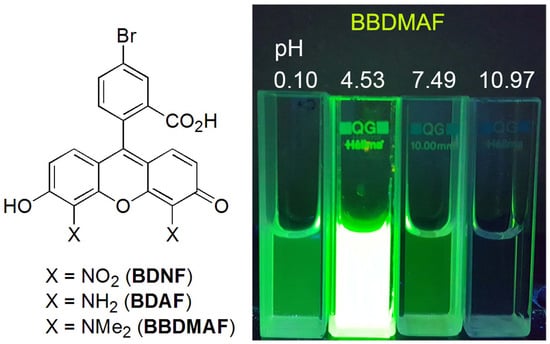
5-Bromo-4′,5′-bis(dimethylamino)fluorescein: Synthesis and Photophysical Studies
Abstract
:1. Introduction
2. Results and Discussion
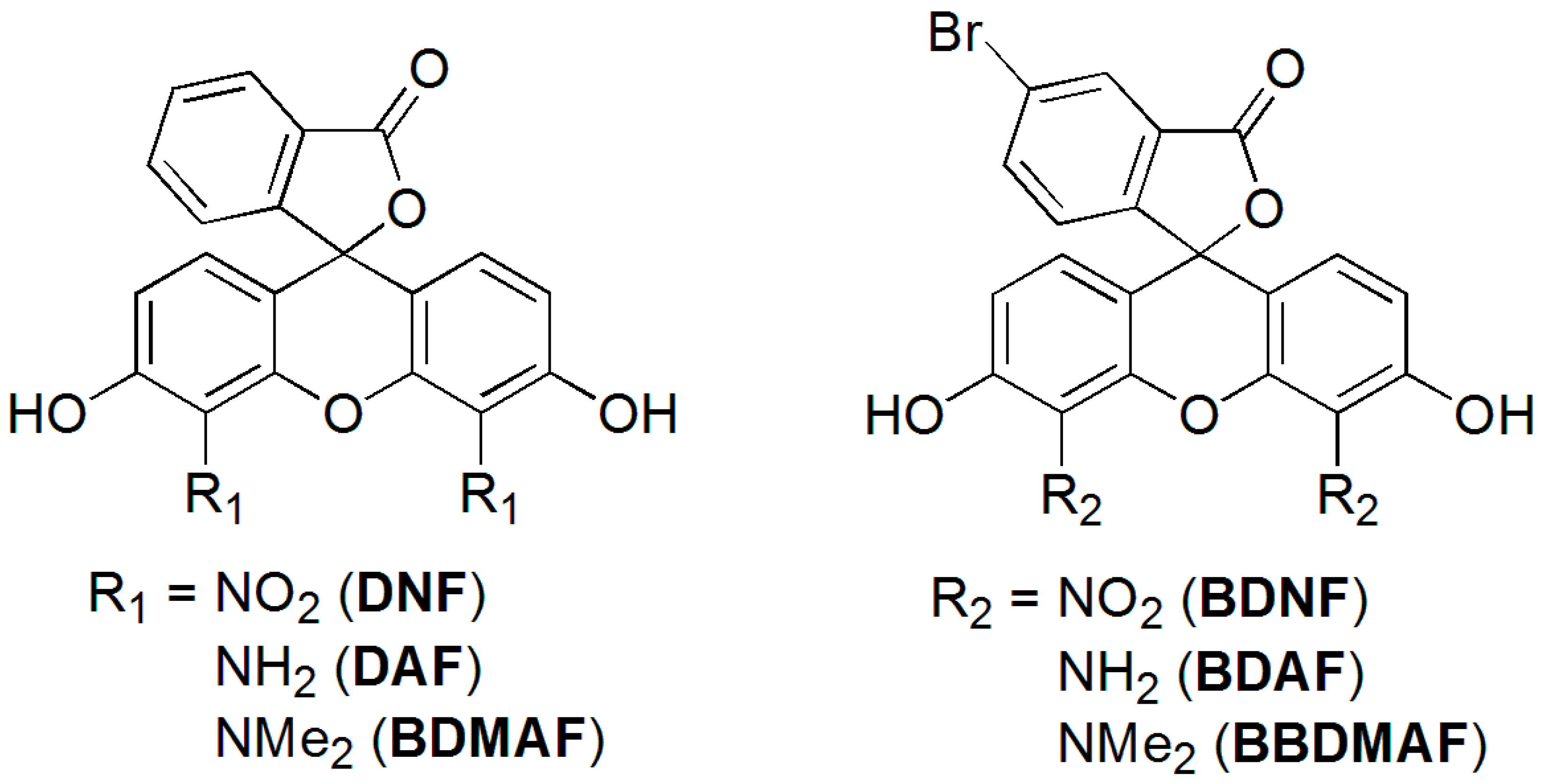
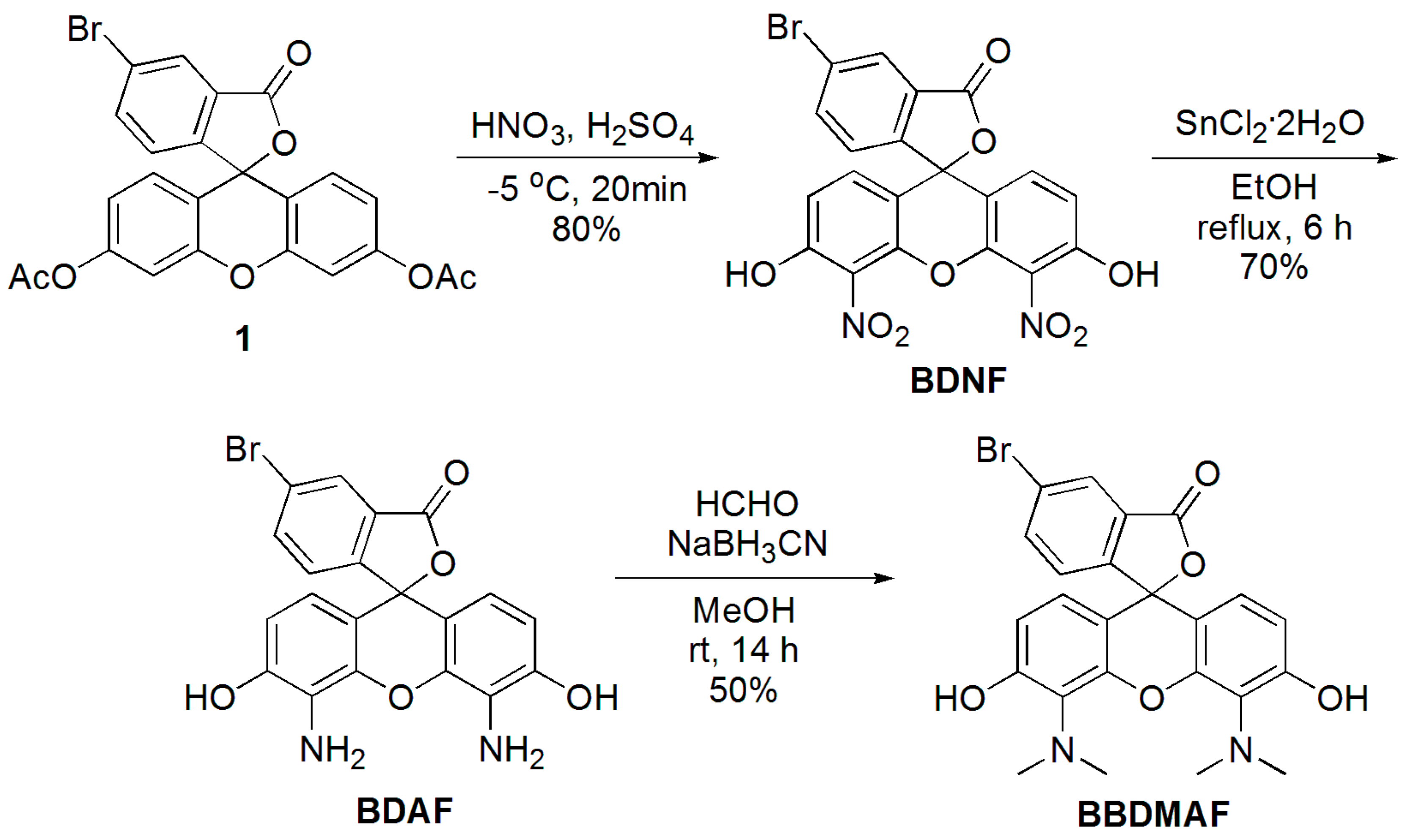
2.1. Synthesis of BDNF, BDAF, and BBDMAF
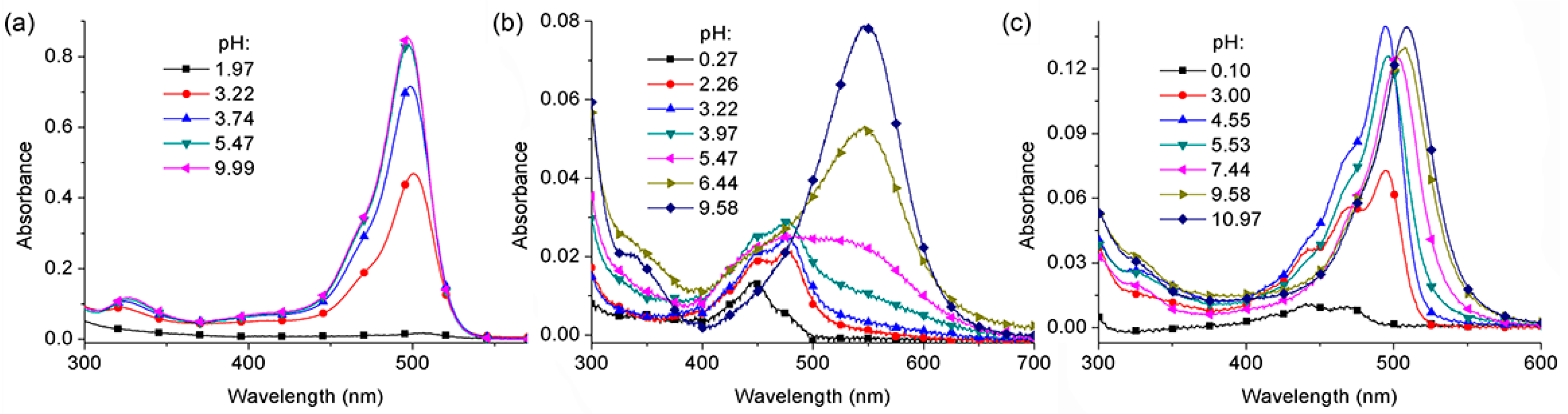
2.2. pKa Determination of BDNF, BDAF, and BBDMAF
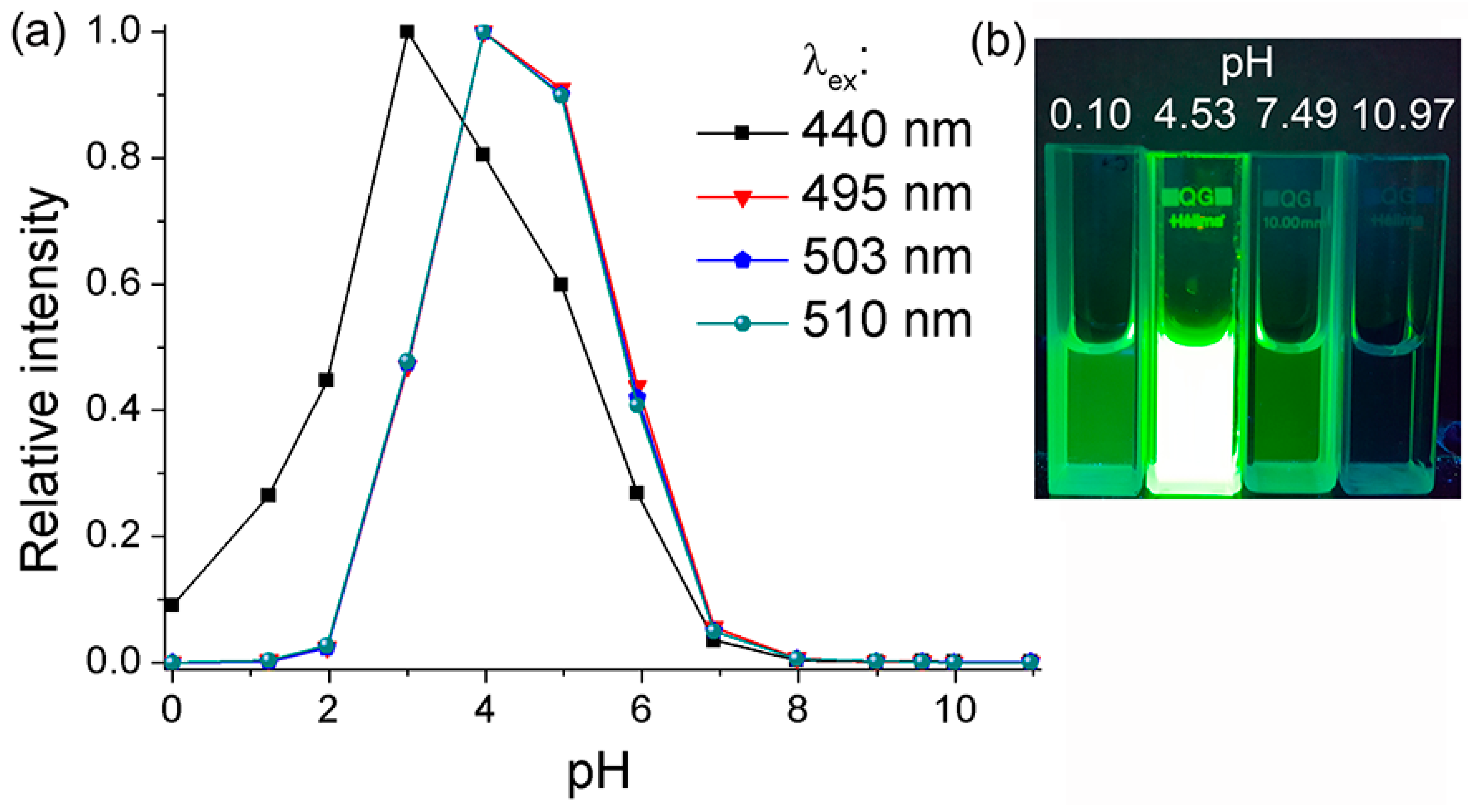
2.3. Fluorescence of BDNF, BDAF, and BBDMAF
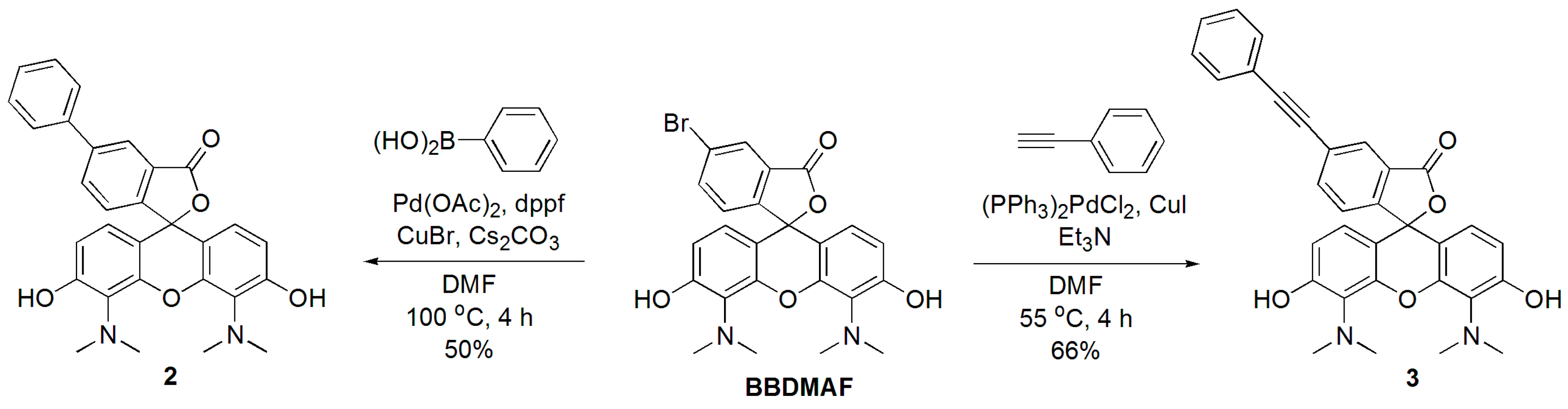
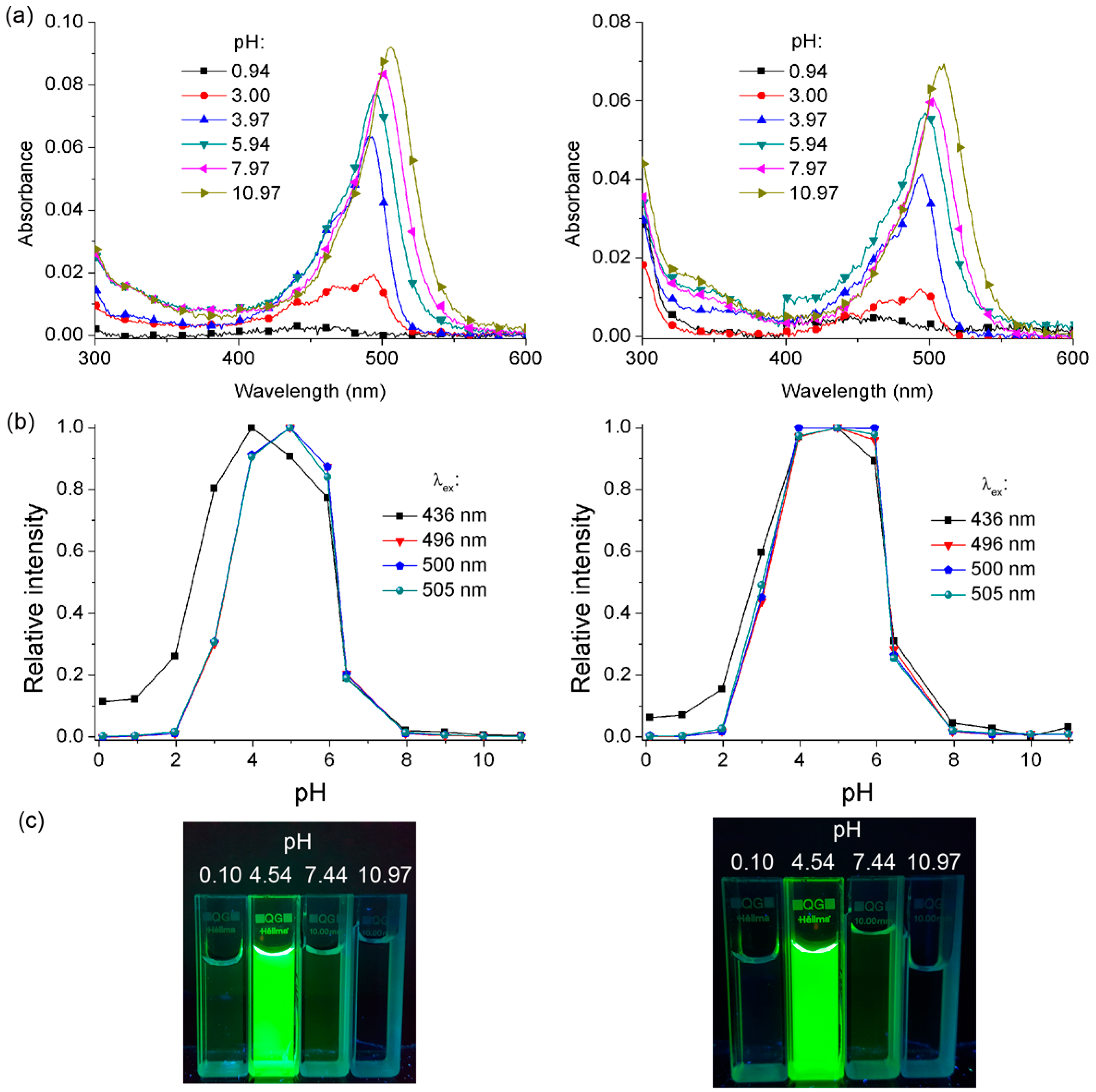
2.4. Suzuki and Sonogashira Reactions of BBDMAF
3. Materials and Methods
3.1. General
3.2. Synthetic Procedures
3.2.1. 5-Bromo-4′,5′-dinitrofluorescein (BDNF)
3.2.2. 5-Bromo-4′,5′-diaminofluorescein (BDAF)
3.2.3. 5-Bromo-4′,5′-bis(dimethylamino)fluorescein (BBDMAF)
3.2.4. 5-Phenyl-4′,5′-bis(dimethylamino)fluorescein (2)
