Fermentation-Guided Natural Products Isolation of a Grape Berry Triacylglyceride that Enhances Ethyl Ester Production
Abstract
:1. Introduction
2. Results
2.1. The PSDVB Loading Process Fractionates Grape Extracts by Polarity
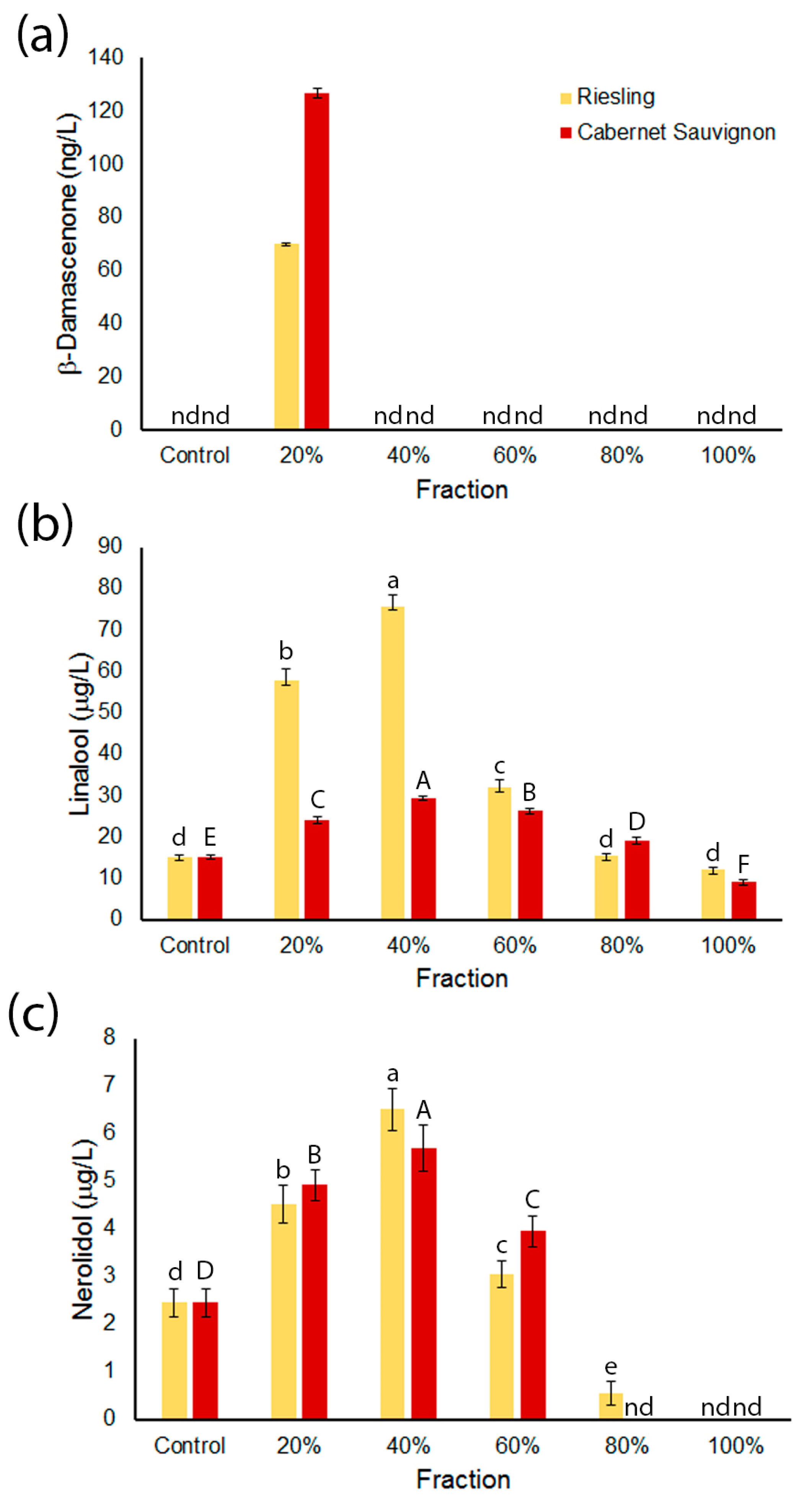
2.2. β-Damascenone and Terpenoids are More Abundant in Wines Made from More Polar Grape Fractions
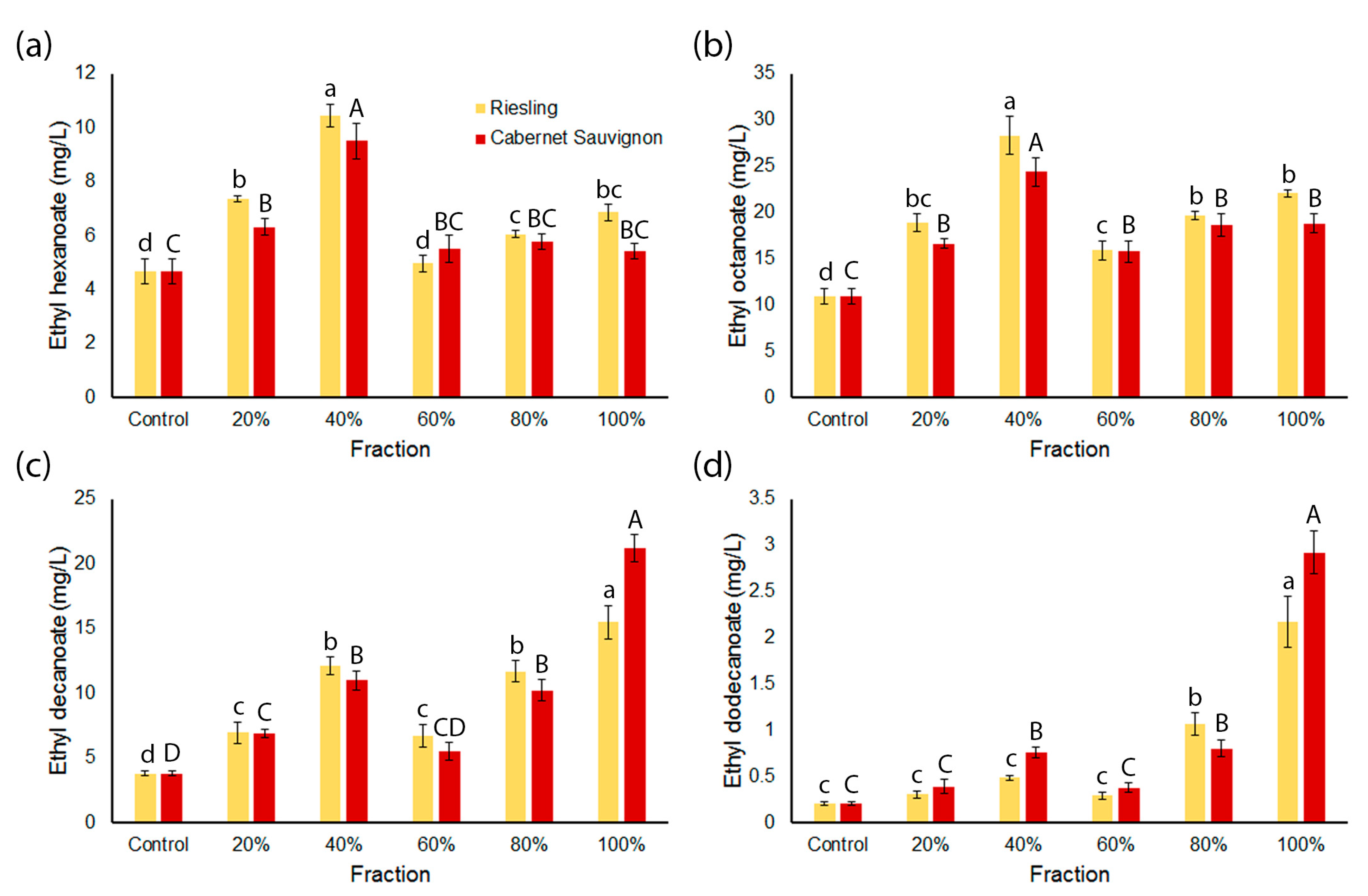
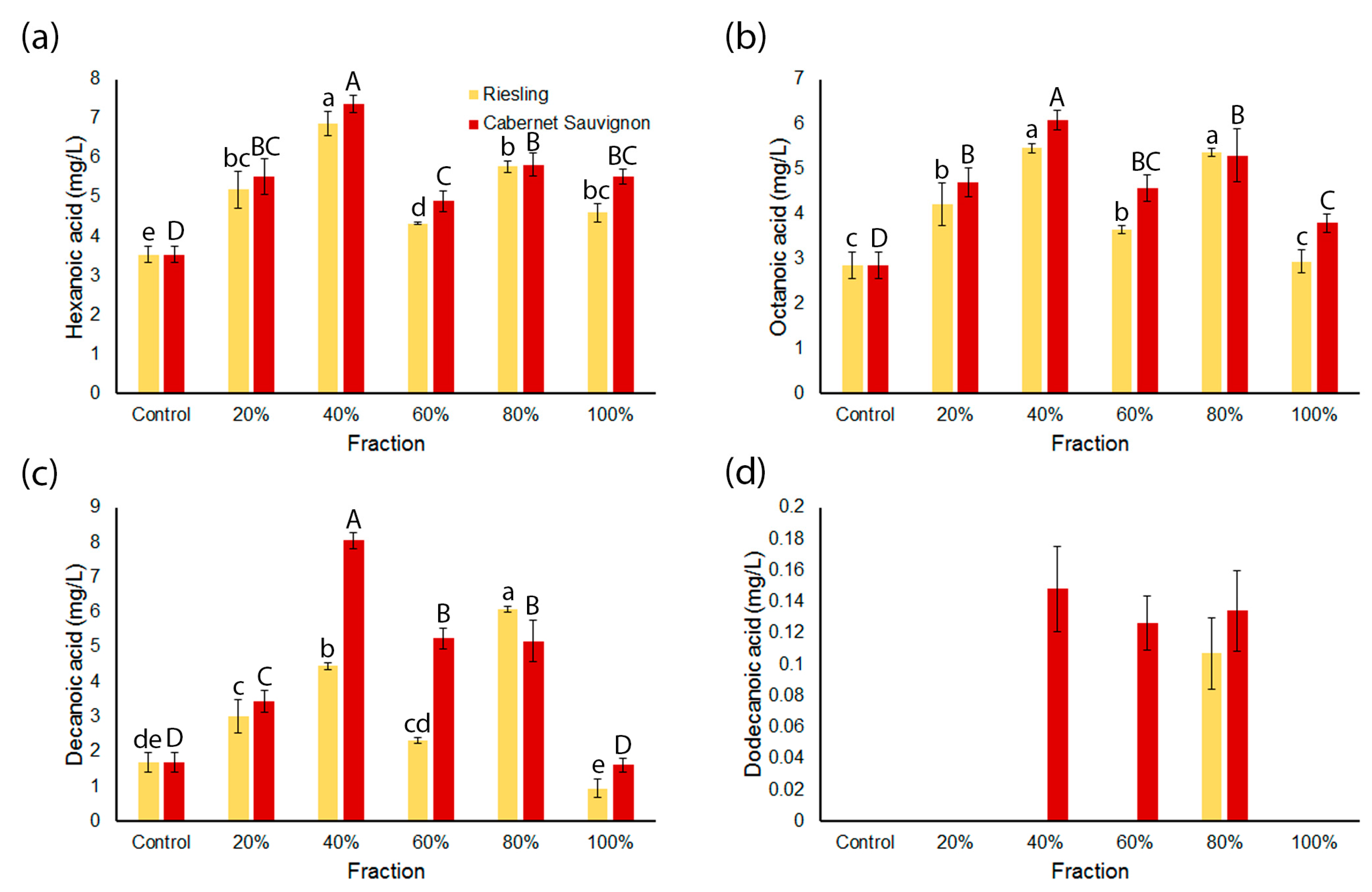
2.3. Concentrations of Medium Chain Ethyl Esters and Medium Chain Fatty Acids in the Wines Are Influenced by Spiked Grape Fractions
2.4. Second Generation Fractionation of the CS100 Fractions on Diol Columns Results in the Identification of Triacylglycerides in a Fraction that Enhances MCEE Production during Fermentation
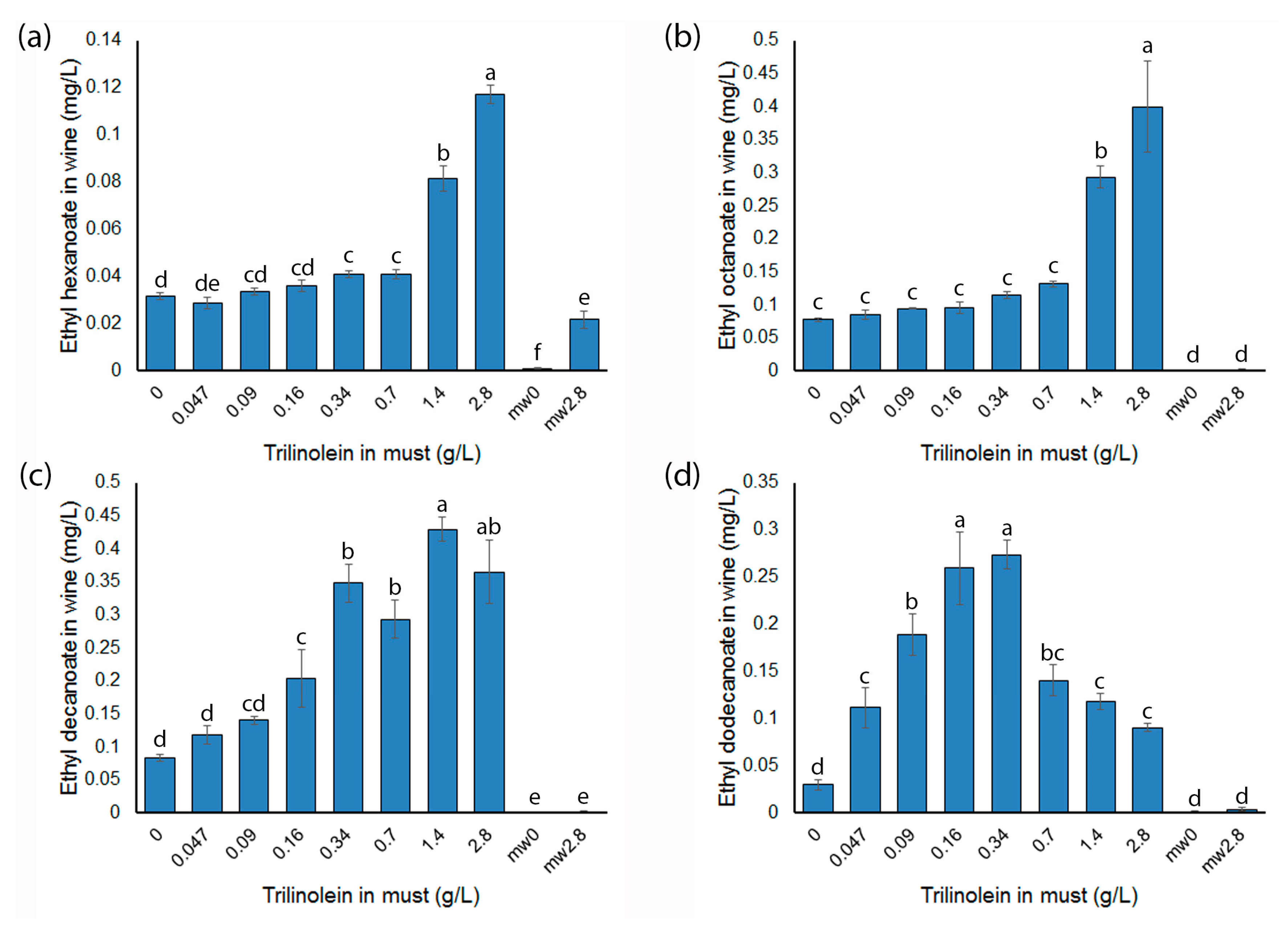
2.5. Trilinolein Additions to Model Musts Influence MCEE in the Resulting Wines
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Grape Materials
4.3. Grape Fractionation
4.3.1. Extraction of Grape Samples
4.3.2. PSDVB Loading and Fractionation of Grape Extracts
4.3.3. Diol Loading and Second Generation Fractionation of Grape Extract Fractions
4.3.4. NMR Spectroscopy
4.3.5. High-Resolution Mass Spectrometry
4.3.6. LC-MS-MS
4.4. Model Must Fermentations
4.4.1. Model Grape Juice Must Preparation
4.4.2. Yeast
4.4.3. Fermentation Conditions: Screen of First Round Fractionation
4.4.4. Fermentation Conditions: Screen of Second Round Fractionation
4.5. Analysis of Volatile Compounds
4.5.1. Volatile Analysis of Wines
4.5.2. Volatile Analysis of Fractionated Grape Extract Samples
4.5.3. Headspace Volatile Analysis of Micro-Fermentation Wines
