Extraction of a Novel Cold-Water-Soluble Polysaccharide from Astragalus membranaceus and Its Antitumor and Immunological Activities
Abstract
:1. Introduction
2. Results
2.1. Isolation, Purification and Chemical Composition Analysis
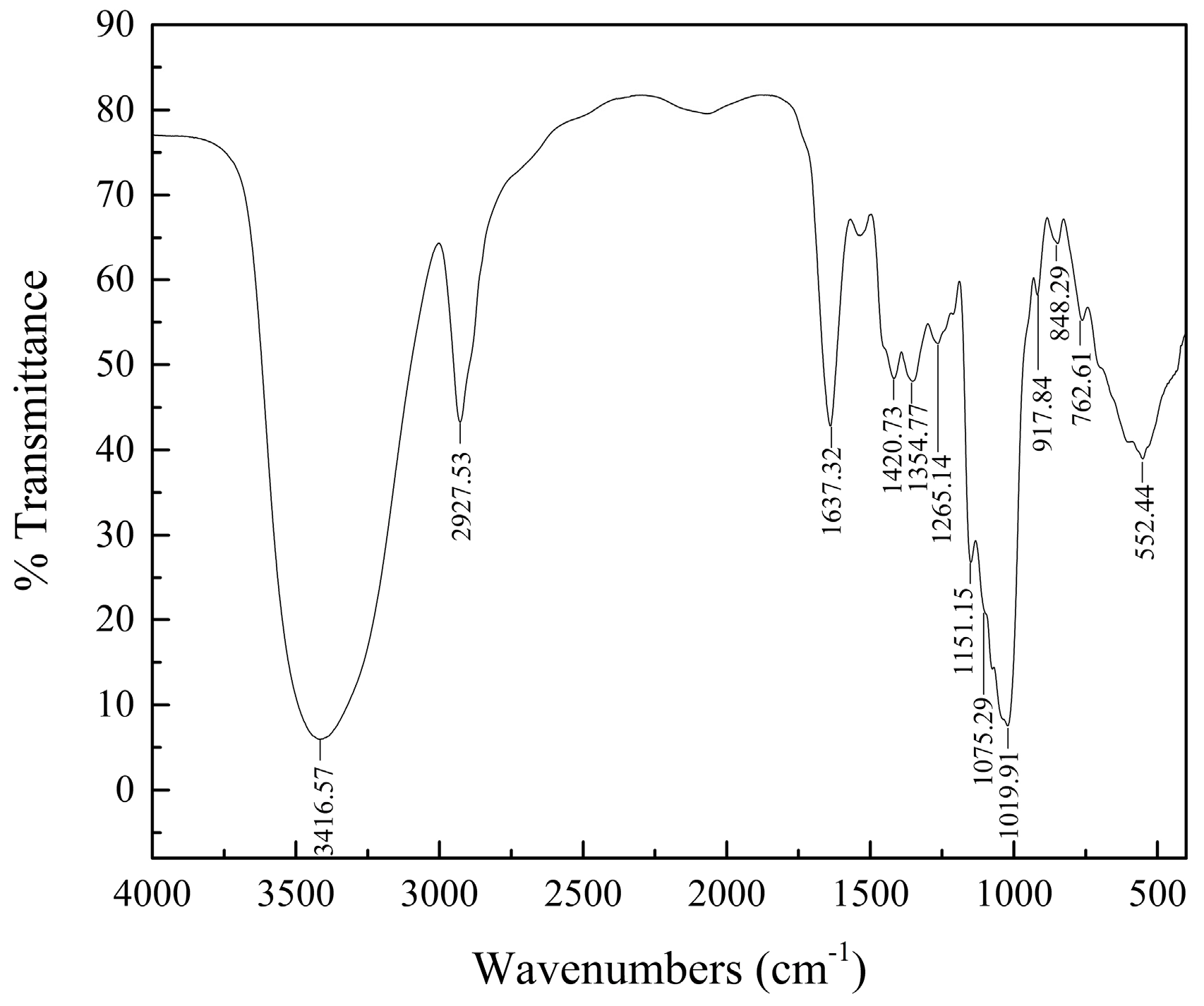
2.2. HPGPC and IR Analyses
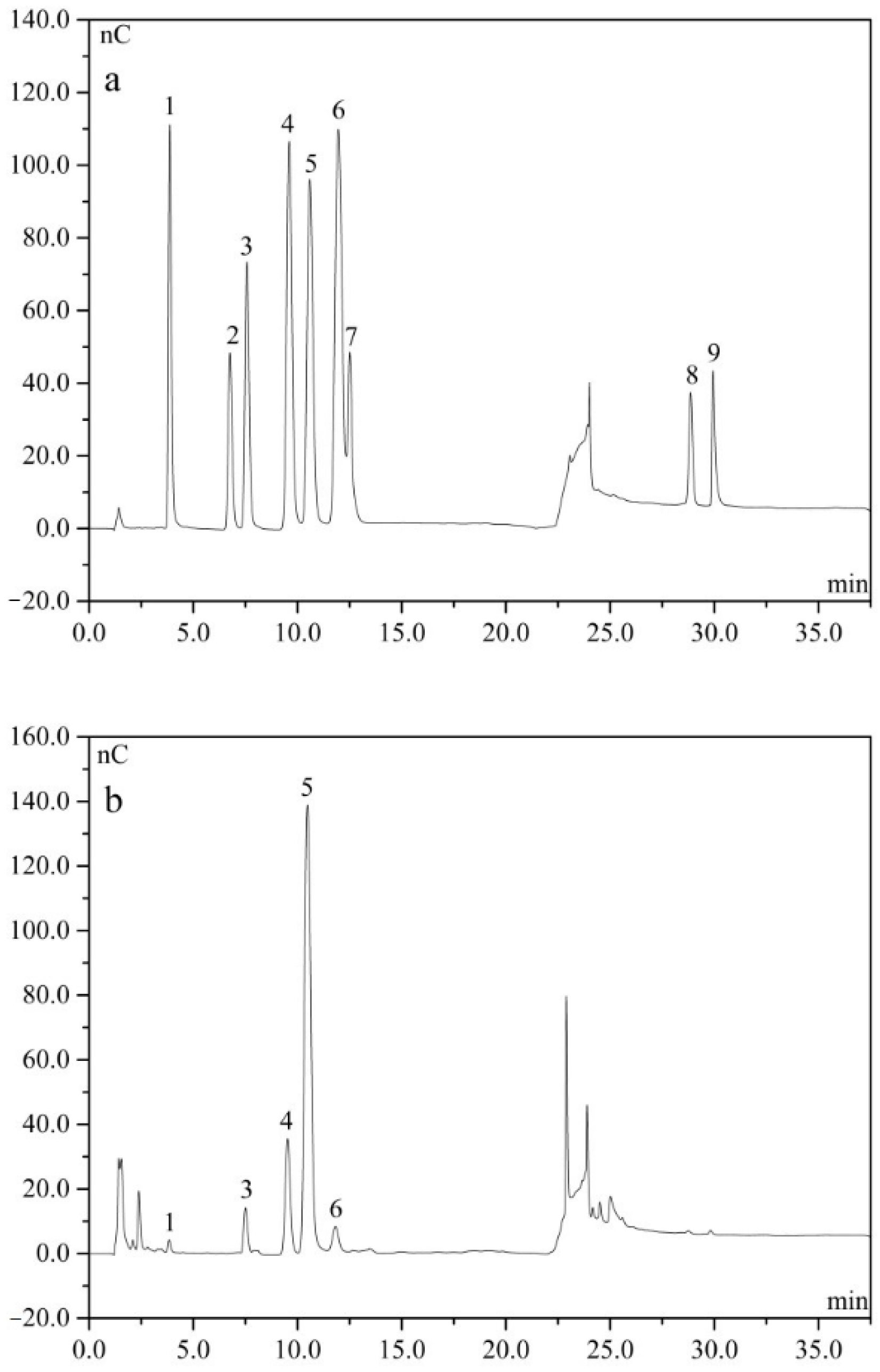
2.3. Monosaccharide
2.4. Antitumor Activity of cAMPs-1A In Vivo
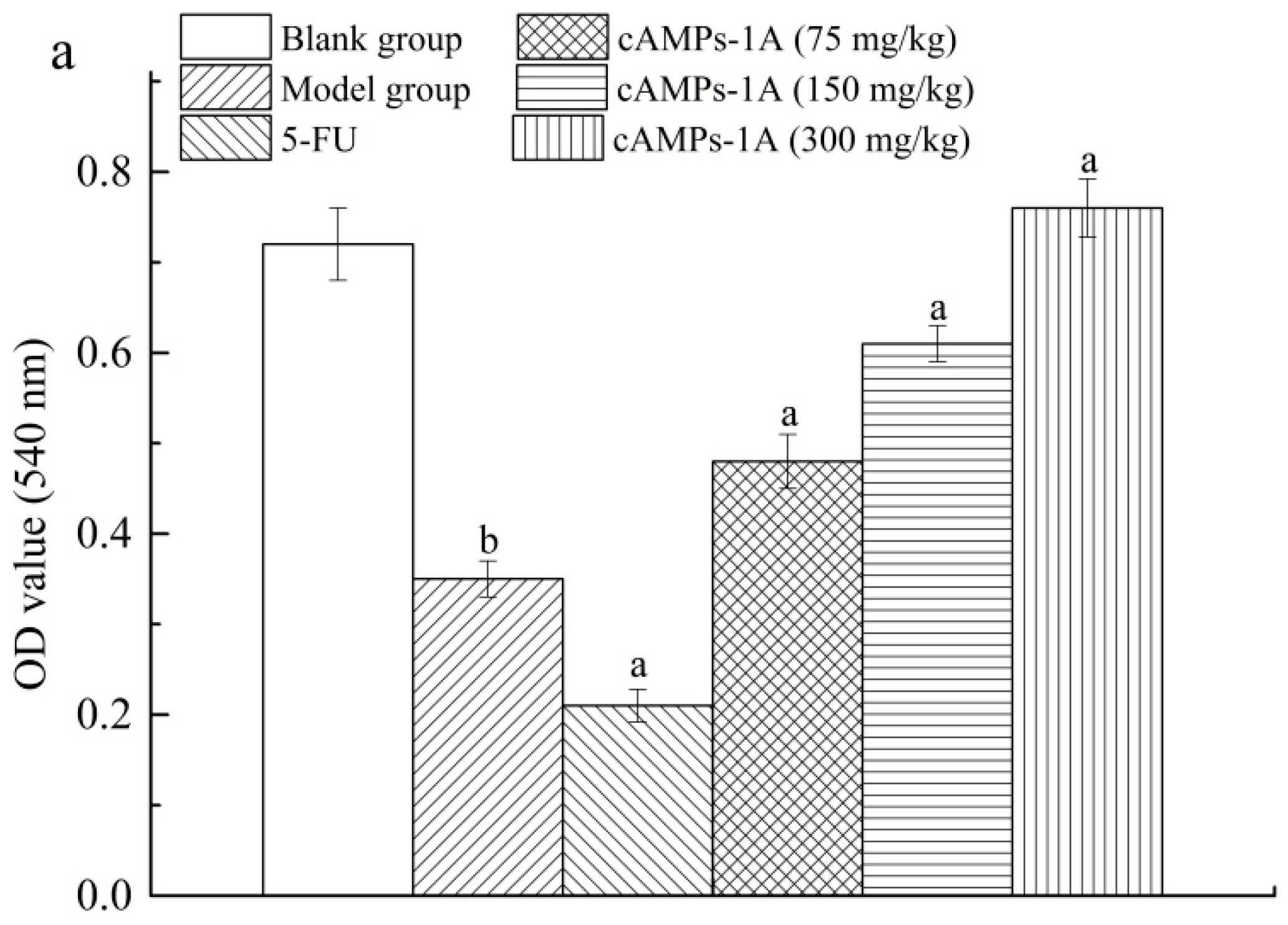
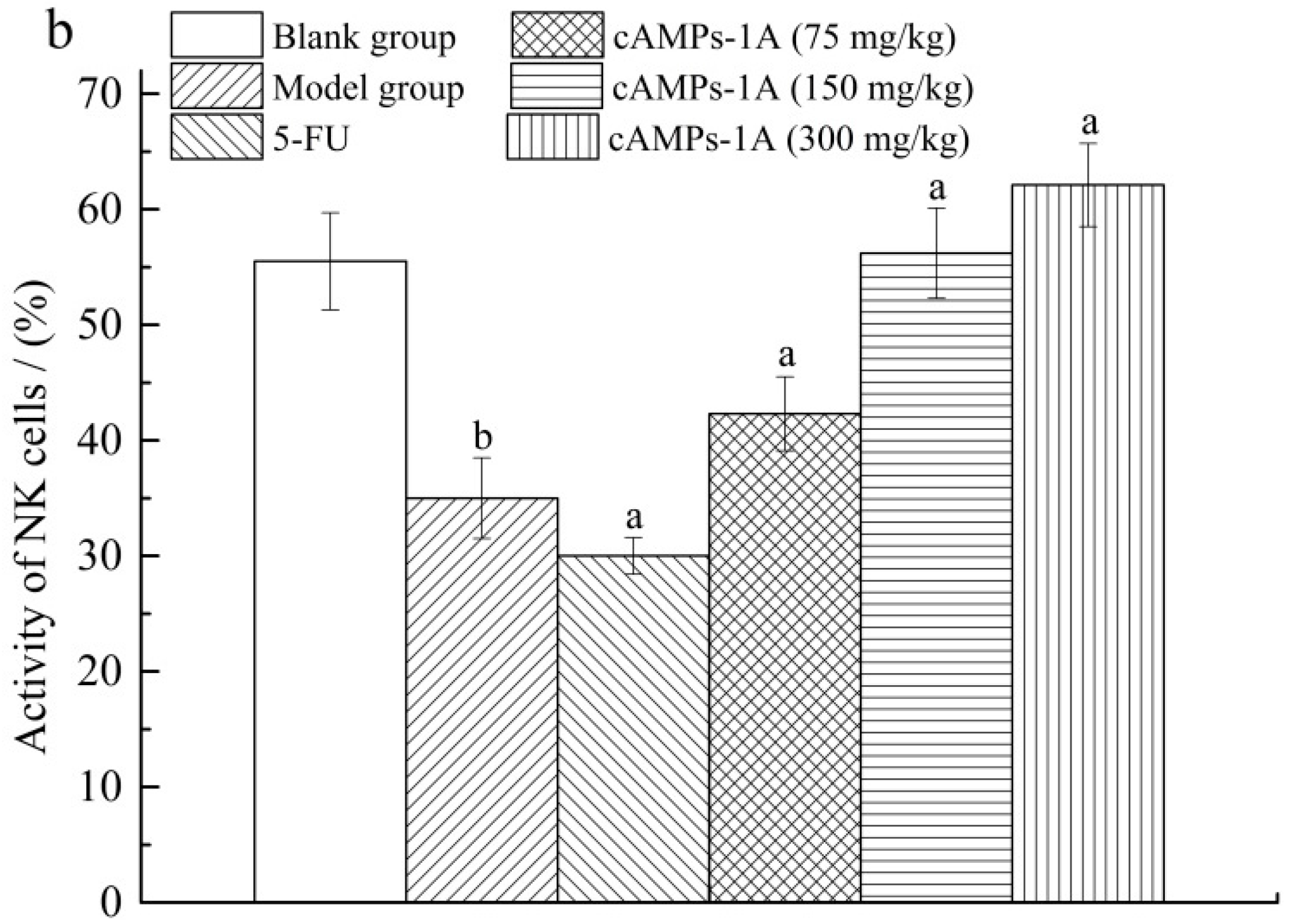
2.5. Effects of cAMPs-1A on the Macrophage Pinocytosis and NK Cytotoxicity
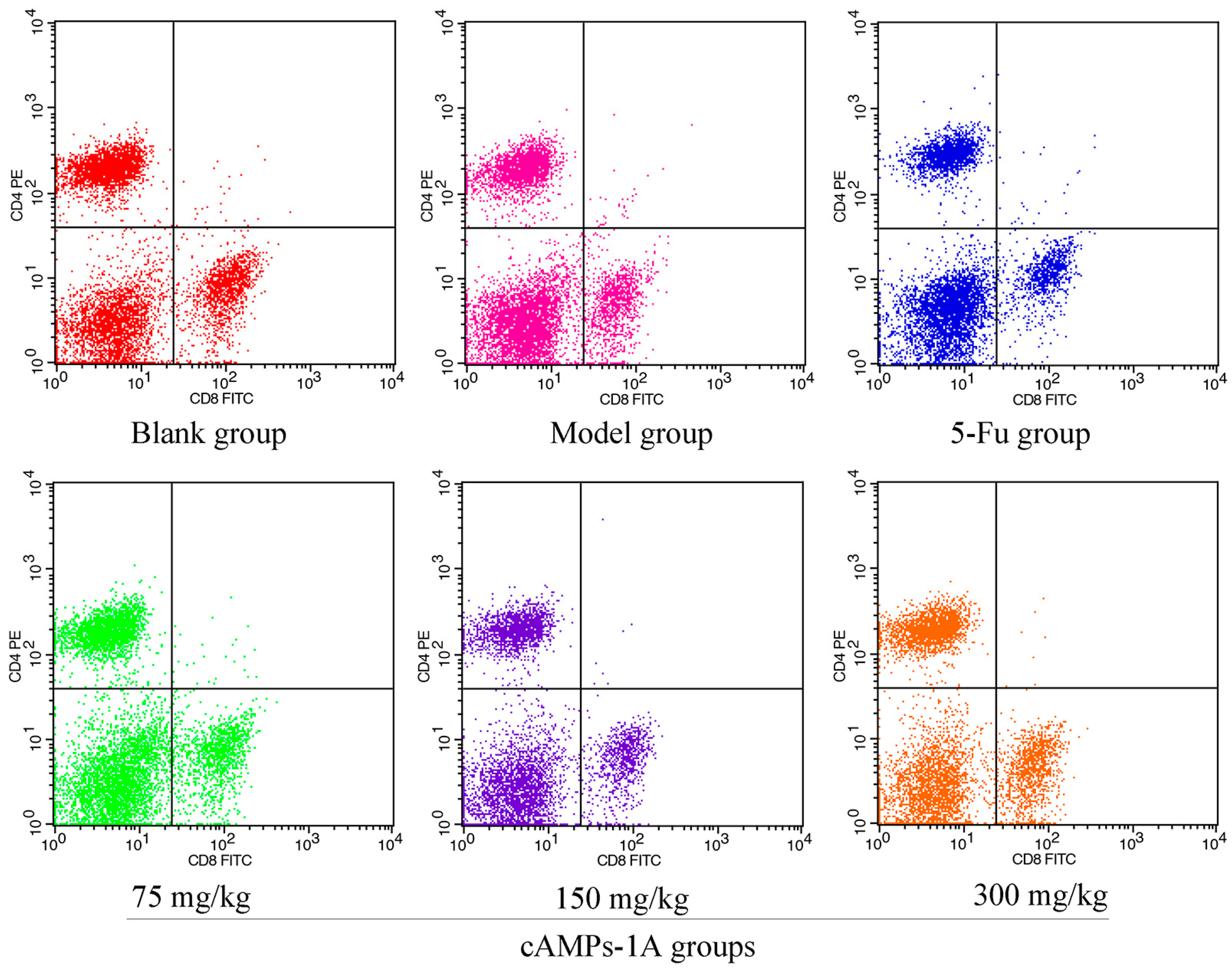
2.6. Effects of cAMPs-1A on the T Cells Subsets in Peripheral Blood
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction, Isolation and Purification of cAMPs
4.3. Partial Characterization and Structure Analysis of cAMPs-1A
Chemical Composition Analysis
4.4. HPGPC and IR Spectrum Assays
4.5. Analysis of Monosaccharide Composition
4.6. Animals and Experimental Design
4.7. Immune Organ Indexes and Tumor Weight
4.8. Macrophage Pinocytosis and NK Cytotoxicity Assays
4.9. Assessment of Lymphocyte Subsets in Peripheral Blood
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xu, H.; Yu, J.; Sun, Y.; Xu, X.; Li, L.; Xue, M.; Du, G. Scutellaria barbata D. Don extract synergizes the antitumor effects of low dose 5-fluorouracil through induction of apoptosis and metabolism. Phytomedicine 2013, 20, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C.; Leung, K.N. In vitro and in vivo anti-tumor effects of Astragalus membranaceus. Cancer Lett. 2007, 252, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zhao, K.; Huang, Q.; Shang, P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2014, 64, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Q.; Shi, Q.; Duan, J.A.; Dong, T.T.X.; Tsim, K.W.K. Chemical analysis of Radix Astragali (Huangqi) in China: A comparison with its adulterants and seasonal variations. J. Agric. Food Chem. 2002, 50, 4861–4866. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Han, N.; Dai, N.Y.T.; Wang, X.L.; Ao, W.L.J. The structural elucidation and antimicrobial activities of two isoflavane glycosides from Astragalus membranaceus (Fisch) Bge. var. mongholicus (Bge) Hsiao. J. Mol. Struct. 2014, 1076, 535–538. [Google Scholar] [CrossRef]
- Jiang, J.B.; Qiu, J.D.; Yang, L.H.; He, J.P.; Smith, G.W.; Li, H.Q. Therapeutic effects of Astragalus polysaccharides on inflammation and synovial apoptosis in rats with adjuvant-induced arthritis. Int. J. Rheum. Dis. 2010, 13, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.Y.; Liu, R.Q.; Si, C.L.; Zhou, F.; Wang, Y.X.; Ding, L.N.; Jing, C.; Liu, A.J.; Zhang, Y.M. Structural analysis and anti-tumor activity comparison of polysaccharides from Astragalus. Carbohydr. Polym. 2011, 85, 895–902. [Google Scholar] [CrossRef]
- Li, R.; Chen, W.C.; Wang, W.P.; Tian, W.Y.; Zhang, X.G. Extraction, characterization of Astragalus polysaccharides and its immune modulating activities in rats with gastric cancer. Carbohydr. Polym. 2009, 78, 738–742. [Google Scholar] [CrossRef]
- Yan, H.; Xie, Y.P.; Sun, S.G.; Sun, X.D.; Ren, F.X.; Shi, Q.R.; Wang, S.H.; Zhang, W.D.; Li, X.M.; Zhang, J. Chemical analysis of Astragalus mongholicus polysaccharides and antioxidant activity of the polysaccharides. Carbohydr. Polym. 2010, 82, 636–640. [Google Scholar] [CrossRef]
- Lai, E.C.H.; Lau, W.Y. The continuing challenge of hepatic cancer in Asia. Surgeon 2005, 3, 210–215. [Google Scholar] [CrossRef]