3.2.5. 5-Phenylethynyl-4′,5′-bis(dimethylamino)fluorescein (3)
3.3. Buffer Solutions
3.4. UV–Vis and Fluorescence Spectroscopy
3.5. Fluorescence Lifetime Measurement
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duan, Y.S.; Liu, M.; Sun, W.; Wang, M.; Liu, S.Z.; Li, Q.X. Recent progress on synthesis of fluorescein probes. Mini-Rev. Org. Chem. 2009, 6, 35–43. [Google Scholar] [CrossRef]
- Han, J.; Burgess, K. Fluorescent indicators for intracellular pH. Chem. Rev. 2010, 110, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, A.; Mizutani, A.; Okinaga, H.; Takano, K.; Yamada, S.; Yamada, S.M.; Nakaguchi, H.; Hoya, K.; Murakami, M.; Takeuchi, M.; et al. Molecular morphology of pituitary cells, from conventional immunohistochemistry to fluorescein imaging. Molecules 2011, 16, 3618–3635. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pradhan, T.; Wang, F.; Kim, J.S.; Yoon, J. Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives. Chem. Rev. 2012, 112, 1910–1956. [Google Scholar] [CrossRef] [PubMed]
- Robertson, T.A.; Bunel, F.; Roberts, M.S. Fluorescein derivatives in intravital fluorescence imaging. Cells 2013, 2, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhan, X.Q.; Bian, Q.N.; Zhang, X.J. Advances in modifying fluorescein and rhodamine fluorophores as fluorescent chemosensors. Chem. Commun. 2013, 49, 429–447. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, M.; Desai, S.; Pulsinelli, W. Dicarboxy-dichlorofluorescein: A new fluorescent probe for measuring acidic intracellular pH. Anal. Biochem. 1990, 187, 109–114. [Google Scholar] [CrossRef]