4.5.4. Data Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Determination of Fraction B1 Composition
References
- Dunlevy, J.D.; Kalua, C.M.; Keyzers, R.A.; Boss, P.K. The production of flavour and aroma compounds in grape berries. In Grapevine Molecular Physiology and Biotechnology, 2nd ed.; Roubelakis-Angelakis, K.A., Ed.; Springer Science+Business Media: Berlin, Germnay, 2009; pp. 293–340. [Google Scholar]
- Ebeler, S.E.; Thorngate, J.H. Wine chemistry and flavor: Looking into the crystal glass. J. Agric. Food Chem. 2009, 57, 8098–8108. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. Am. J. Enol. Vitic. 2014, 65, 1–24. [Google Scholar] [CrossRef]
- Ganss, S.; Kirsch, F.; Winterhalter, P.; Fischer, U.; Schmarr, H.G. Aroma changes due to second fermentation and glycosylated precursors in Chardonnay and Riesling sparkling wines. J. Agric. Food Chem. 2011, 59, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- Loscos, N.; Hernandez-Orte, P.; Cacho, J.; Ferreira, V. Release and formation of varietal aroma compounds during alcoholic fermentation from nonfloral grape odorless flavor precursors fractions. J. Agric. Food Chem. 2007, 55, 6674–6684. [Google Scholar] [CrossRef] [PubMed]
- Loscos, N.; Hernandez-Orte, P.; Cacho, J.; Ferreira, V. Evolution of the aroma composition of wines supplemented with grape flavour precursors from different varietals during accelerated wine ageing. Food Chem. 2010, 120, 205–216. [Google Scholar] [CrossRef]