- Liu, A.; Liu, D.; Zhao, S.; Zheng, J.; Cao, D.; Zhang, H. Up regulation of annexin A2 on murine H22 hepatocarcinoma cells induced by cartilage polysaccharide. Cancer Epidemiol. 2011, 35, 490–496. [Google Scholar] [CrossRef]
- Lim, J.D.; Yu, C.Y.; Kim, S.H.; Chung, I.M. Structural characterization of an intestinal immune system-modulating arabino-3,6-galactan-like polysaccharide from the above-ground part of Astragalus membranaceus (Bunge). Carbohydr. Polym. 2016, 136, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Yang, X.M.; Ma, H.L.; Yan, J.K.; Guo, D.Z. Purification, characterization and antitumor activity of polysaccharides extracted from Phellinus igniarius mycelia. Carbohydr. Polym. 2015, 133, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Hromádková, Z.; Ebringerová, A.; Valachovic, P. Ultrasound-assisted extraction of water-soluble polysaccharides from the roots of valerian (Valeriana officinalis L.). Ultrason. Sonochem. 2002, 9, 37–42. [Google Scholar] [CrossRef]
- Chen, R.Z.; Tan, L.; Jin, C.G.; Lu, J.; Tian, L.; Chang, Q.Q.; Wang, K. Extraction, isolation, characterization and antioxidant activity of polysaccharides from Astragalus membranaceus. Ind. Crops Prod. 2015, 77, 434–443. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Liu, X.C.; Fang, X.N.; Sun, H.Q.; Yang, X.Y.; Zhang, Y.M. Structural characterization and anti-tumor activity of polysaccharide produced by Hirsutella sinensis. Int. J. Biol. Macromol. 2016, 82, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Meng, Y.; Liu, L.; Li, J.; Ren, D.; Guo, Y. Purification, characterization and antioxidant activities of polysaccharides from thinned-young apple. Int. J. Biol. Macromol. 