Qiao, J.; Sun, Y.; Qiu, Y. Strategies to design bipolar small molecules for OLEDs: Donor-acceptor structure and non-donor-acceptor structure. Adv. Mater. 2011, 23, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.L.; Chan, L.H.; Lee, R.H.; Yen, M.Y.; Kuo, W.J.; Chen, C.T.; Jeng, R.J. Highly efficient carbazole-π-dimesitylborane bipolar fluorophores for nondoped blue organic light-emitting diodes. Adv. Mater. 2008, 20, 3947–3952. [Google Scholar] [CrossRef]
- Shi, H.-P.; Dai, J.-X.; Xu, L.; Shi, L.-W.; Fang, L.; Shuanga, S.-M.; Dong, C. A boron-containing carbazole dimer: Synthesis, photophysical properties and sensing properties. Org. Biomol. Chem. 2012, 10, 3852–3858. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.-P.; Dai, J.-X.; Wu, X.-H.; Shi, L.-W.; Yuan, J.-D.; Fang, L.; Miao, Y.-Q.; Du, X.-G.; Wang, H.; Dong, C. A novel dimesitylboron-substituted indolo[3,2-b]carbazole derivative: Synthesis, electrochemical, photoluminescent and electroluminescent properties. Org. Electron. 2013, 14, 868–874. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, W.; Dong, X.; Wu, X.; Wu, Y.; Fang, L.; Miao, Y.; Wang, H. A novel carbazole derivative containing dimesitylboron units: Synthesis, photophysical, aggregation induced emission and electroluminescent properties. Dyes Pigments 2014, 104, 34–40. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yuan, J.; Wu, X.; Dong, X.; Fang, L.; Miao, Y.; Wang, H.; Cheng, F. Two novel indolo[3,2-b]carbazole derivatives containing dimesitylboron moieties: Synthesis, photoluminescent and electroluminescent properties. New J. Chem. 2014, 38, 2368–2378. [Google Scholar] [CrossRef]
- Zhang, W.; He, Z.; Wanga, Y.; Zhao, S. Multifunctional electroluminescent material based on dimesitylboron and α-naphthylamino fluorene bridge. Synth. Met. 2011, 161, 2323–2328. [Google Scholar] [CrossRef]
- Xu, X.; Ye, S.; He, B.; Chen, B.; Xiang, J.; Zhou, J.; Lu, P.; Zhao, Z.; Qiu, H. Dimesitylboryl-functionalized fluorene derivatives: Promising luminophors with good electron-transporting ability for deep blue organic light-emitting diodes. Dyes Pigments 2014, 101, 136–141. [Google Scholar] [CrossRef]
- Chen, L.; Lin, G.; Peng, H.; Nie, H.; Zhuang, Z.; Shen, S.; Ding, S.; Huang, D.; Hu, R.; Chen, S.; et al. Dimesitylboryl-functionalized tetraphenylethene derivatives: Efficient solid-state luminescent materials with enhanced electron-transporting ability for nondoped OLEDs. J. Mater. Chem. C 2016, 4, 5241–5247. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, C.; Lin, G.; Nie, H.; Luo, W.; Zhuang, Z.; Ding, S.; Hu, R.; Su, S.; Huang, F.; et al. Solution-processible, star-shaped bipolar tetraphenylethene derivatives for the fabrication of efficient nondoped OLEDs. J. Mater. Chem. C 2016, 4, 2775–2783. [Google Scholar] [CrossRef]
- Chen, L.; Lin, G.; Peng, H.; Ding, S.; Luo, W.; Hu, R.; Chen, S.; Huang, F.; Qin, A.; Zhao, Z.; et al. Sky-blue nondoped OLEDs based on new AIEgens: Ultrahigh brightness, remarkable efficiency and low efficiency roll-off. Mater. Chem. Front. 2017, 1, 176–180. [Google Scholar] [CrossRef]
