Discrimination of Stereoisomers by Their Enantioselective Interactions with Chiral Cholesterol-Containing Membranes
Abstract
:1. Introduction
2. Experimental
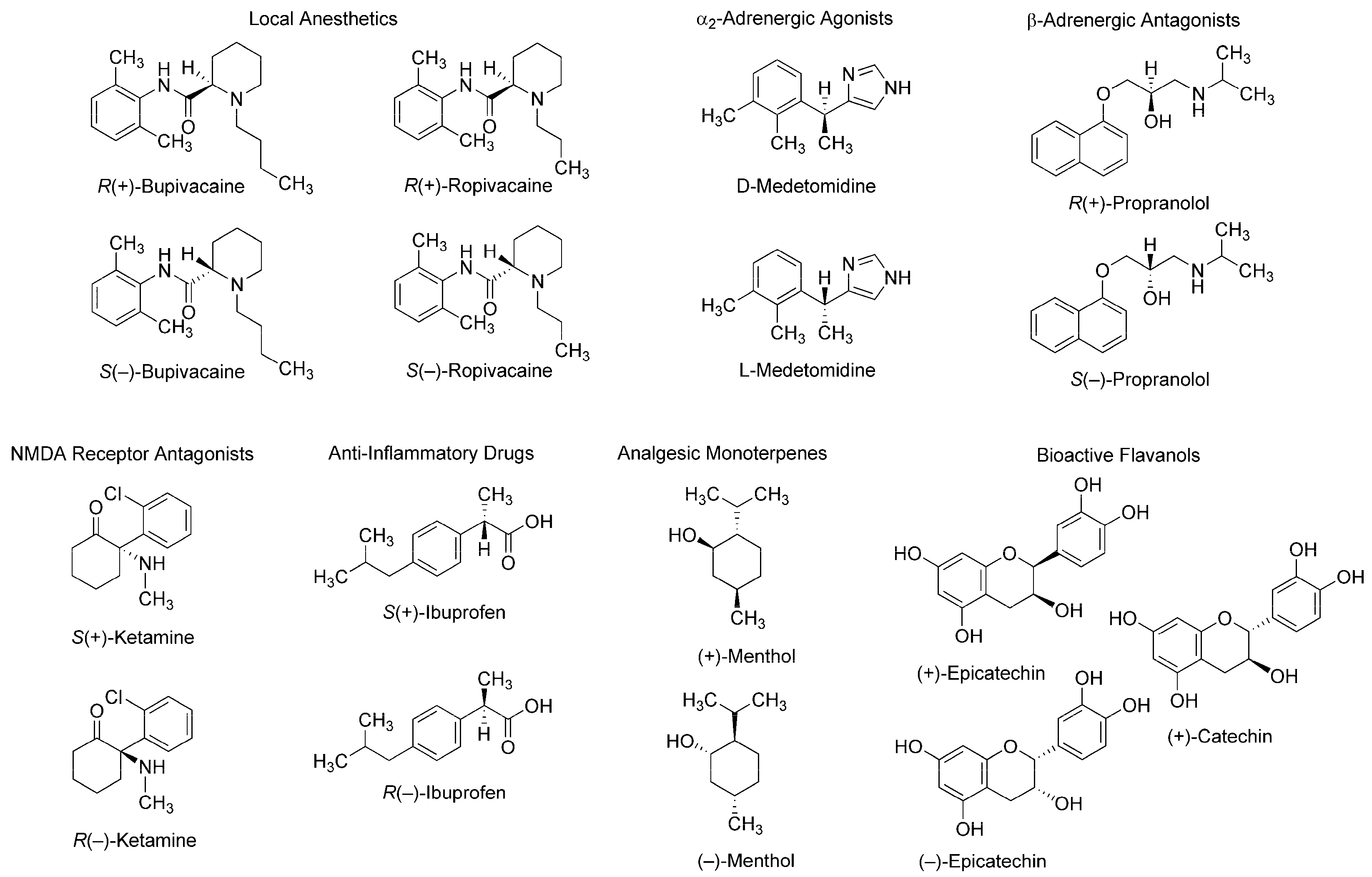
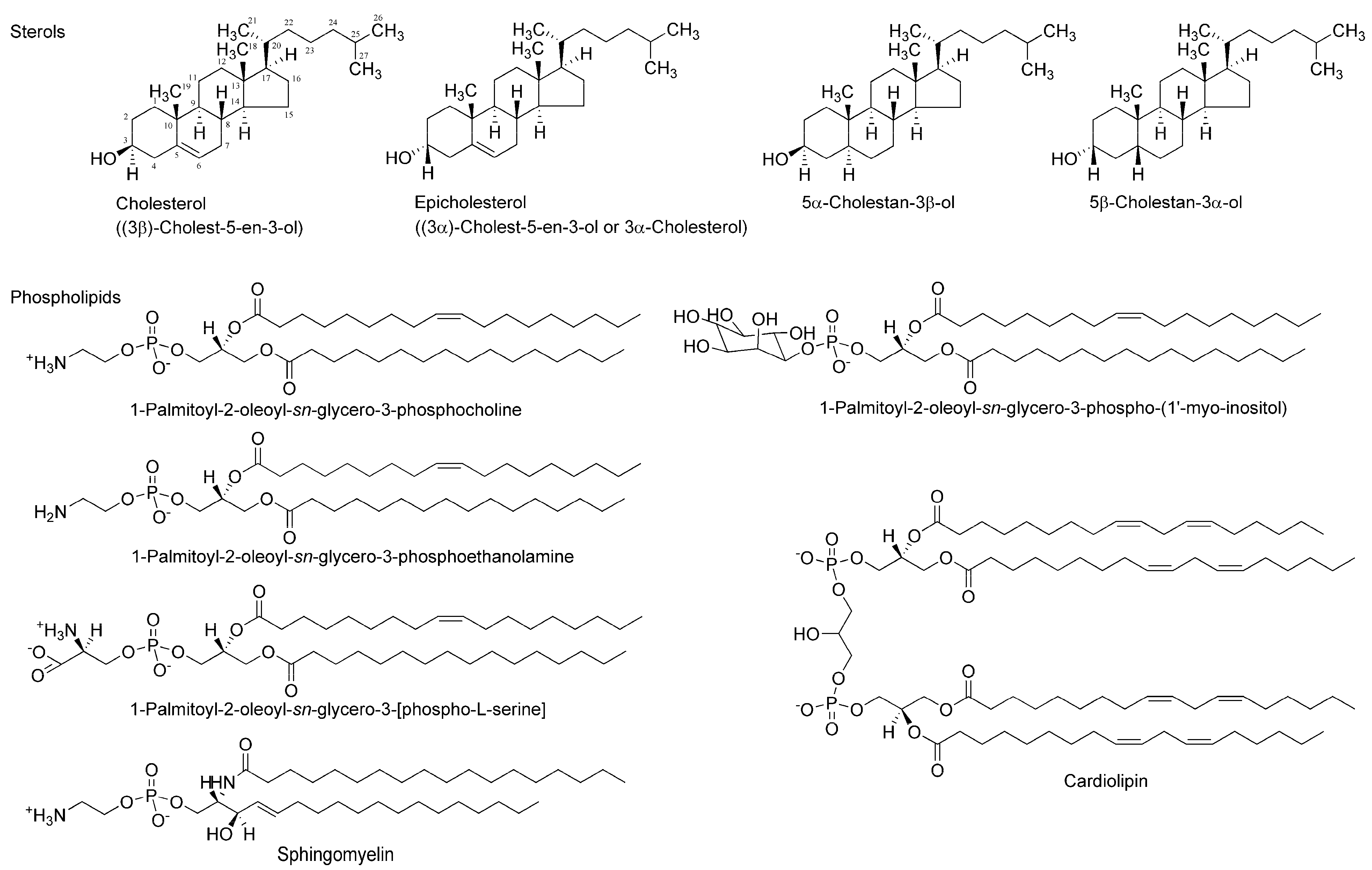
2.1. Materials
2.2. Biomimetic Membranes
2.3. Membrane Interactions
2.4. Specific Membrane Regions
2.5. Statistical Analysis
3. Results and Discussion
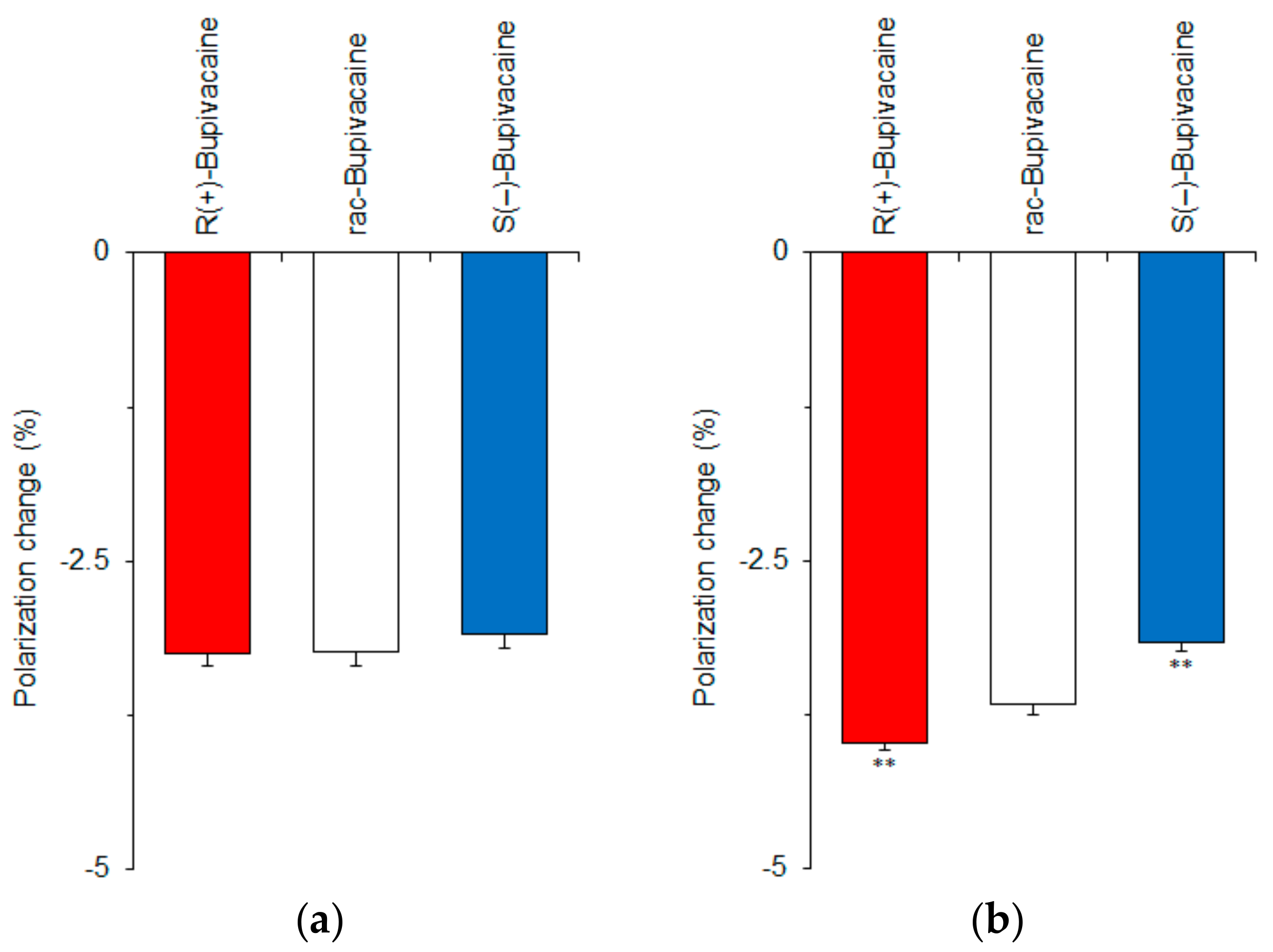
3.1. Membrane Interactions in the Absence or Presence of Cholesterol
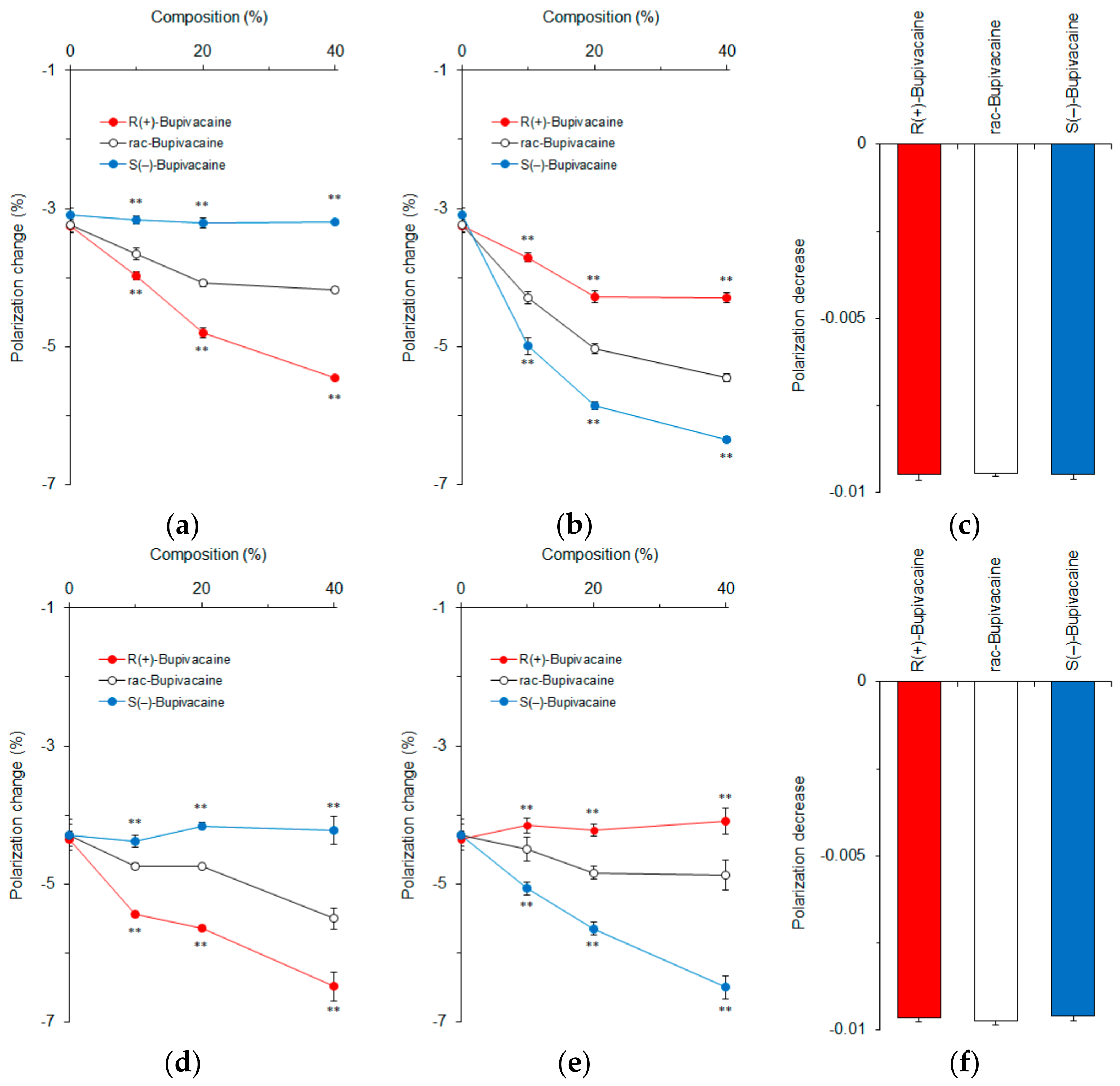
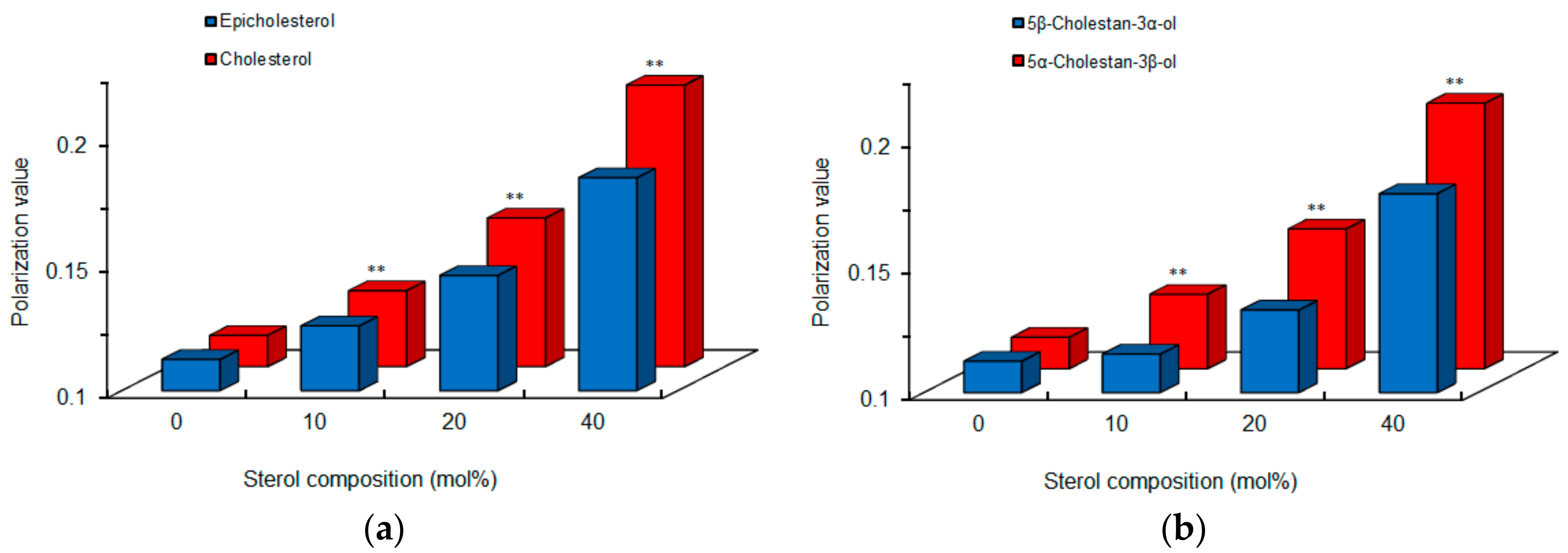
3.2. Effects of Sterols on Membrane Interactions
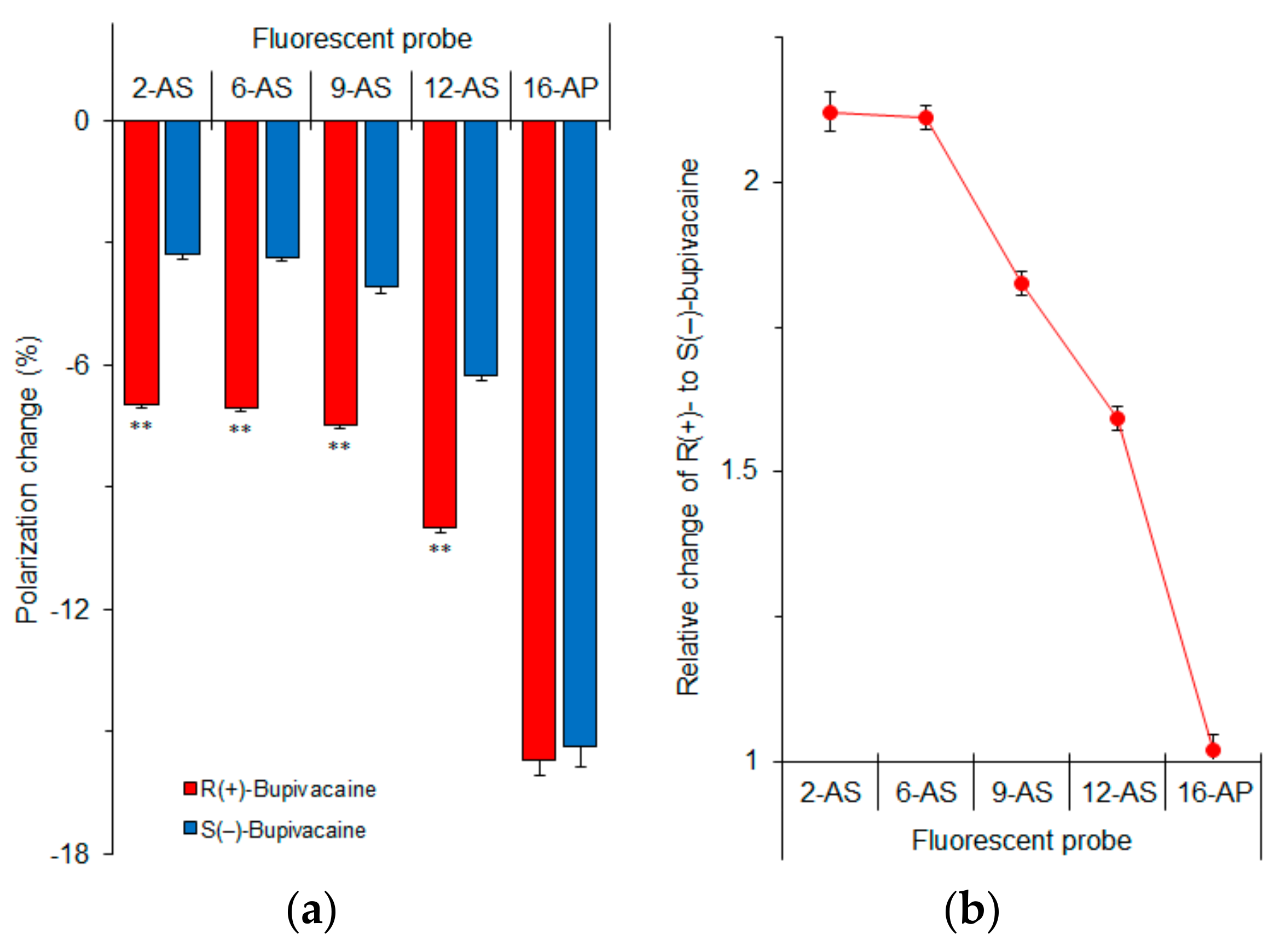
3.3. Membrane Interactions of Different Classes of Stereoisomers
3.4. Membrane Region Responsible for Enantioselective Interaction
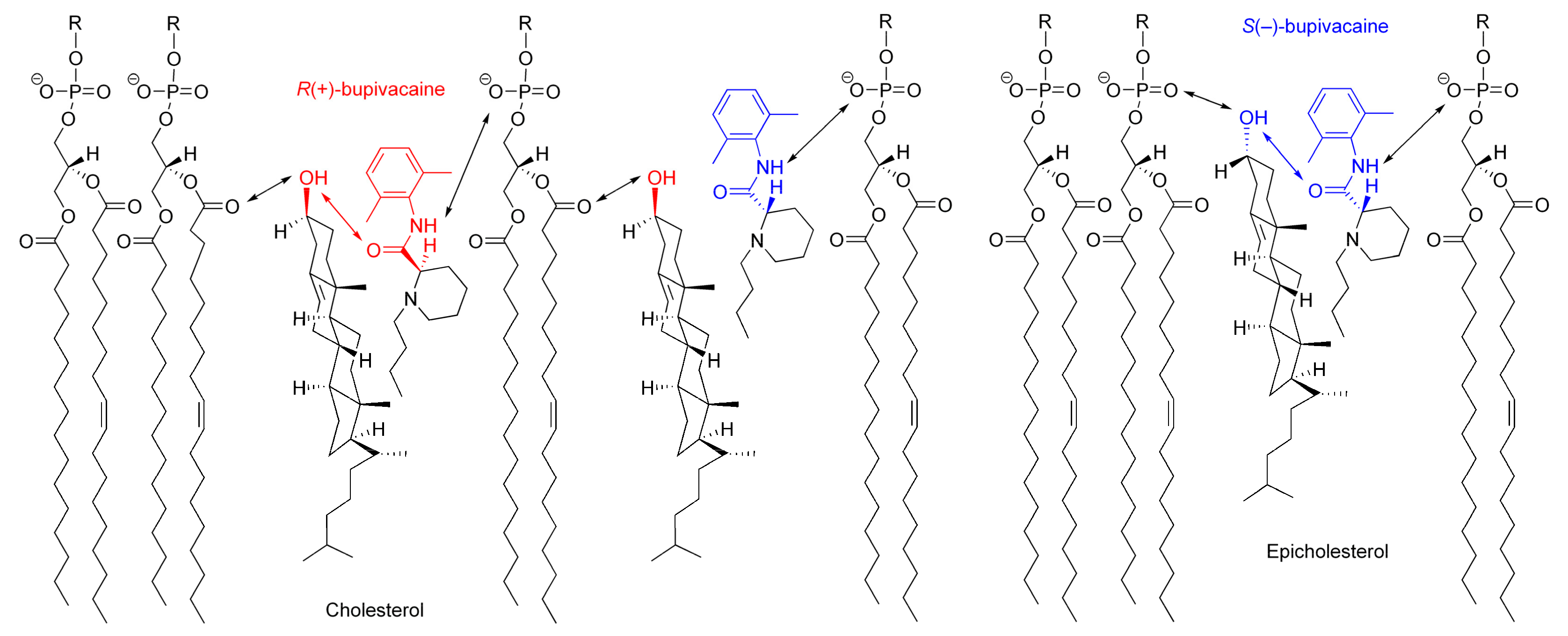
3.5. Possible Membrane Interaction
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rentsch, K.M. The importance of stereoselective determination of drugs in the clinical laboratory. J. Biochem. Biophys. Methods 2002, 54, 1–9. [Google Scholar] [CrossRef]
- Heavner, J.E. Cardiac toxicity of local anaesthetics in the intact isolated heart model: A review. Reg. Anesth. Pain Med. 2002, 27, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Kuusela, E.; Vainio, O.; Kaistinen, A.; Kobylin, S.; Raekallio, M. Sedative, analgesic, and cardiovascular effects of levomedetomidine alone and in combination with dexmedetomidine in dogs. Am. J. Vet. Res. 2001, 62, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Stoschitzky, K.; Lindner, W.; Kiowski, W. Stereoselective vascular effects of the (R)- and (S)-enantiomers of propranolol and atenolol. J. Cardiovasc. Pharmacol. 