- Boss, P.K.; Pearce, A.D.; Zhao, Y.J.; Nicholson, E.L.; Dennis, E.G.; Jeffery, D.W. Potential grape-derived contributions to volatile ester concentrations in wine. Molecules 2015, 20, 7845–7873. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.G.; Keyzers, R.A.; Kalua, C.M.; Maffei, S.M.; Nicholson, E.L.; Boss, P.K. Grape contribution to wine aroma: Production of hexyl acetate, octyl acetate, and benzyl acetate during yeast fermentation is dependent upon precursors in the must. J. Agric. Food Chem. 2012, 60, 2638–2646. [Google Scholar] [CrossRef] [PubMed]
- Keyzers, R.A.; Boss, P.K. Changes in the volatile compound production of fermentations made from musts with increasing grape content. J. Agric. Food Chem. 2010, 58, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Bonnlander, B.; Baderschneider, B.; Messerer, M.; Winterhalter, P. Isolation of two novel terpenoid glucose esters from Riesling wine. J. Agric. Food Chem. 1998, 46, 1474–1478. [Google Scholar] [CrossRef]
- Sanchez-Palomo, E.; Alonso-Villegas, R.; Vinas, M.A.G. Characterisation of free and glycosidically bound aroma compounds of La Mancha Verdejo white wines. Food Chem. 2015, 173, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.; Strauss, C.R.; Wilson, B.; Massy-Westropp, R.A. Use of C18 reversed-phase liquid chromatography for the isolation of monoterpene glycosides and nor-isoprenoid precursors from grape juice and wines. J. Chromatogr. A 1982, 235, 471–480. [Google Scholar] [CrossRef]
- Mateo, J.J.; Jimenez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Schneider, R.; Razungles, A.; Augier, C.; Baumes, R. Monoterpenic and norisoprenoidic glycoconjugates of Vitis vinifera L. cv. Melon B. as precursors of odorants in Muscadet wines. J. Chromatogr. A 2001, 936, 145–157. [Google Scholar] [CrossRef]
