In Silico and In Vitro Study of the Bromelain-Phytochemical Complex Inhibition of Phospholipase A2 (Pla2)
Abstract
:1. Introduction
2. Results
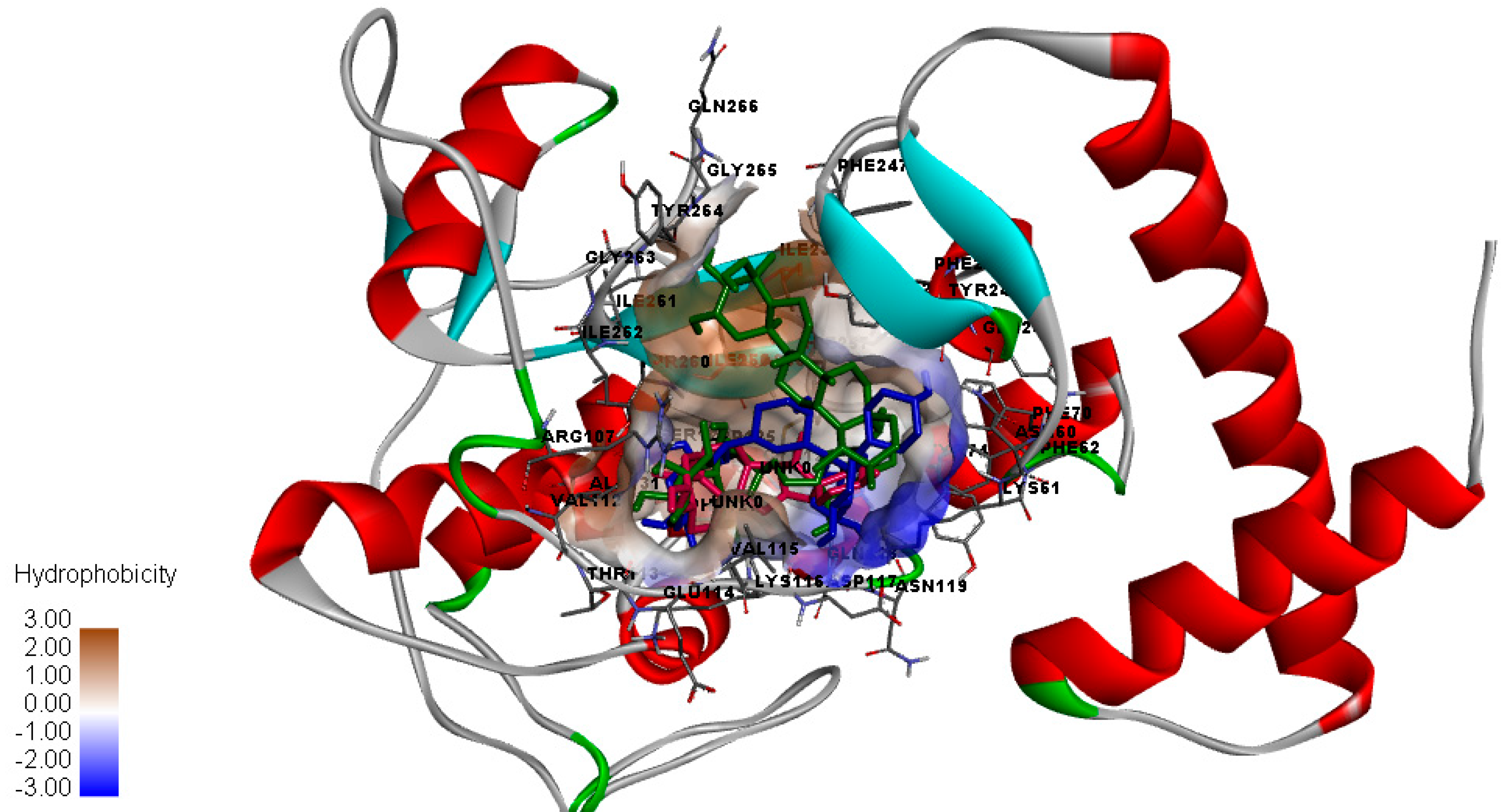
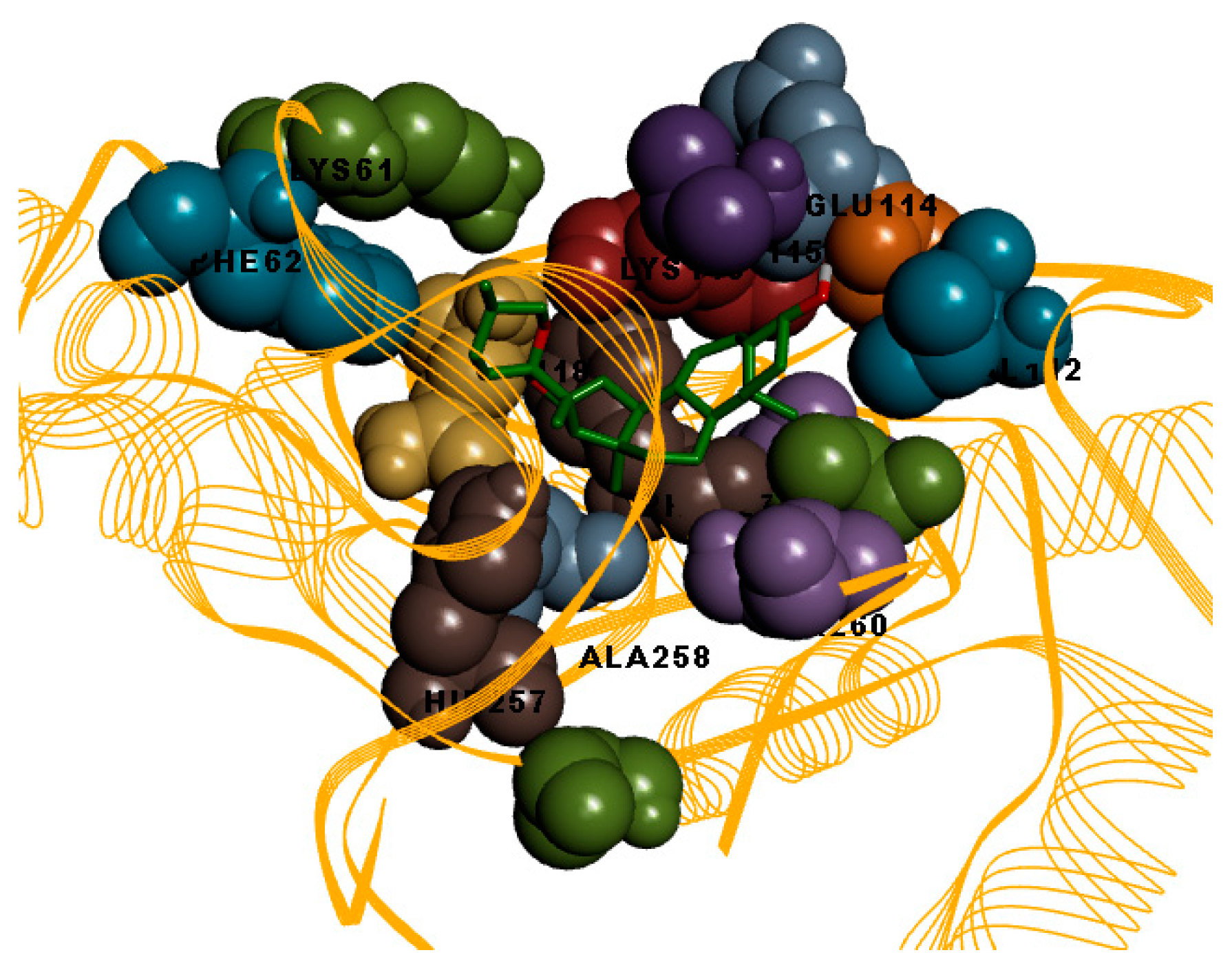
2.1. Top Ten Ranking Compounds and Molecular Docking Results
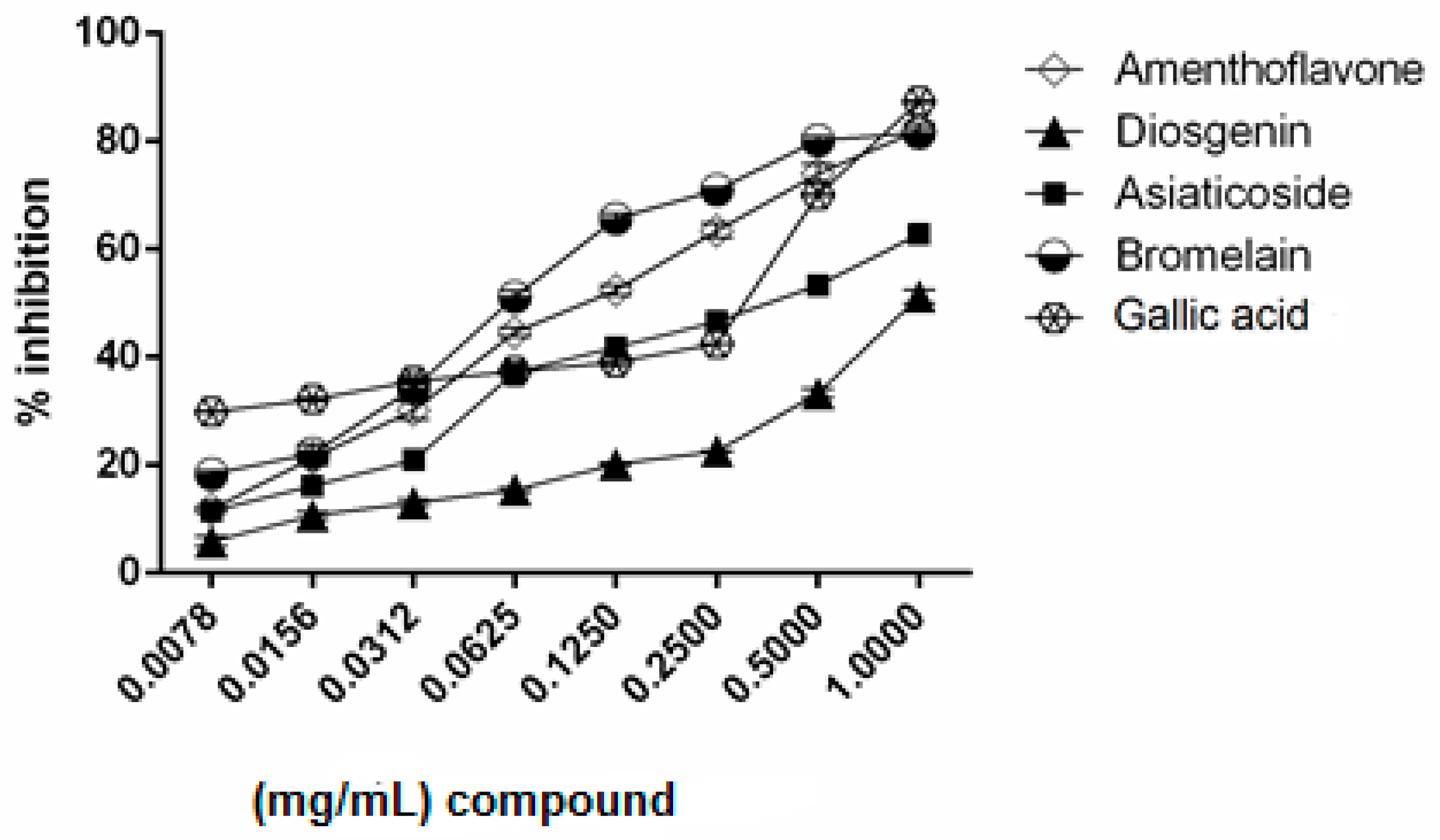
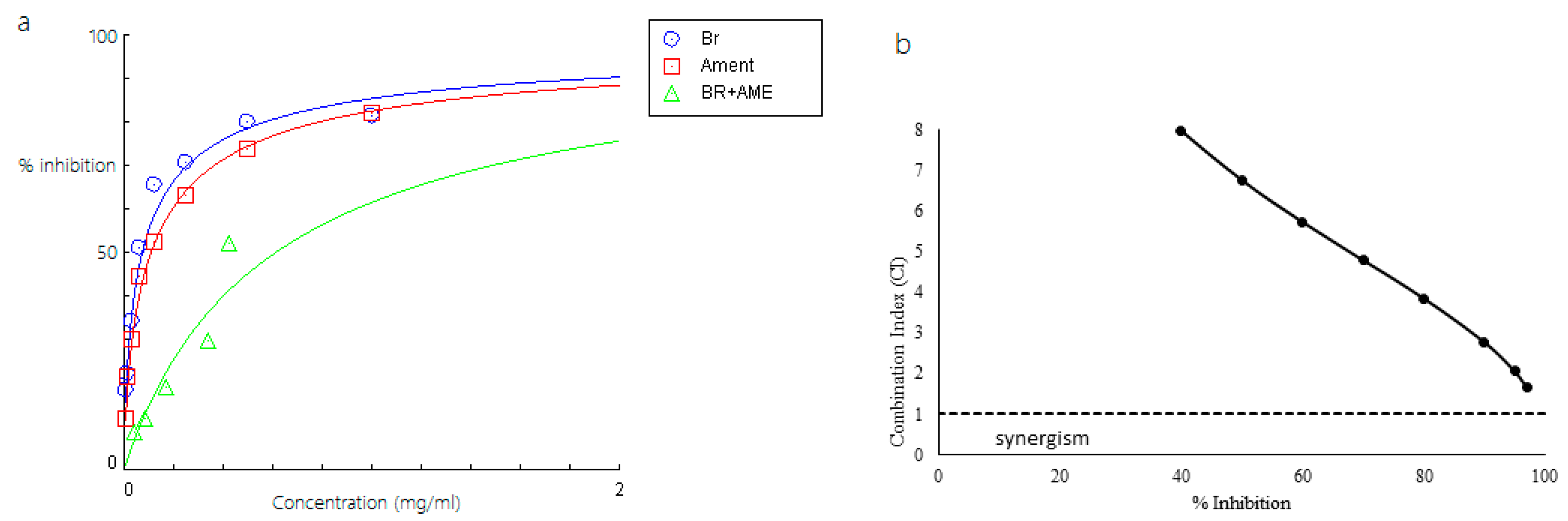
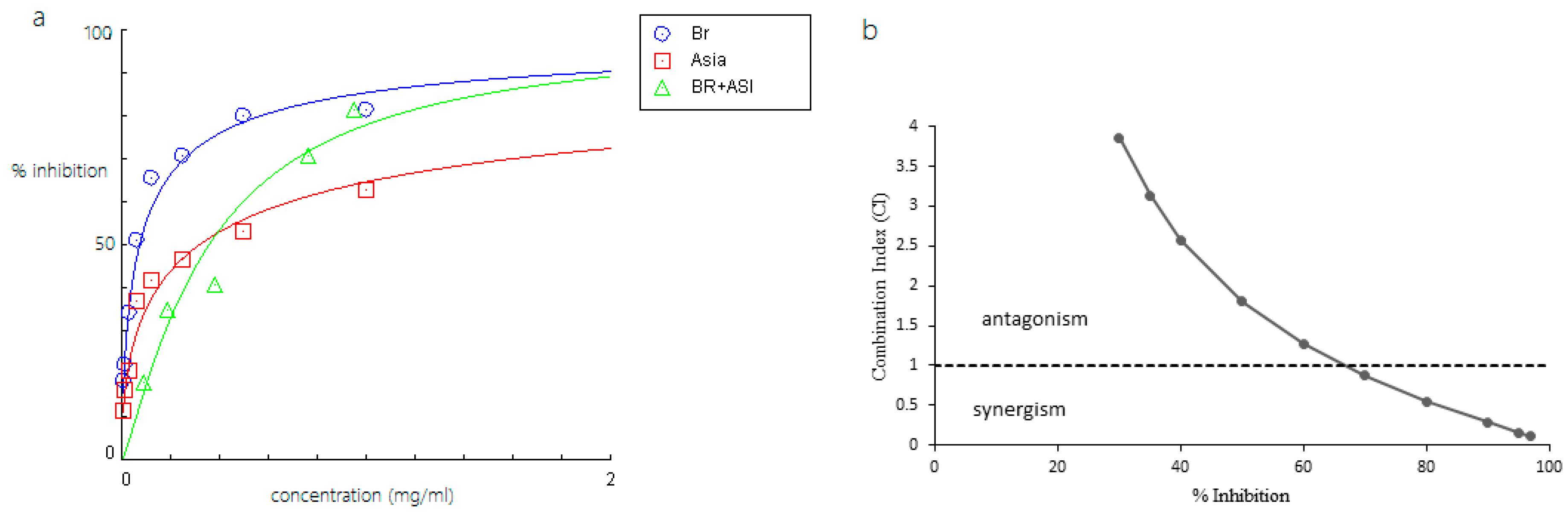
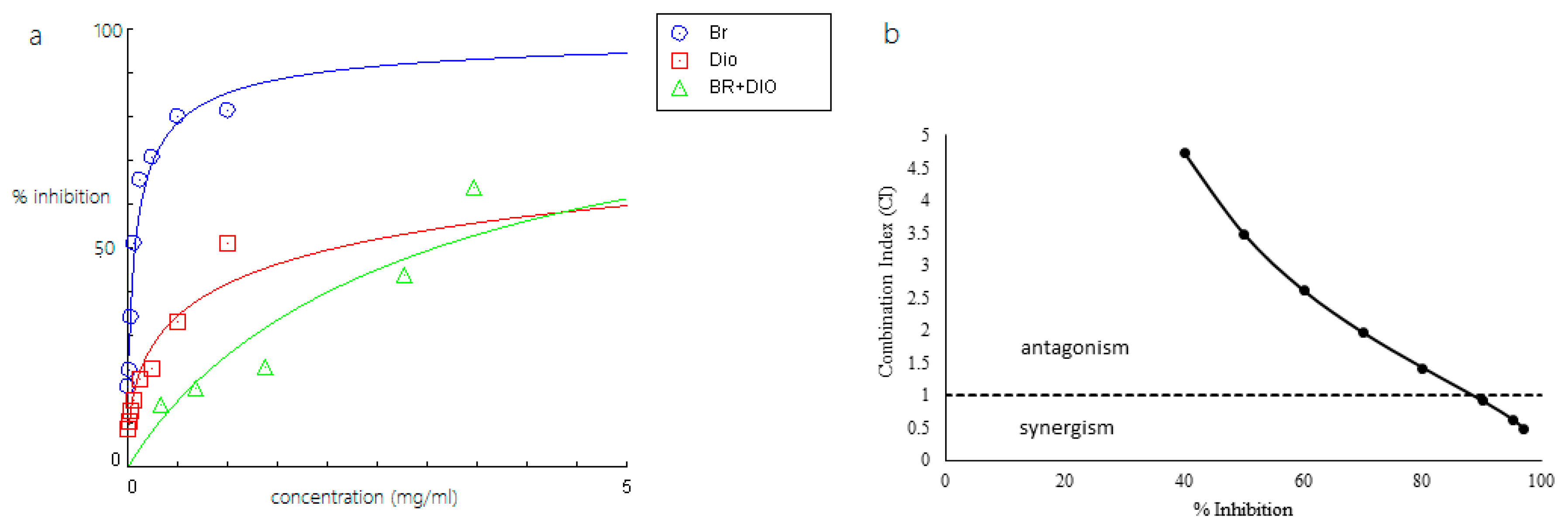
2.2. Inhibitory Activity of Bromelain, Amenthoflavone, Asiaticoside, and Diosgenin Against Pla2
2.3. Combined Effects of Phytochemicals and Bromelain Against Pla2
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Virtual Screening
4.2.1. Protein and Ligand Library Preparation
4.2.2. Molecular Docking
4.3. Inhibition of Pla2 Activity
4.4. Experimental Design of Combination Study
4.5. Median Effect, Combination Index (CI), and Dose Reduction Index Analysis
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dave, S.; Kaur, N.J.; Nanduri, R.; Dkhar, H.K.; Kumar, A.; Gupta, P. Inhibition of adipogenesis and induction of apoptosis and lipolysis by stem bromelain in 3T3-L1 adipocytes. PLoS ONE 2012, 7, e30831. [Google Scholar] [CrossRef] [PubMed]