2015, 72, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Mao, G.H.; Feng, W.W.; Mao, R.W.; Gua, X.Y.; Lia, T.; Lid, Q.; Bao, Y.T.; Yang, L.Q.; Wue, X.Y. Isolation, characterization and antioxidant activity of polysaccharide from Schisandra sphenanthera. Carbohydr. Polym. 2014, 105, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.A.; Bourne, E.J.; Stacey, M.; Whiffen, H.D. Infrared spectra of carbohydrates. Part I. Some derivatives of d-glucopyranose. J. Chem. Soc. 1954, 171–176. [Google Scholar] [CrossRef]
- Wang, B.; Lei, C.J.; Wu, R.; Li, L.; Deng, C.M.; Chen, W.X.; Hu, F.R.; Long, H.C.; Tao, Z.Z.; Zeng, C.; et al. Anti-tumor effect of LTA combined with 5-FU on H22 tumor bearing mice. Asian Pac. J. Trop. Med. 2015, 8, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Audrey, V.; Gordon, S. Alternative activation of macrophages: Immune function and cellular biology. Immunobiology 2009, 214, 630–641. [Google Scholar]
- Tosato, F.; Bernardi, D.; Sanzari, M.C.; Pantano, G.; Plebani, M. Biological variability of lymphocyte subsets of human adults’ blood. Clin. Chim. Acta 2013, 424, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liang, M.F.; Yang, P.P.; Guo, F.X.; Pan, D.Q.; Huang, X.; Li, Y.F.; Wu, C.; Qu, T.H.; Zhu, R. Taishan Pinus massoniana pollen polysaccharides promote immune responses of recombinant Bordetella avium ompA in BALB/c mice. Int. Immunopharmacol. 2013, 17, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.J.; Meng, K.; Qi, C.H.; Yang, X.Y.; Wang, Y.G.; Fan, W.T.; Yan, Z.G.; Zhao, X.N.; Liu, J. Immune-enhancing activity of polysaccharides isolated from Atractylodis macrocephalae Koidz. Carbohydr. Polym. 2015, 126, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Synytsya, A.; Kim, H.B.; Choi, D.J.; Lee, S.; Lee, J.; Kim, W.J.; Jang, S.; Park, Y.I. Purification, characterization and immunomodulating activity of a pectic polysaccharide isolated from Korean mulberry fruit Oddi (Morus alba L.). Int. Immunopharmacol. 