- Stahl-Biskup, E.; Intert, F.; Holthuijzen, J.; Stengele, M.; Schulz, G. Glycosidically bound volatiles: A review 1986–1991. Flavour Fragr. J. 1993, 8, 61–80. [Google Scholar] [CrossRef]
- Cullere, L.; Aznar, M.; Cacho, J.; Ferreira, V. Fast fractionation of complex organic extracts by normal-phase chromatography on a solid-phase extraction polymeric sorbent: Optimization of a method to fractionate wine flavor extracts. J. Chromatogr. A 2003, 1017, 17–26. [Google Scholar] [CrossRef]
- Pineau, B.; Barbe, J.C.; Van Leeuwen, C.; Dubourdieu, D. Examples of perceptive interactions involved in specific “red-” and “black-berry” aromas in red wines. J. Agric. Food Chem. 2009, 57, 3702–3708. [Google Scholar] [CrossRef] [PubMed]
- Marston, A.; Hostettmann, K. Modern separation methods. Nat. Prod. Rep. 1991, 8, 391–413. [Google Scholar] [CrossRef]
- Sticher, O. Natural product isolation. Nat. Prod. Rep. 2008, 25, 517–554. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.J.; Field, J.J.; Atkinson, P.H.; Northcote, P.T.; Miller, J.H. From Marine Organism to Potential Drug: Using Innovative Techniques to Identify and Characterize Novel Compounds—A Bottom-Up Approach. In Bioactive Natural Products: Chemistry and Biology; Brahmachari, G., Ed.; Wiley: Weinheim, Germany, 2015; pp. 443–472. [Google Scholar]
- van Wyk, A.W.; Gray, C.A.; Keyzers, R.A.; Rivett, D.E.; Caira, M.R.; Nader, B.S.; Davis, G.E.; Werk, T.L.; Davies-Coleman, M.T. Transformations of hispanolone. Novel Michael adducts with in planta activity against rice blast. Tetrahedron 2005, 61, 8493–8498. [Google Scholar] [CrossRef]
