VSP-17, a New PPARγ Agonist, Suppresses the Metastasis of Triple-Negative Breast Cancer via Upregulating the Expression of E-Cadherin
Abstract
:1. Introduction
2. Results
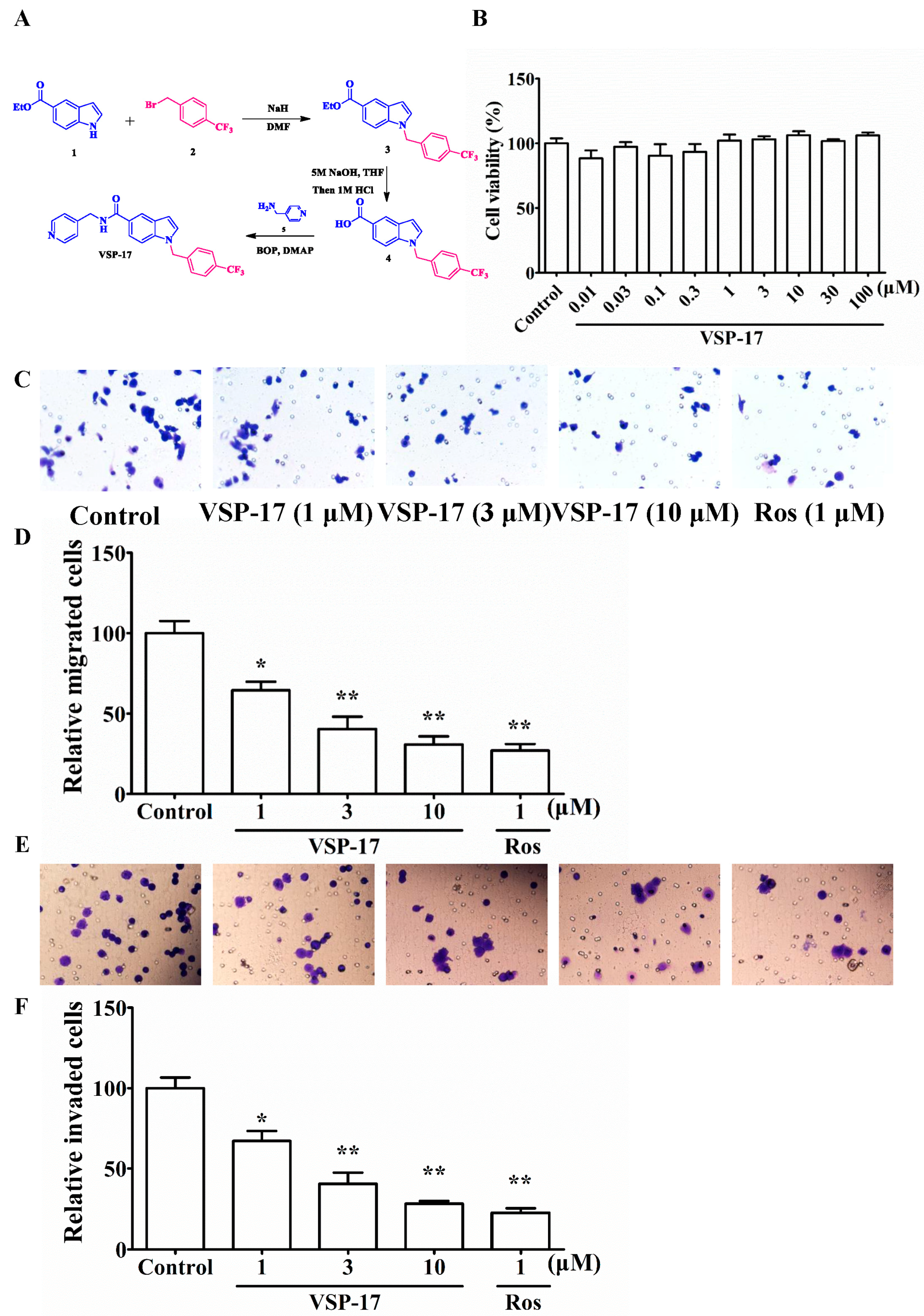
2.1. The Synthetic Routes of VSP-17
2.2. Effect of VSP-17 on the Cell Viability of MDA-MB-231 Cells
2.3. Effect of VSP-17 on the Migration and Invasion of MDA-MB-231 Cells
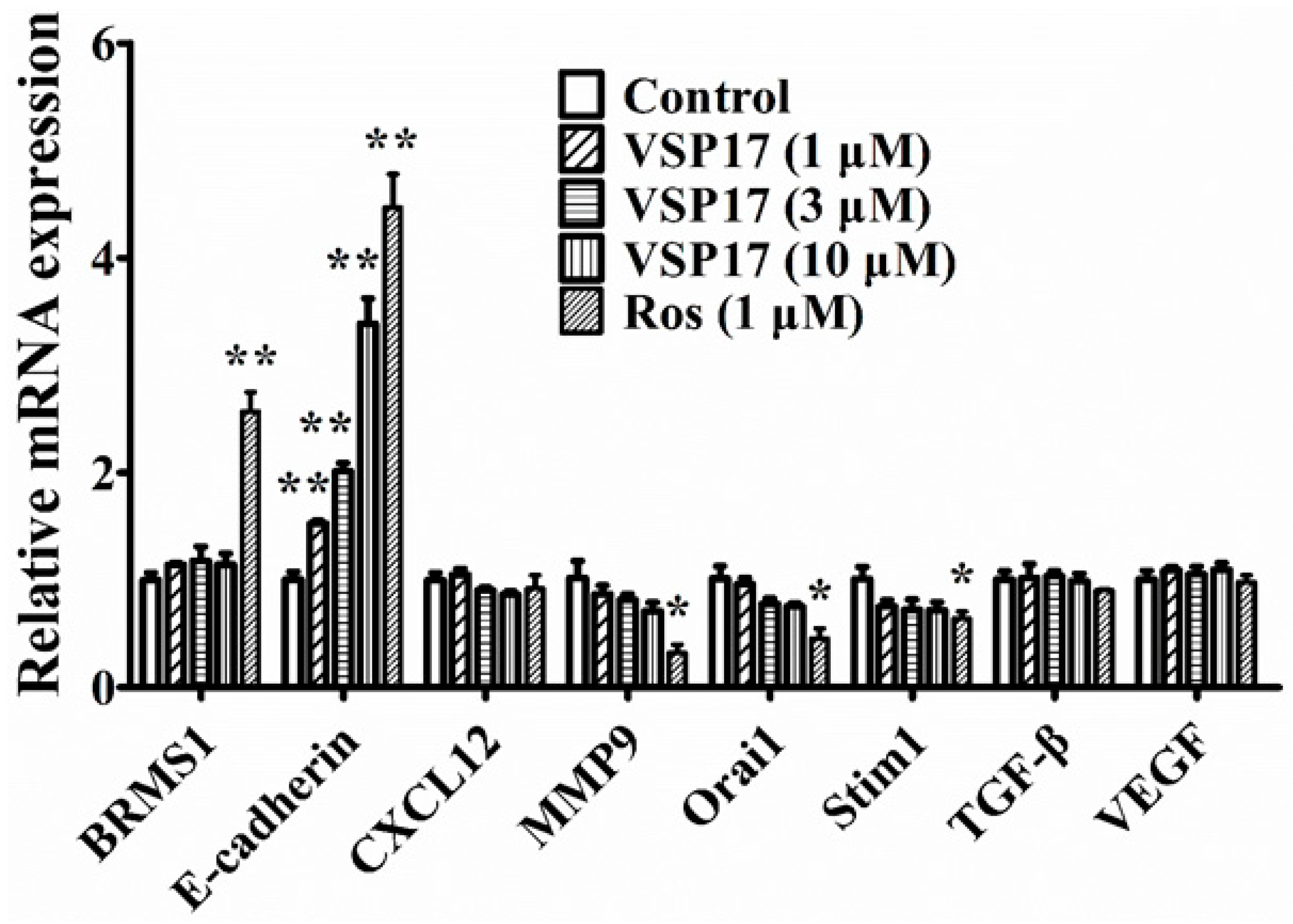
2.4. The Key Role That E-Cadherin Plays in the Anti-Migration and Anti-Invasion Effect of VSP-17
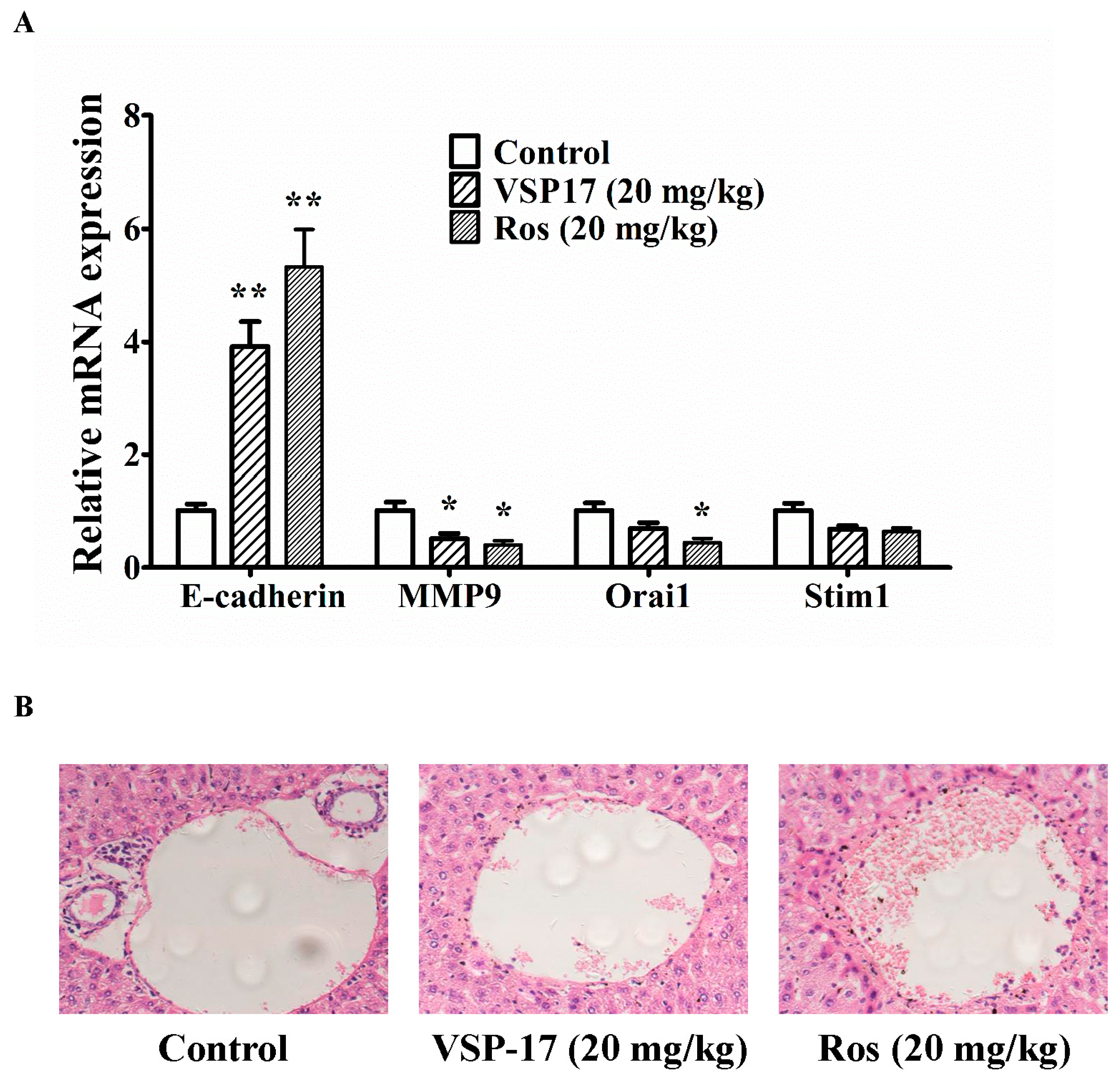
2.5. The Effect of VSP-17 on the Metastasis Markers and Liver Metastasis in MDA-MB-231 Xenograft Model
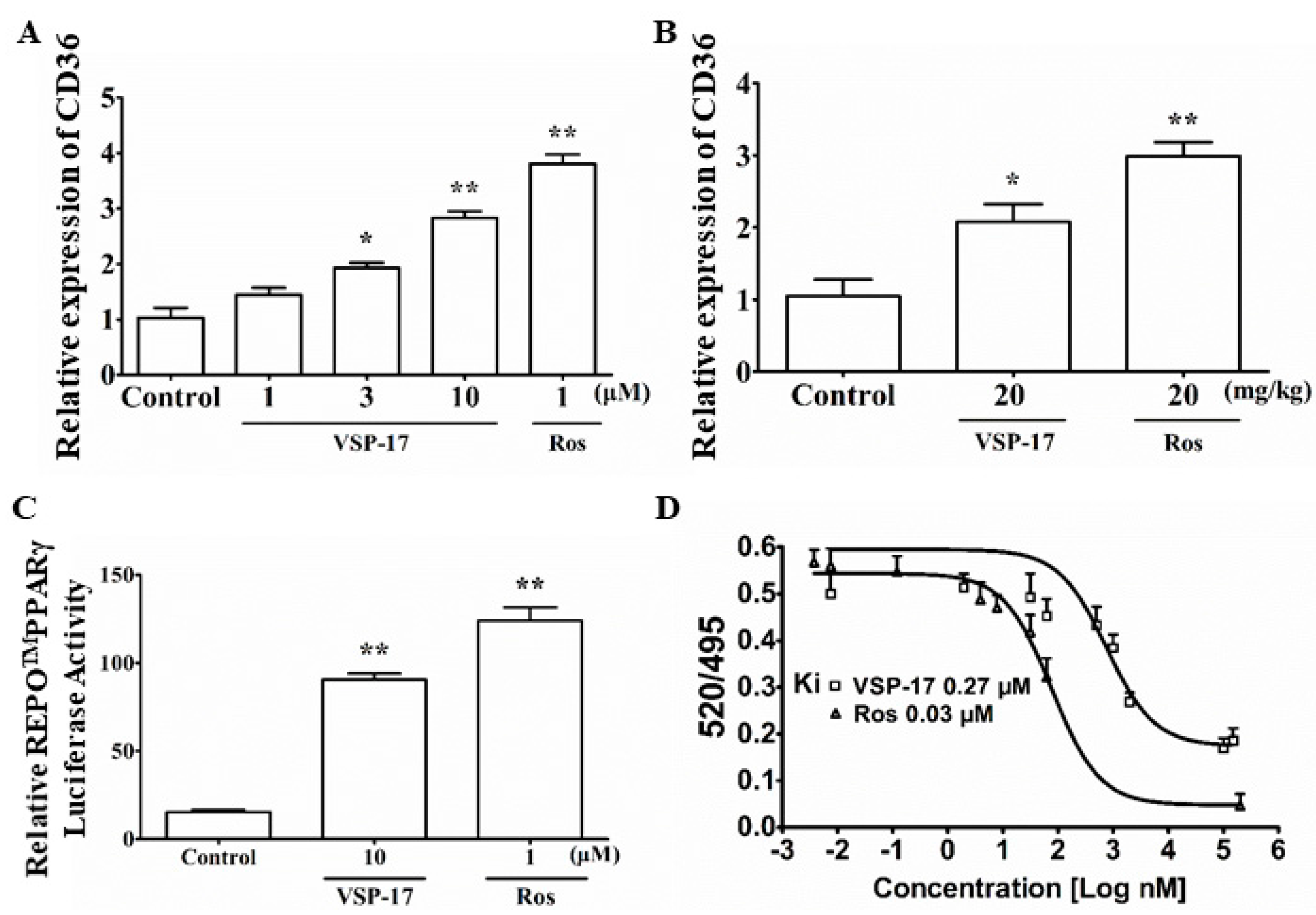
2.6. VSP-17 Could Activate PPARγ
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Cell Migration Assay
4.4. Cell Invasion Assay
4.5. Quantitative Real-Time Polymerase Chain Reaction (Q-PCR)
4.6. Luciferase Reporter Assay
4.7. PPARγ Competitive Binding Assay
4.8. Animals and Treatment
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bonotto, M.; Gerratana, L.; Poletto, E.; Driol, P.; Giangreco, M.; Russo, S.; Minisini, A.M.; Andreetta, C.; Mansutti, M.; Pisa, F.E.; et al. Measures of outcome in metastatic breast cancer: Insights from a real-world scenario. Oncologist 2014, 19, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Gregório, A.C.; Lacerda, M.; Figueiredo, P.; Simões, S.; Dias, S.; Moreira, J.N. Therapeutic Implications of the Molecular and Immune Landscape of Triple-Negative Breast Cancer. Pathol. Oncol. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zardavas, D.; Baselga, J.; Piccart, M. Emerging targeted agents in metastatic breast cancer. Nat. Rev. Clin. Oncol. 2013, 10, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.N.; Schwab, R.B.; Martinez, M.E. Reproductive risk factors and breast cancer subtypes: A review of the literature. Breast Cancer Res. Treat. 2014, 144, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gerratana, L.; Fanotto, V.; Bonotto, M.; Bolzonello, S.; Minisini, A.M.; Fasola, G.; Puglisi, F. Pattern of metastasis and outcome in patients with breast cancer. Clin. Exp. Metastasis 2015, 32, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.; Fritsche, H.; Mennel, R.; Norton, L.; Ravdin, P.; Taube, S.; Somerfield, M.R.; Hayes, D.F.; Bast, R.C., Jr. American Society of Clinical Oncology. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J. Clin. Oncol. 2007, 25, 5287–5312. [Google Scholar] [CrossRef] [PubMed]
- Jamdade, V.S.; Sethi, N.; Mundhe, N.A.; Kumar, P.; Lahkar, M.; Sinha, N. Therapeutic targets of triple-negative breast cancer: A review. Br. J. Pharmacol. 2015, 172, 4228–4237. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, S.; Glück, S. Molecularly targeted therapies for metastatic triple-negative breast cancer. Breast Cancer Res. Treat. 2013, 138, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Ondrey, F. Peroxisome proliferator-activated receptor gamma pathway targeting in carcinogenesis: Implications for chemoprevention. Clin. Cancer. Res. 2009, 15, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Yee, L.D.; Williams, N.; Wen, P.; Young, D.C.; Lester, J.; Johnson, M.V.; Farrar, W.B.; Walker, M.J.; Povoski, S.P.; Suster, S.; et