1995, 25, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Klepstad, P.; Maurset, A.; Moberg, E.R.; Oye, I. Evidence of a role for NMDA receptors in pain perception. Eur. J. Pharmacol. 1990, 187, 513–518. [Google Scholar] [CrossRef]
- Evans, A.M. Comparative pharmacology of S(+)-ibuprofen and (RS)-ibuprofen. Clin. Rheumatol. 2001, 20 (Suppl. 1), 9–14. [Google Scholar] [CrossRef]
- Hall, A.C.; Turcotte, C.M.; Betts, B.A.; Yeung, W.Y.; Agyeman, A.S.; Burk, L.A. Modulation of human GABAA and glycine receptor currents by menthol and related monoterpenoids. Eur. J. Pharmacol. 2004, 506, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Nyfeler, F.; Moser, U.K.; Walter, P. Stereospecific effects of (+)- and (–)-catechin on glycogen metabolism in isolated rat hepatocytes. Biochim. Biophys. Acta 1983, 763, 50–57. [Google Scholar] [CrossRef]
- Punke, M.A.; Friederich, P. Lipophilic and stereospecific interactions of amino-amide local anesthetics with human Kv1.1 channels. Anesthesiology 2008, 109, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar] [PubMed]
- Izake, E.L. Chiral discrimination and enantioselective analysis of drugs: An overview. J. Pharm. Sci. 2007, 96, 1659–1676. [Google Scholar] [CrossRef] [PubMed]
- Arnett, E.D.; Gold, J.M. Chiral Aggregation Phenomena. 4. A search for stereospecific interactions between highly purified enantiomeric and racemic dipalmitoyl phosphatidylcholines and other chiral surfactants in monolayers, vesicles, and gels. J. Am. Chem. Soc. 1982, 104, 636–639. [Google Scholar] [CrossRef]