- Quach, N.D.; Arnold, R.D.; Cummings, B.S. Secretory phospholipase A2 enzymes as pharmacological targets for treatment of disease. Biochem. Pharmacol. 2014, 90, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009, 50, S237–S242. [Google Scholar] [CrossRef] [PubMed]
- Nanda, B.L.; Nataraju, A.; Rajesh, R.; Rangappa, K.S.; Shekar, M.A.S.; Vishwanath, B.S. PLA2 mediated arachidonate free radicals: PLA2 inhibition and neutralization of free radicals by anti-oxidants—A new role as anti-inflammatory molecule. Curr. Top. Med. Chem. 2007, 7, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.S.; Kazunori, S.; Katsumi, M.; Takuma, Y.; Eiryu, S.; Tomoyuki, S.; Ryo, N.; Tatsuro, A.; Shohei, O.; Tomoyasu, K.; et al. Elevated circulating levels of inflammatory markers in patients with acute coronary syndrome. Int. J. Vasc. Med. 2015, 2015. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, M.; Miladi, S.; Ali, Y.-B.; Damak, M.; Gargouri, Y.; Bezzine, S. In vitro study of the PLA2 inhibition and antioxidant activities of Aloe vera leaf skin extracts. Lipids Health Dis. 2011, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Bushra, R.; Aslam, N. An overview of clinical pharmacology of ibuprofen. Oman Med. J. 2010, 25, 155–1661. [Google Scholar] [PubMed]
- Poh, S.S.; Abd, M.F.A. Thermal stability of free bromelain and bromelain-polyphenol complex in pineapple juice. Int. Food Res. J. 2011, 18, 1051–1060. [Google Scholar]
- Zhang, Z.; Sun, T.; Niu, J.; He, Z.; Liu, Y.; Wang, F. Amentoflavone protects hippocampal neurons: Anti-inflammatory, antioxidative, and antiapoptotic effects. Neural Regen. Res. 2015, 10, 1125–1133. [Google Scholar] [PubMed]
- Wan, J.; Gong, X.; Jiang, R.; Zhang, Z.; Zhang, L. Antipyretic and anti-inflammatory effects of asiaticoside in lipopolysaccharide-treated rat through up-regulation of heme oxygenase-1. Phyther. Res. 2013, 27, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Hamid, A.A.; Maurya, A.K.; Prakash, O.; Khan, F.; Kumar, A.; Aiyelaagbe, O.O.; Negi, A.S.; Bawankule, D.U. Synthesis