al. Pilot study of Rosiglitazone therapy in women with breast cancer: Effects of short-term therapy on tumor tissue and serum markers. Clin. Cancer Res. 2007, 13, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zang, C.; Fenner, M.H.; Possinger, K.; Elstner, E. PPARgamma ligands and ATRA inhibit the invasion of human breast cancer cells in vitro. Breast Cancer Res. Treat. 2003, 79, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Bojková, B.; Garajová, M.; Kajo, K.; Péc, M.; Kubatka, P.; Kassayová, M.; Kisková, T.; Orendás, P.; Ahlersová, E.; Ahlers, I. Pioglitazone in chemically induced mammary carcinogenesis in rats. Eur. J. Cancer Prev. 2010, 19, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Colmers, I.N.; Bowker, S.L.; Johnson, J.A. Thiazolidinedione use and cancer incidence in type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2012, 38, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Fogo, A.B. Potential for peroxisome proliferator-activated receptor-gamma agonists in progression: Beyond metabolism. Curr. Opin. Nephrol. Hypertens. 2008, 17, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, A.; Genua, M.; Malaguarnera, R. PPAR-γ agonists and their effects on IGF-I receptor signaling: Implications for cancer. PPAR Res. 2009, 2009, 830501. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, A.; Lewis, J.D.; Quesenberry, C.P., Jr.; Peng, T.; Strom, B.L.; Van Den Eeden, S.K.; Ehrlich, S.F.; Habel, L.A. Cohort study of pioglitazone and cancer incidence in patients with diabetes. Diabetes