- Mannock, D.A.; McIntosh, T.J.; Jiang, X.; Covey, D.F.; McElhaney, N. Effects of natural and enantiomeric cholesterol on the thermotropic phase behavior and structure of egg sphingomyelin bilayer membranes. Biophys. J. 2003, 84, 1038–1046. [Google Scholar] [CrossRef]
- Tsuchiya, H. Membrane interactions of phytochemicals as their molecular mechanism applicable to the discovery of drug leads from plants. Molecules 2015, 20, 18923–18966. [Google Scholar] [CrossRef] [PubMed]
- Nandi, N.; Vollhardt, D. Chiral discrimination and recognition in Langmuir monolayers. Curr. Opin. Colloid Interface Sci. 2008, 13, 40–46. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Mizogami, M. The membrane interaction of drugs as one of mechanisms for their enantioselective effects. Med. Hypotheses 2012, 79, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Zunino, M.P.; Turina, A.V.; Zygadlo, J.A.; Perillo, M.A. Stereoselective effects of monoterpenes on the microviscosity and curvature of model membranes assessed by DPH steady-state fluorescence anisotropy and light scattering analysis. Chirality 2011, 23, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Okimoto, Y.; Watanabe, A.; Niki, E.; Yamashita, T.; Noguchi, N. A novel fluorescent probe diphenyl-1-pyrenylphosphine to follow lipid peroxidation in cell membranes. FEBS Lett. 2000, 474, 137–140. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Ueno, T.; Mizogami, M.; Takakura, K. Local anesthetics structure-dependently interact with anionic phospholipid membranes to modify the fluidity. Chem. Biol. Interact. 2010, 183, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, H.; Tanaka, K.; Takeda, M.; Katsu, T.; Mima, S.; Mizushima, T. Geranylgeranylacetone protects membranes against nonsteroidal anti-inflammatory drugs. Mol. Pharmacol. 2005, 68, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Marczak, A. Fluorescence anisotropy of membrane fluidity probes in human erythrocytes incubated with anthracyclines and glutaraldehyde. Bioelectrochemistry 2009, 74, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H. Effects of red wine flavonoid components on biomembranes and cell proliferation. Int. J. Wine Res. 2011, 3, 9–17. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kishi, Y.; Ishigami, T.; Suga, K.; Umakoshi, H. Chiral selective adsorption of ibuprofen on a liposome membrane. J. Phys. Chem. B 2016, 120, 2790–2795. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, T.; Suga, K.; Umakoshi, H. Chiral recognition of l-amino acids on liposomes prepared with l-phospholipid. ACS Appl. Mater. Interfaces 2015, 7, 21065–21072. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, S.; Neely, W.C.; Myers, L.J.; Vodyanoy, V. Chiral recognition of odorants (+)- and (–)-carvone by phospholipid monolayers. J. Am. Chem. Soc. 1992, 114, 1404–1405. [Google Scholar] [CrossRef]