- Shi, H.; Yang, J.; Dong, X.; Wu, X.; Zhou, P.; Cheng, F.; Choi, M.M.F. A novel tetraphenylethene–carbazole type compound containing the dimesitylboron moiety: Aggregation-induced emission enhancement and electroluminescence properties. RSC Adv. 2014, 4, 19418–19421. [Google Scholar] [CrossRef]
- Kalluvettukuzhy, N.K.; Thilagar, P. Bistable polyaromatic aminoboranes: Bright solid state emission and mechanochromism. Organometallics 2017, 36, 2692–2701. [Google Scholar] [CrossRef]
- Cinar, M.E.; Ozturk, T. Thienothiophenes, dithienothiophenes, and thienoacenes: Syntheses, oligomers, polymers, and properties. Chem. Rev. 2015, 115, 3036–3140. [Google Scholar] [CrossRef] [PubMed]
- Dikcal, F.; Ozturk, T.; Cinar, M.E. Fused thiophenes: An overview of the computational investigations. Org. Commun. 2017, 10, 56–71. [Google Scholar] [CrossRef]
- Turkoglu, G.; Cinar, M.E.; Ozturk, T. Synthesis, photophysical and anion sensing properties of triarylborane-substituted cross-conjugated and conjugated thienothiophenes. Eur. J. Org. Chem. 2017, 4552–4561. [Google Scholar] [CrossRef]
- Mazzeo, M.; Vitale, V.; Sala, F.F.; Anni, M.; Barbarella, G.; Favaretto, L.; Sotgiu, G.; Cingolani, R.; Gigli, G. Bright white organic light-emitting devices from a single active molecular material. Adv. Mater. 2005, 17, 34–39. [Google Scholar] [CrossRef]
- Turkoglu, G.; Cinar, M.E.; Buyruk, A.; Tekin, E.; Mucur, S.P.; Kaya, K.; Ozturk, T. Novel organoboron compounds derived from thieno[3,2-b]thiophene and triphenylamine units for OLED devices. J. Mater. Chem. C 2016, 4, 6045–6053. [Google Scholar] [CrossRef]
- Zhou, Z.; Wakamiya, A.; Kushida, T.; Yamaguchi, S. Planarized triarylboranes: Stabilization by structural constraint and their plane-to-bowl conversion. J. Am. Chem. Soc. 2012, 134, 4529–4532. [Google Scholar] [CrossRef] [PubMed]
- Shuto, A.; Kushida, T.; Fukushima, T.; Kaji, H.; Yamaguchi, S. π-Extended planarized triphenylboranes with thiophene spacers. Org. Lett. 2013, 15, 6234–6237. [Google Scholar] [CrossRef] [PubMed]
- Hertz, V.M.; Ando, N.; Hirai, M.; Bolte, M.; Lerner, H.-W.; Yamaguchi, S.; Wagner, M. Steric shielding vs structural constraint in a boron-containing polycyclic aromatic hydrocarbon. Organometallics 2017, 36, 2512–2519. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, G.; Wong, W.-Y. Functionalization of phosphorescent emitters and their host materials by main-group elements for phosphorescent organic light-emitting devices. Chem. Soc. Rev. 2015, 44, 8484–8575. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-S.; Chi, L.-C.; Chang, H.-W.; Huang, Y.-H.; Tien, K.-C.; Chen, C.-C.; Chang, C.-H.; Wu, C.-C.; Chaskar, A.; Chou, S.-H.; et al. A diarylborane-substituted carbazole as a universal bipolar host material for highly efficient electrophosphorescence devices. J. Mater. Chem. 2012, 22, 870–876. [Google Scholar] [CrossRef]
- Shi, H.; Xin, D.; Dong, X.; Dai, J.-X.; Wu, X.; Miao, Y.; Fang, L.; Wang, H.; Choi, M.M.F. A star-shaped bipolar host material based on carbazole and dimesitylboron moieties for fabrication of highly efficient red, green and blue electrophosphorescent devices. J. Mater. Chem. C 2014, 2, 2160–2168. [Google Scholar] [CrossRef]