of diosgenin analogues as potential anti-inflammatory agents. J. Steroid Biochem. Mol. Biol. 2014, 143, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Pavan, R.; Jain, S.; Shraddha; Kumar, A. Properties and therapeutic application of bromelain: A review. Biotechnol. Res. Int. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gohil, K.J.; Patel, J.A.; Gajjar, A.K. Pharmacological review on centella asiatica: A potential herbal cure-all. Indian J. Pharm. Sci. 2010, 72, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Tohda, C.; Yang, X.; Matsui, M.; Inada, Y.; Kadomoto, E.; Nakada, S.; Watari, H.; Shibahara, N. Diosgenin-rich yam extract enhances cognitive function: A placebo-controlled, randomized, double-blind, crossover study of healthy adults. Nutrients 2017, 9, 1160. [Google Scholar] [CrossRef] [PubMed]
- Talib, S.Z.; Hasyim, K.K. Stability of bromelain-polyphenol complex in pineapple juice. Chem. Eng. 2008, 49, 27–38. [Google Scholar]
- Bolten, W.W.; Glade, M.J.; Raum, S.; Ritz, B.W. The safety and efficacy of an enzyme combination in managing knee osteoarthritis pain in adults: A randomized, double-blind, placebo-controlled trial. Arthritis 2015, 2015, 251521. [Google Scholar] [CrossRef] [PubMed]
- Rathnavelu, V.; Alitheen, N.B.; Sohila, S.; Kanagesan, S.; Ramesh, R. Potential role of bromelain in clinical and therapeutic applications. Biomed. Rep. 2016, 5, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Ben Bdira, F.; Jiang, J.; Kallemeijn, W.; de Haan, A.; Florea, B.I.; Bleijlevens, B.; Boot, R.; Overkleeft, H.S.; Aerts, J.M.; Ubbink, M. Hydrophobic interactions contribute to conformational stabilization of endoglycoceramidase II by mechanism-based probes. Biochemistry 2016, 55, 4823–4835. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, O.; Marsden, B.; Pogacic, V.; Rellos, P.; Müller, S.; Bullock, A.N.; Schwaller, J.; Sundström, M.; Knapp, S. A systematic interaction map of validated kinase inhibitors with Ser/Thr kinases. Proc. Natl. Acad. Sci. USA 2007, 104, 20523–20528. [Google Scholar] [CrossRef] [PubMed]