Care. 2011, 34, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Demetri, G.D.; Mueller, E.; Sarraf, P.; Spiegelman, B.M.; Winer, E.P. Use of the peroxisome proliferator-activated receptor (PPAR) gamma ligand troglitazone as treatment for refractory breast cancer: A phase II study. Breast Cancer Res. Treat. 2003, 79, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Thiazolidinediones and liver toxicity. Diabetes Metab. 2001, 27, 305–313. [Google Scholar] [PubMed]
- Ravinuthala, R.S.; Nori, U. Rosiglitazone toxicity. Ann. Intern. Med. 2000, 133, 658. [Google Scholar] [CrossRef]
- Choi, J.H.; Banks, A.S.; Kamenecka, T.M.; Busby, S.A.; Chalmers, M.J.; Kumar, N.; Kuruvilla, D.S.; Shin, Y.; He, Y.; Bruning, J.B.; et al. Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature 2011, 477, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.W.; Liu, Y.; Siefert, S.A.; Moskaluk, C.A.; Petroni, G.R.; Jones, D.R. Breast cancer metastasis suppressor 1 (BRMS1) suppresses metastasis and correlates with improved patient survival in non-small cell lung cancer. Cancer Lett. 2009, 276, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Mareel, M.; Boterberg, T.; Noë, V.; Van Hoorde, L.; Vermeulen, S.; Bruyneel, E.; Bracke, M. E-cadherin/catenin/cytoskeleton complex: A regulator of cancer invasion. J. Cell. Physiol. 1997, 173, 271–274. [Google Scholar] [CrossRef]