- Jang, H.-G.; Kim, B.S.; Lee, J.Y.; Hwang, S.-H. Synthesis of dimesitylborane-substituted phenylcarbazoles as bipolar host materials and the variation of the green PHOLED performance with the substituent position of the boron atom. Dalton Trans. 2014, 43, 7712–7715. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.Y.; Zysman-Colman, E. Purely organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Adv. Mater. 2017, 29, 1605444. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Sato, K.; Yoshimura, K.; Kai, T.; Kawada, A.; Miyazaki, H.; Adachi, C. Efficient up-conversion of triplet excitons into a singlet state and its application for organic light emitting diodes. Appl. Phys. Lett. 2011, 98, 083302. [Google Scholar] [CrossRef]
- Nakagawa, T.; Ku, S.-Y.; Wong, K.-T.; Adachi, C. Electroluminescence based on thermally activated delayed fluorescence generated by a spirobifluorene donor–acceptor structure. Chem. Commun. 2012, 48, 9580–9582. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, Y.; Namikawa, T.; Ikemizu, D.; Miyata, Y.; Suzuki, T.; Kita, H.; Sato, T.; Oi, S. Light blue and green thermally activated delayed fluorescence from 10H-phenoxaborin-derivatives and their application to organic light-emitting diodes. J. Mater. Chem. C 2015, 3, 9122–9130. [Google Scholar] [CrossRef]
- Kitamoto, Y.; Namikawa, T.; Suzuki, T.; Miyata, Y.; Kita, H.; Sato, T.; Oi, S. Dimesitylarylborane-based luminescent emitters exhibiting highly efficient thermally activated delayed fluorescence for organic light emitting diodes. Org. Electron. 2016, 34, 208–217. [Google Scholar] [CrossRef]
- Kitamoto, Y.; Namikawa, T.; Suzuki, T.; Miyata, Y.; Kita, H.; Sato, T.; Oi, S. Design and synthesis of efficient blue thermally activated delayed fluorescence molecules bearing triarylborane and 10,10-dimethyl-5,10-dihydrophenazasiline moieties. Tetrahedron Lett. 2016, 57, 4914–4917. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, G.; Wu, K.; Luo, Z.; Zhou, T.; Zeng, X.; Yu, J.; Gong, S.; Yang, C. Boosting reverse intersystem crossing by increasing donors in triarylboron/phenoxazine hybrids: TADF emitters for high-performance solution-processed OLEDs. J. Mater. Chem. C 2016, 4, 4402–4407. [Google Scholar] [CrossRef]
- Li, S.-Y.; Sun, Z.-B.; Zhao, C.-H. Charge-transfer emitting triarylborane π-electron systems. Inorg. Chem. 2017, 56, 8705–8717. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-B.; Li, S.-Y.; Liu, Z.-Q.; Zhao, C.-H. Triarylborane p-electron systems with intramolecular charge-transfer transitions. Chin. Chem. Lett. 2016, 27, 1131–1138. [Google Scholar] [CrossRef]
- Fu, G.-L.; Zhang, H.-Y.; Yan, Y.-Q.; Zhao, C.-H. p-Quaterphenyls laterally substituted with a dimesitylboryl group: A promising class of solid-state blue emitters. J. Org. Chem. 2012, 77, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Fu, G.-L.; Zhao, Y.-H.; Zhao, C.-H. Through-space charge-transfer emitting biphenyls containing a boryl and an amino group at the o,o′-positions. Org. Lett. 2011, 13, 4830–4833. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, Q.