2013, 17, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.W.; Feng, M.; Zhai, X.; Hu, M.; You, L.; Luo, W.; Zhao, M. Optimization for the extraction of polysaccharides from Ganoderma lucidum and their antioxidant and antiproliferative activities. J. Taiwan Inst. Chem. E 2013, 44, 886–894. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, C.; Pan, W.; Wang, J.; Wang, W. Carboxylate groups play a major role in antitumor activity of Ganoderma applanatum polysaccharide. Carbohydr. Polym. 2015, 123, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Hou, Y.; Hou, W. Structure feature and antitumor activity of a novel polysaccharide isolated from Lactarius deliciosus Gray. Carbohydr. Polym. 2012, 89, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, F.; Yu, Z.; Lin, J.; Yang, L. Chemical characterization and in vitro antitumor activity of a single-component polysaccharide from Taxus chinensis var. mairei. Carbohydr. Polym. 2015, 133, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Giuliano Ramadori, S.C. Effects of systemic chemotherapy on the liver. Ann. Hepatol. 2010, 9, 133–143. [Google Scholar]
- Naidu, M.U.; Ramana, G.V.; Rani, P.U.; Mohan, I.K.; Suman, A.; Roy, P. Chemotherapy-induced and/or radiation therapy-induced oral mucositis—Complicating the treatment of cancer. Neoplasia 2004, 6, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Ugolini, S.; Blaise, D.; Chabannon, C.; Brossay, L. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 2012, 12, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, T.; Peng, B.; Luo, X.; Liu, X.; Hu, L.; Liu, Y.; Di, D.; Song, Y.; Deng, Y. Targeted delivery of epirubicin to tumor-associated macrophages by sialic acid-cholesterol conjugate modified liposomes with improved antitumor activity. Int. J. Pharm. 