- Roberti, M.P.; Arriaga, J.M.; Bianchini, M.; Quintá, H.R.; Bravo, A.I.; Levy, E.M.; Mordoh, J.; Barrio, M.M. Protein expression changes during human triple negative breast cancer cell line progression to lymph node metastasis in a xenografted model in nude mice. Cancer Biol. Ther. 2012, 13, 1123–1140. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- Mehner, C.; Hockla, A.; Miller, E.; Ran, S.; Radisky, D.C.; Radisky, E.S. Tumor cell-produced matrix metalloproteinase 9 (MMP9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget 2014, 5, 2736–2749. [Google Scholar] [CrossRef] [PubMed]
- Motiani, R.K.; Hyzinski-García, M.C.; Zhang, X.; Henkel, M.M.; Abdullaev, I.F.; Kuo, Y.H.; Matrougui, K.; Mongin, A.A.; Trebak, M. STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflugers Arch. 2013, 465, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Sanders, A.J.; Katoh, M.; Ungefroren, H.; Gieseler, F.; Prince, M.; Thompson, S.K.; Zollo, M.; Spano, D.; Dhawan, P.; et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin. Cancer Biol. 2015, 35, S244–S275. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Sun, H.; Deng, C.X. Potential therapeutic targets of triple-negative breast cancer based on its intrinsic subtype. Oncotarget 2017, 8, 73329–73344. [Google Scholar] [CrossRef] [PubMed]
- Bulfoni, M.; Gerratana, L.; Del Ben, F.; Marzinotto, S.; Sorrentino, M.; Turetta, M.; Scoles, G.; Toffoletto, B.; Isola, M.; Beltrami, C.A.; et al. In patients with metastatic breast cancer the identification of circulating tumor cells in epithelial-to-mesenchymal transition is associated with a poor prognosis. Breast Cancer Res. 2016, 18, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.I.; Liu, Z.; Chen, Y.; Xu, K.; Dong, J. PPARγ activation reduces ischemia/reperfusion-induced metastasis in a murine