- Zalacain, A.; Marín, J.; Alonso, G.; Salinas, M. Analysis of wine primary aroma compounds by stir bar sorptive extraction. Talanta 2007, 71, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Maicas, S.; Mateo, J.J. Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: A review. Appl. Microbiol. Biotechnol. 2005, 67, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.; Strauss, C.R.; Wilson, B.; Massy-Westropp, R.A. Novel monoterpene disaccharide glycosides of Vitis vinifera grapes and wines. Phytochemistry 1982, 21, 2013–2020. [Google Scholar] [CrossRef]
- Carrau, F.M.; Medina, K.; Boido, E.; Farina, L.; Gaggero, C.; Dellacassa, E.; Versini, G.; Henschke, P.A. De novo synthesis of monoterpenes by Saccharomyces cerevisiae wine yeasts. FEMS Microbiol. Lett. 2005, 243, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Saerens, S.M.G.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Saerens, S.M.G.; Verstrepen, K.J.; Van Laere, S.D.M.; Voet, A.R.D.; Van Dijck, P.; Delvaux, F.R.; Thevelein, J.M. The Saccharomyces cerevisiae EHT1 and EEB1 genes encode novel enzymes with medium-chain fatty acid ethyl ester synthesis and hydrolysis capacity. J. Biol. Chem. 2006, 281, 4446–4456. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, K.; Yasui, Y.; Hirose, S.; Ohnishi, M. Fatty acids in must prepared from 11 grapes grown in Japan: Comparison with wine and effect on fatty acid ethyl ester formation. Lipids 2005, 40, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Edwards, P.J.; Jouanneau, S.; Kilmartin, P.A.; Gardner, R.C.; Villas-Boas, S.G. Sauvignon blanc metabolomics: Grape juice metabolites affecting the development of varietal thiols and other aroma compounds in wines. Metabolomics 2014, 10, 556–573. [Google Scholar] [CrossRef]