2017, 523, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B. Innate immunity: An overview. Mol. Immunol. 2004, 40, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Hwang, D.; Hong, H.D.; Shin, K.S. Antitumor and antimetastatic activities of pectic polysaccharides isolated from persimmon leaves mediated by enhanced natural killer cell activity. J. Funct. Foods 2017, 37, 460–466. [Google Scholar] [CrossRef]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Kawakami, S.; Higuchi, Y.; Yamashita, F.; Hashida, M. The involvement of NK cell activation following intranasal administration of CpG DNA lipoplex in the prevention of pulmonary metastasis and peritoneal dissemination in mice. Clin. Exp. Metastasis 2012, 29, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.H.; Zhu, L.; Jiang, J.G. Immunoregulatory actions of polysaccharides from Chinese herbal medicine. Expert Opin. Ther. Targets 2010, 14, 1367–1402. [Google Scholar] [CrossRef] [PubMed]
- Franchini, A.; Ottaviani, E. Thymus: Conservation in evolution. Gen. Comp. Endocr. 2017, 246 (Suppl. C), 46–50. [Google Scholar] [CrossRef] [PubMed]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, C.; Galvez-Cancino, F.; Oyarce, C.; Contreras, F.; Prado, C.; Valeria, C.; Cruz, S.; Lladser, A.; Pacheco, R. Inhibition of dopamine receptor D3 signaling in dendritic cells increases antigen cross-presentation to CD8+ T-cells favoring anti-tumor immunity. J. Neuroimmunol. 