-W.; Zhang, W.-N.; Peng, Q.; Zhao, C.-H. Charge-transfer emission in organoboron-based biphenyls: Effect of substitution position and conformation. J. Org. Chem. 2015, 80, 10914–10924. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yuan, Y.; Li, S.-Y.; Sun, Z.-B.; Jiang, Z.-Q.; Zhao, C.-H. A highly twisted triarylborane-based biphenyl as an efficient host for blue and green phosphorescent oleds. J. Mater. Chem. C 2016, 4, 7607–7613. [Google Scholar] [CrossRef]
- He, X.; Baumgartner, T. Conjugated main-group polymers for optoelectronics. RSC Adv. 2013, 3, 11334–11350. [Google Scholar] [CrossRef]
- Matsumi, N.; Naka, K.; Chujo, Y. Extension of π-conjugation length via the vacant p-orbital of the boron atom. Synthesis of novel electron deficient π-conjugated systems by hydroboration polymerization and their blue light emission. J. Am. Chem. Soc. 1998, 120, 5112–5113. [Google Scholar] [CrossRef]
- Zhao, C.H.; Wakamiya, A.; Yamaguchi, S. Highly emissive poly(aryleneethynylene)s containing 2,5-diboryl-1,4-phenylene as a building unit. Macromolecules 2007, 40, 3898–3900. [Google Scholar] [CrossRef]
- Turkoglu, G.; Cinar, M.E.; Ozturk, T. Organoboron copolymers containing thienothiophene and selenophenothiophene analogues: Optical, electrochemical and fluoride sensing properties. RSC Adv. 2017, 7, 23197–23207. [Google Scholar] [CrossRef]
- Reitzenstein, D.; Lambert, C. Localized versus backbone fluorescence in N-p-(diarylboryl)phenyl-substituted 2,7-and 3,6-linked polycarbazoles. Macromolecules 2009, 42, 773–782. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Lin, Y.-Y.; Chen, Y.-C.; Lin, J.T.; Lee, R.-H.; Kuo, W.-J.; Jeng, R.-J. Carbazole/fluorene copolymers with dimesitylboron pendants for blue light-emitting diodes. Polymer 2011, 52, 976–986. [Google Scholar] [CrossRef]
- Yin, X.; Guo, F.; Lalancette, R.A.; Jäkle, F. Luminescent main-chain organoborane polymers: Highly robust, electron-deficient poly(oligothiophene borane)s via Stille coupling polymerization. Macromolecules 2016, 49, 537–546. [Google Scholar] [CrossRef]
- Sevinis, E.B.; Sahin, C.; Cinar, M.E.; Eroglu, M.S.; Ozturk, T. Copolymers possessing dithienothiophene and boron for optoelectronic applications. Polym. Eng. Sci. 2016, 56, 1390–1398. [Google Scholar] [CrossRef]
- Sahin, O.; Cinar, M.E.; Tekin, E.; Mucur, S.P.; Topal, S.; Suna, G.; Eroglu, M.S.; Ozturk, T. White light emitting polymers possessing thienothiophene and boron units. ChemistrySelect 2017, 2, 2889–2894. [Google Scholar] [CrossRef]
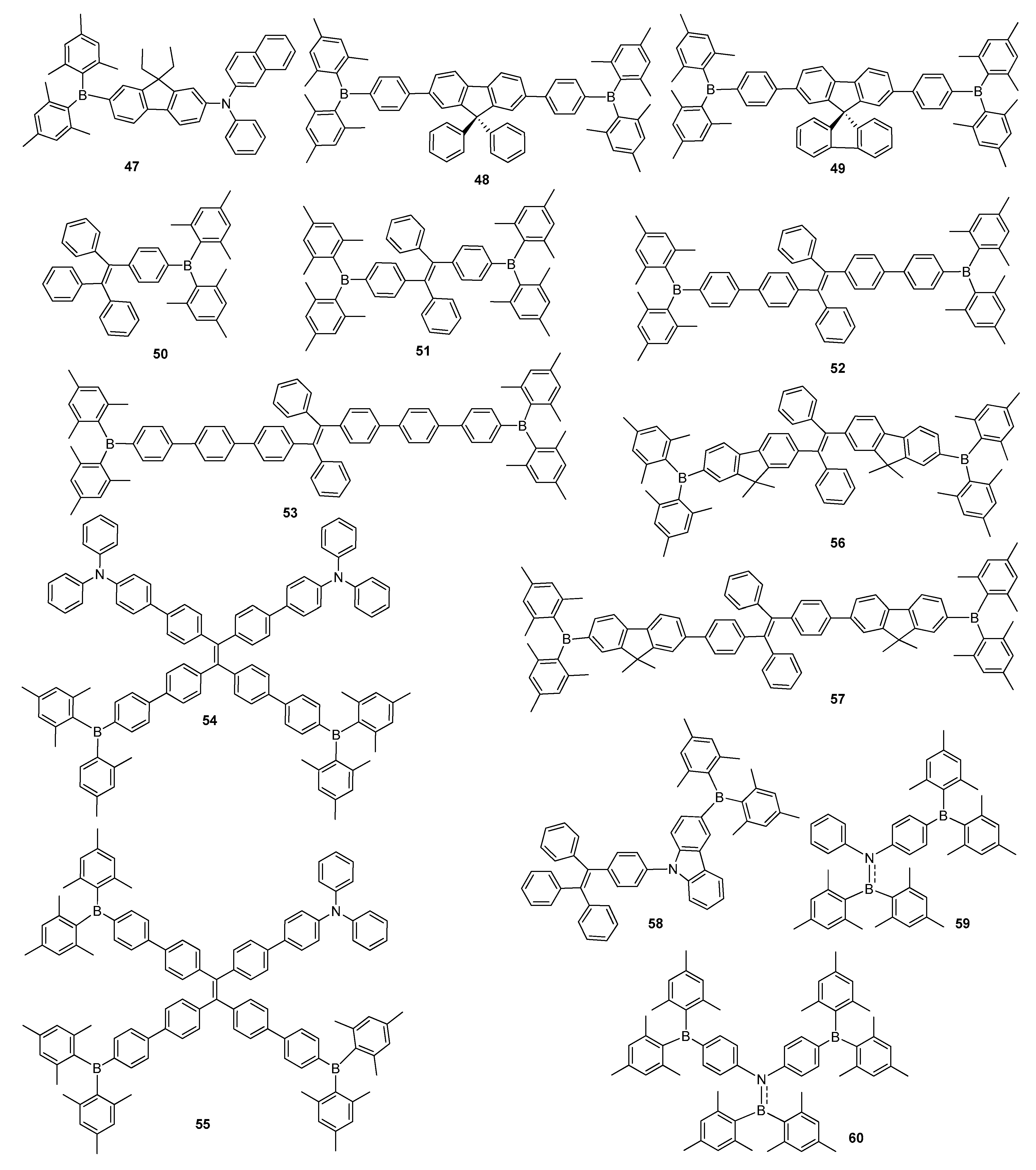

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turkoglu, G.; Cinar, M.E.; Ozturk, T. Triarylborane-Based Materials for OLED Applications. Molecules 2017, 22, 1522. https://doi.org/10.3390/molecules22091522
Turkoglu G, Cinar ME, Ozturk T. Triarylborane-Based Materials for OLED Applications. Molecules. 2017; 22(9):1522. https://doi.org/10.3390/molecules22091522
Chicago/Turabian StyleTurkoglu, Gulsen, M. Emin Cinar, and Turan Ozturk. 2017. "Triarylborane-Based Materials for OLED Applications" Molecules 22, no. 9: 1522. https://doi.org/10.3390/molecules22091522