- Pasenkiewicz-Gierula, M.; Róg, T.; Grochowski, J.; Serda, P.; Czarnecki, R.; Librowski, T.; Lochyński, S. Effects of a carane derivative local anesthetic on a phospholipid bilayer studied by molecular dynamics simulation. Biophys. J. 2003, 85, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Róg, T.; Pasenkiewicz-Gierula, M. Effects of epicholesterol on the phosphatidylcholine bilayer: A molecular simulation study. Biophys. J. 2003, 84, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Róg, T.; Pasenkiewicz-Gierula, M.; Vattulainen, I.; Karttunen, M. Ordering effects of cholesterol and its analogues. Biochim. Biophys. Acta 2009, 1788, 97–121. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.M.; Martin, E.; Bosnjak, Z.J.; Stowe, D.F. Stereospecific effect of bupivacaine isomers on atrioventricular conduction in the isolated perfused guinea pig heart. Anesthesiology 1997, 86, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Groban, L.; Deal, D.D.; Vernon, J.C.; James, R.L.; Butterworth, J. Cardiac resuscitation after incremental overdosage with lidocaine, bupivacaine, levobupivacaine, and ropivacaine in anesthetized dogs. Anesth. Analg. 2001, 92, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.G.; Dominguez, J.J.; Frascarolo, P.; Reiz, S. A comparison of the electrocardiographic cardiotoxic effects of racemic bupivacaine, levobupivacaine, and ropivacaine in anesthetized swine. Anesth. Analg. 