2017, 303, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.; Gupta, P.C. Structure of a Water-Soluble Polysaccharide from the Seeds of Cassia angustifolia. Planta Med. 1986, 52, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Barbosa, H.; Slater, N.K.H.; Marcos, C.J. Protein quantification in the presence of poly (ethylene glycol) and dextran using the Bradford method. Anal. Chem. 2009, 395, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Bitter, T.; Muir, H.M. The determination of hydroxyproline in urine hydrolysate. Anal. Biochem. 1962, 62, 330–334. [Google Scholar] [CrossRef]
- Liu, L.; Wang, D.; Wang, J.; Ji, H.; Zhang, Y. NOAD, a novel nitric oxide donor, induces G2/M phase arrest and apoptosis in human hepatocellular carcinoma Bel-7402 cells. Toxicol. In Vitro 2015, 29, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xiao, B.; Sun, T. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2013, 62, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, W.D.; Li, Y. Antitumor and immunomodulatory activity of polysaccharide isolated from Trametes orientalis. Carbohydr. Polym. 2015, 131, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, W.; Song, W.; Chen, H.; Teng, A.; Liu, A. Partial characterization and anti-tumor activity of an acidic polysaccharide from Gracilaria lemaneiformis. Carbohydr. Polym. 2012, 88, 1313–1318. [Google Scholar] [CrossRef]
- Kong, F.; Li, F.E.; He, Z.; Jiang, Y.; Hao, R.; Sun, X.; Tong, H. Anti-tumor and macrophage activation induced by alkali-extracted polysaccharide from Pleurotus ostreatus. Int. J. Biol. Macromol. 2014, 69, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, J.; Hu, Y.; Cheng, G.; Zhu, X.; Liu, F.; Zhang, Y.; Liu, Z.; Xu, J. Effects of Astragalus polysaccharide on the immune response to foot-and-mouth disease vaccine in mice. Carbohydr. Polym. 2010, 82, 680–686. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Treatment | Dose (mg/kg) | Thymus Index (mg/g) | Spleen Index (mg/g) | Tumor Weight (g) | Inhibitory Rate (%) |