- Lin, H.J.; Szmacinski, H.; Lakowicz, J.R. Lifetime-based pH sensors: Indicators for acidic environments. Anal. Biochem. 1999, 269, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Shim, S.; Hwang, G.T. 4′,5′-Bis(dimethylamino)fluorescein exhibits pH-dependent emission behavior distinct from that of fluorescein. Asian J. Org. Chem. 2018, 7, 150–154. [Google Scholar] [CrossRef]
- Han, J.W.; Castro, J.C.; Burgess, K. Microwave-assisted functionalization of bromo-fluorescein and bromorhodamine derivatives. Tetrahedron Lett. 2003, 44, 9359–9362. [Google Scholar] [CrossRef]
- Han, J.; Loudet, A.; Barhoumi, R.; Burghardt, R.C.; Burgess, K. A ratiometric pH reporter for imaging protein-dye conjugates in living cells. J. Am. Chem. Soc. 2009, 131, 1642–1643. [Google Scholar] [CrossRef] [PubMed]
- Yesilgul, N.; Uyar, T.B.; Seven, O.; Akkaya, E.U. Singlet oxygen generation with chemical excitation of an erythrosine-luminol conjugate. ACS Omega 2017, 2, 1367–1371. [Google Scholar] [CrossRef]
- Jiao, G.-S.; Han, J.W.; Burgess, K. Syntheses of regioisomerically pure 5-or 6-halogenated fluoresceins. J. Org. Chem. 2003, 68, 8264–8267. [Google Scholar] [CrossRef] [PubMed]
- Mchedlov-Petrosyan, N.O.; Vodolazkaya, N.A.; Martynova, V.P.; Samoilov, D.V.; El’tsov, A.V. Protolytic properties of thiofluorescein and its derivatives. Russ. J. Gen. Chem. 2002, 72, 785–792. [Google Scholar] [CrossRef]
- Scarminio, I.; Kubista, M. Analysis of correlated spectral data. Anal. Chem. 1993, 65, 409–416. [Google Scholar] [CrossRef]
- Ghasemi, J.; Lotfi, S.; Safaeian, M.; Niazi, A.; Mazloum-Ardakani, M.; Noroozi, M. Spectrophotometric determination of acidity constants of alizarine red S in mixed aqueous-organic solvents. J. Chem. Eng. Data 2006, 51, 1530–1535. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Ghasemi, J.B.; Saaidpour, S.; Mohajeri, A. Spectrophotometric study of the effects of surfactants and ethanol on the acidity constants of fluorescein. Spectrochim. Acta A 2008, 71, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Swamy, K.M.K.; Yoon, J. Study on various fluorescein derivatives as pH sensors. Tetrahedron Lett. 2011, 52, 2340–2343. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O.; Cheipesh, T.A.; Vodolazkaya, N.A. Acid-base dissociation and tautomerism of two aminofluorescein dyes in solution. J. Mol. Liq. 2017, 225, 696–705. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O.; Cheipesh, T.A.; Shekhovtsov, S.V.; Redko, A.N.; Rybachenko, V.I.; Omelchenko, I.V.; Shishkin, O.V. Ionization and tautomerism of methyl fluorescein and related dyes. Spectrochim. Acta A 2015, 150, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.Z.; Paone, D.V.; Ginnetti, A.T.; Kurihara, H.; Dreher, S.D.; Weissman, S.A.; Stauffer, S.R.; Burgey, C.S. Copper-facilitated Suzuki reactions: Application to 2-heterocyclic boronates. Org. Lett. 2009, 11, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Son, Y.-S.; Lee, J.Y.; Kim, M.H.; Woo, S.K.; Lee, K.C.; Lee, Y.J.; Hwang, G.T. pH-sensitive fluorescent deoxyuridines labeled with 2-aminofluorene derivatives. Tetrahedron 2016, 72, 5595–5601. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC technical report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
Sample Availability: Samples of BDNF, BDAF, and BBDMAF are available from the authors. |
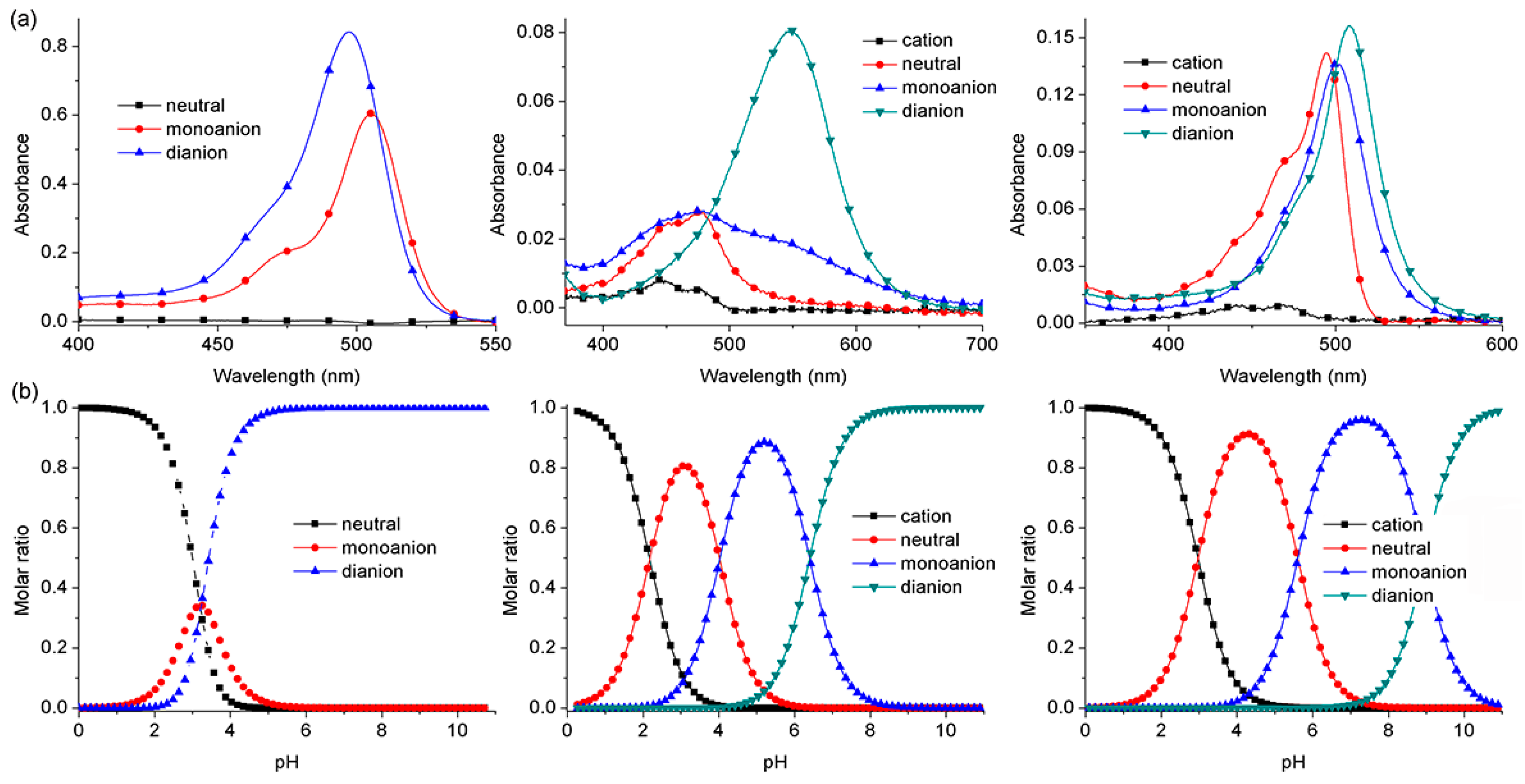

| Compound | pKa1 | pKa2 | pKa3 |
|---|---|---|---|
| DNF 2 | 5.36 | 5.00 | n.d. 3 |
| BDNF | 3.19 | 3.22 | n.d. 3 |
| DAF 2 | 2.68 | 4.27 | 6.40 |
| BDAF | 2.37 | 3.99 | 6.51 |
| BDMAF 2 | 3.37 | 5.83 | 8.68 |
| BBDMAF | 2.96 | 5.77 | 9.50 |
| 2 | 3.42 | 5.87 | 9.02 |
| 3 | 3.52 | 5.53 | 8.84 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.Y.; Lee, J.-Y.; Cho, C.-W.; Choi, W.; Lee, Y.; Shim, S.; Hwang, G.T. 5-Bromo-4′,5′-bis(dimethylamino)fluorescein: Synthesis and Photophysical Studies. Molecules 2018, 23, 219. https://doi.org/10.3390/molecules23010219
Hwang JY, Lee J-Y, Cho C-W, Choi W, Lee Y, Shim S, Hwang GT. 5-Bromo-4′,5′-bis(dimethylamino)fluorescein: Synthesis and Photophysical Studies. Molecules. 2018; 23(1):219. https://doi.org/10.3390/molecules23010219
Chicago/Turabian StyleHwang, Jun Yeon, Jung-Yean Lee, Chang-Woo Cho, Wonjun Choi, Yejin Lee, Sangdeok Shim, and Gil Tae Hwang. 2018. "5-Bromo-4′,5′-bis(dimethylamino)fluorescein: Synthesis and Photophysical Studies" Molecules 23, no. 1: 219. https://doi.org/10.3390/molecules23010219