2000, 90, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Lee-Son, S.; Wang, G.K.; Concus, A.; Crill, E.; Strichartz, G. Stereoselective inhibition of neuronal sodium channels by local anesthetics. Evidence for two sites of action? Anesthesiology 1992, 77, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Vladimirov, M.; Nau, C.; Mok, W.M.; Strichartz, G. Potency of bupivacaine stereoisomers tested in vitro and in vivo: Biochemical, electrophysiological, and neurobehavioral studies. Anesthesiology 2000, 93, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Savola, J.M.; Virtanen, R. Central α2-adrenoceptors are highly stereoselective for dexmedetomidine, the dextro enantiomer of medetomidine. Eur. J. Pharmacol. 1991, 195, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kuusela, E.; Raekallio, M.; Väisänen, M.; Mykkänen, K.; Ropponen, H.; Vainio, O. Comparison of medetomidine and dexmedetomidine as premedicants in dogs undergoing propofol-isoflurane anesthesia. Am. J. Vet. Res. 2001, 62, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Selmi, A.L.; Mendes, G.M.; Figueiredo, J.P.; Barbudo-Selmi, G.R.; Lins, B.T. Comparison of medetomidine-ketamine and dexmedetomidine-ketamine anesthesia in golden-headed lion tamarins. Can. Vet. J. 2004, 45, 481–485. [Google Scholar] [PubMed]
- White, P.F.; Schüttler, J.; Shafer, A.; Stanski, D.R.; Horai, Y.; Trevor, A.J. Comparative pharmacology of the ketamine isomers. Br. J. Anaesth. 1985, 57, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.M. Enantioselective pharmacodynamics and pharmacokinetics of chiral non-steroidal anti-inflammatory drugs. Eur. J. Clin. Pharmacol. 1992, 42, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Stiufiuc, R.; Iacovita, C.; Lucaciu, C.M.; Stiufiuc, G.; Nicoara, R.; Oltean, M.; Chis, V.; Bodoki, E. Adsorption geometry of propranolol enantiomers on silver nanoparticles. J. Mol. Struct. 2013, 1031, 201–206. [Google Scholar] [CrossRef]