- Varela, C.; Torrea, D.; Schmidt, S.; Ancin-Azpilicueta, C.; Henschke, P. Effect of oxygen and lipid supplementation on the volatile composition of chemically defined medium and Chardonnay wine fermented with Saccharomyces cerevisiae. Food Chem. 2012, 135, 2863–2871. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Shi, Y.; Jiang, R.; Yang, Q.; Wang, Y.; Liu, P.; Duan, C.-Q.; Yan, G. Effects of adding unsaturated fatty acids on fatty acid composition of saccharomyces cerevisiae and major volatile compounds in wine. S. Afr. J. Enol. Vitic. 2015, 36, 285–295. [Google Scholar] [CrossRef]
- Tumanov, S.; Zubenko, Y.; Greven, M.; Greenwood, D.R.; Shmanai, V.; Villas-Boas, S.G. Comprehensive lipidome profiling of Sauvignon blanc grape juice. Food Chem. 2015, 180, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Rosi, I.; Bertuccioli, M. Iinfluences of lipid addition on fatty-acid composition of Saccharomyces cerevisiae and aroma characteristics of experimental wines. J. Inst. Brew. 1992, 98, 305–314. [Google Scholar] [CrossRef]
- Nisbet, M.A.; Tobias, H.J.; Brenna, J.T.; Sacks, G.L.; Mansfield, A.K. Quantifying the contribution of grape hexoses to wine volatiles by high-precision [U13C]-glucose tracer studies. J. Agric. Food Chem. 2014, 62, 6820–6827. [Google Scholar] [CrossRef] [PubMed]
- Dufour, J.; Malcorps, P.; Silcock, P. Control of ester synthesis during brewery fermentation. In Brewing Yeast Fermentation Performance; Smart, K., Ed.; Blackwell Publishing: Oxford, UK, 2008; pp. 213–233. [Google Scholar]