model of hepatocellular carcinoma. Exp. Ther. Med. 2016, 11, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.W.; Lin, D.Q.; Cao, L.Q. Peroxisome proliferator-activated receptor-γ inhibits pancreatic cancer cell invasion and metastasis via regulating MMP-2 expression through PTEN. Mol. Med. Rep. 2015, 12, 6255–6260. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, S.; Li, B.; Sun, A.; Zou, Y.; Ge, J. A protective role of ciglitazone in ox-LDL-induced rat microvascular endothelial cells via modulating PPARγ-dependent AMPK/eNOS pathway. J. Cell. Mol. Med. 2015, 19, 92–102. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: The compound, VSP-17, is available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhu, M.; Yuan, B.; Zhang, K.; Zhong, M.; Yi, W.; Xu, X.; Duan, X. VSP-17, a New PPARγ Agonist, Suppresses the Metastasis of Triple-Negative Breast Cancer via Upregulating the Expression of E-Cadherin. Molecules 2018, 23, 121. https://doi.org/10.3390/molecules23010121
Wang Y, Zhu M, Yuan B, Zhang K, Zhong M, Yi W, Xu X, Duan X. VSP-17, a New PPARγ Agonist, Suppresses the Metastasis of Triple-Negative Breast Cancer via Upregulating the Expression of E-Cadherin. Molecules. 2018; 23(1):121. https://doi.org/10.3390/molecules23010121
Chicago/Turabian StyleWang, Yuhui, Menglin Zhu, Bo Yuan, Kefeng Zhang, Mingli Zhong, Wei Yi, Xiaotian Xu, and Xiaoqun Duan. 2018. "VSP-17, a New PPARγ Agonist, Suppresses the Metastasis of Triple-Negative Breast Cancer via Upregulating the Expression of E-Cadherin" Molecules 23, no. 1: 121. https://doi.org/10.3390/molecules23010121