- Corvalán, N.A.; Zygadlo, J.A.; Garciá, D.A. Stereo-selective activity of menthol on GABAA receptor. Chirality 2009, 21, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Ohvo-Rekilä, H.; Ramstedt, B.; Leppimäki, P.; Slotte, J.P. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 2002, 41, 66–97. [Google Scholar] [CrossRef]
- Bukiya, A.N.; Belani, J.D.; Rychnovsky, S.; Dopico, A.M. Specificity of cholesterol and analogs to modulate BK channels points to direct sterol-channel protein interactions. J. Gen. Physiol. 2010, 137, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Weizenmann, N.; Huster, D.; Scheidt, H.A. Interaction of local anesthetics with lipid bilayers investigated by 1H MAS NMR spectroscopy. Biochim. Biophys. Acta 2012, 1818, 3010–3018. [Google Scholar] [CrossRef] [PubMed]
- Steinkopf, S.; Hanekam, L.; Schaathun, M.; Budnjo, A.; Haug, B.E.; Nerdal, W. Interaction of local anaesthetic articaine enantiomers with brain lipids: A Langmuir monolayer study. Eur. J. Pharm. Sci. 2012, 47, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Westover, E.J.; Covey, D.F. The enantiomer of cholesterol. J. Membr. Biol. 2004, 202, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ge, M.; Ciani, L.; Kuriakose, G.; Westover, E.J.; Dura, M.; Covey, D.F.; Freed, J.H.; Maxfield, F.R.; Lytton, J.; et al. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: Implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J. Biol. Chem. 2004, 279, 37030–37039. [Google Scholar] [CrossRef] [PubMed]