- Weete, J. Fungal Lipid Biochemistry: Distribution and Metabolism; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Redón, M.; Guillamón, J.M.; Mas, A.; Rozès, N. Effect of lipid supplementation upon Saccharomyces cerevisiae lipid composition and fermentation performance at low temperature. Eur. Food Res. Technol. 2009, 228, 833–840. [Google Scholar] [CrossRef]
- Bird, S.S.; Marur, V.R.; Stavrovskaya, I.G.; Kristal, B.S. Qualitative characterization of the rat liver mitochondrial lipidome using LC-MS profiling and high energy collisional dissociation (HCD) all ion fragmentation. Metabolomics 2013, 9, 67–83. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blackford, C.L.; Dennis, E.G.; Keyzers, R.A.; Schueuermann, C.; Trengove, R.D.; Boss, P.K. Fermentation-Guided Natural Products Isolation of a Grape Berry Triacylglyceride that Enhances Ethyl Ester Production. Molecules 2018, 23, 152. https://doi.org/10.3390/molecules23010152
Blackford CL, Dennis EG, Keyzers RA, Schueuermann C, Trengove RD, Boss PK. Fermentation-Guided Natural Products Isolation of a Grape Berry Triacylglyceride that Enhances Ethyl Ester Production. Molecules. 2018; 23(1):152. https://doi.org/10.3390/molecules23010152
Chicago/Turabian StyleBlackford, Christopher L., Eric G. Dennis, Robert A. Keyzers, Claudia Schueuermann, Robert D. Trengove, and Paul K. Boss. 2018. "Fermentation-Guided Natural Products Isolation of a Grape Berry Triacylglyceride that Enhances Ethyl Ester Production" Molecules 23, no. 1: 152. https://doi.org/10.3390/molecules23010152