|---|---|---|---|---|---|
| Blank group | - | 3.30 ± 0.66 | 4.52 ± 0.47 | - | - |
| Model group | - | 1.51 ± 0.17 b | 7.93 ± 0.46 b | 2.63 ± 0.24 | - |
| 5-Fu | 30 | 1.21 ± 0.11 a | 3.87 ± 0.15 a | 1.41 ± 0.13 a | 46.39 |
| cAMPs-1A group | 75 | 1.89 ± 0.16 a | 6.45 ± 0.32 a | 2.09 ± 0.16 a | 20.53 |
| cAMPs-1A group | 150 | 2.47 ± 0.21 a | 5.89 ± 0.40 a | 1.67 ± 0.14 a | 36.50 |
| cAMPs-1A group | 300 | 3.08 ± 0.36 a | 4.62 ± 0.29 a | 1.46 ± 0.11 a | 44.49 |
| Groups | Dose (mg/kg) | CD4+ (%) | CD8+ (%) |
|---|---|---|---|
| Blank group | - | 37.66 ± 2.68 | 18.25 ± 1.40 |
| Model group | - | 29.22 ± 2.61 b | 15.26 ± 1.38 b |
| 5-Fu | 30 | 28.43 ± 2.24 | 14.84 ± 1.41 |
| cAMPs-1A groups | 75 | 31.43 ± 3.11 | 16.26 ± 1.12 |
| 150 | 33.79 ± 2.70 a | 17.83 ± 0.93 a | |
| 300 | 37.24 ± 3.74 a | 19.63 ± 3.42 a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, A.-j.; Yu, J.; Ji, H.-y.; Zhang, H.-c.; Zhang, Y.; Liu, H.-p. Extraction of a Novel Cold-Water-Soluble Polysaccharide from Astragalus membranaceus and Its Antitumor and Immunological Activities. Molecules 2018, 23, 62. https://doi.org/10.3390/molecules23010062
Liu A-j, Yu J, Ji H-y, Zhang H-c, Zhang Y, Liu H-p. Extraction of a Novel Cold-Water-Soluble Polysaccharide from Astragalus membranaceus and Its Antitumor and Immunological Activities. Molecules. 2018; 23(1):62. https://doi.org/10.3390/molecules23010062
Chicago/Turabian StyleLiu, An-jun, Juan Yu, Hai-yu Ji, Hong-cui Zhang, Yan Zhang, and Hui-ping Liu. 2018. "Extraction of a Novel Cold-Water-Soluble Polysaccharide from Astragalus membranaceus and Its Antitumor and Immunological Activities" Molecules 23, no. 1: 62. https://doi.org/10.3390/molecules23010062