- Jafurulla, M.; Rao, B.D.; Sreedevi, S.; Ruysschaert, J.M.; Covey, D.F.; Chattopadhyay, A. Stereospecific requirement of cholesterol in the function of the serotonin1A receptor. Biochim. Biophys. Acta 2014, 1838, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Pesek, J.J.; Matyska, M.T.; Dawson, G.B.; Wilsdorf, A.; Marc, P.; Padki, M. Cholesterol bonded phase as a separation medium in liquid chromatography. Evaluation of properties and applications. J. Chromatogr. A 2003, 986, 253–262. [Google Scholar] [CrossRef]
- Espiritu, R.A.; Matsumori, N.; Tsuda, M.; Murata, M. Direct and stereospecific interaction of amphidinol 3 with sterol in lipid bilayers. Biochemistry 2014, 53, 3287–3293. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuchiya, H.; Mizogami, M. Discrimination of Stereoisomers by Their Enantioselective Interactions with Chiral Cholesterol-Containing Membranes. Molecules 2018, 23, 49. https://doi.org/10.3390/molecules23010049
Tsuchiya H, Mizogami M. Discrimination of Stereoisomers by Their Enantioselective Interactions with Chiral Cholesterol-Containing Membranes. Molecules. 2018; 23(1):49. https://doi.org/10.3390/molecules23010049
Chicago/Turabian StyleTsuchiya, Hironori, and Maki Mizogami. 2018. "Discrimination of Stereoisomers by Their Enantioselective Interactions with Chiral Cholesterol-Containing Membranes" Molecules 23, no. 1: 49. https://doi.org/10.3390/molecules23010049