- Krstin, S.; Peixoto, H.S.; Wink, M. Combinations of alkaloids affecting different molecular targets with the saponin digitonin can synergistically enhance trypanocidal activity against Trypanosoma brucei brucei. Antimicrob. Agents Chemother. 2015, 59, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.S.; van Staden, J. COX-1 inhibitory activity in extracts from Eucomis L’Herit. species. J. Ethnopharmacol. 2001, 75, 257–265. [Google Scholar] [CrossRef]
- Ramachandran, C.; Wilk, B.; Melnick, S.J.; Eliaz, I. Synergistic antioxidant and anti-inflammatory effects between modified citrus pectin and honokiol. Evid. Based. Complement. Alternat. Med. 2017, 2017, 8379843. [Google Scholar] [CrossRef] [PubMed]
- Alasmary, F.A.S.; Alnahdi, F.S.; Ben Bacha, A.; El-Araby, A.M.; Moubayed, N.; Alafeefy, A.M.; El-Araby, M.E. New quinoxalinone inhibitors targeting secreted phospholipase A2 and α-glucosidase. J. Enzyme Inhib. Med. Chem. 2017, 32, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Cotrim, C.A.; de Oliveira, S.C.B.; Diz Filho, E.B.S.; Fonseca, F.V.; Baldissera, L.; Antunes, E.; Ximenes, R.M.; Monteiro, H.S.A.; Rabello, M.M.; Hernandes, M.Z.; et al. Quercetin as an inhibitor of snake venom secretory phospholipase A2. Chem. Biol. Interact. 2011, 189, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.V.; Tresoldi, I.; Modesti, A.; Bei, R. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, J.; Ahn, H.S.; Cheong, J.H.; dela Peña, I. Natural product-derived treatments for attention-deficit/hyperactivity disorder: Safety, efficacy, and therapeutic potential of combination therapy. Neural Plast. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- LigPrep; Schrodinger, LLC: New York, NY, USA, 2017.
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein–ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Protein Eng. Des. Sel. 1995, 8, 127–134. [Google Scholar] [CrossRef]
- De Araújo, A.L.; Radvanyi, F. Determination of phospholipase A2 activity by a colorimetric assay using a pH indicator. Toxicon 1987, 25, 1181–1188. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
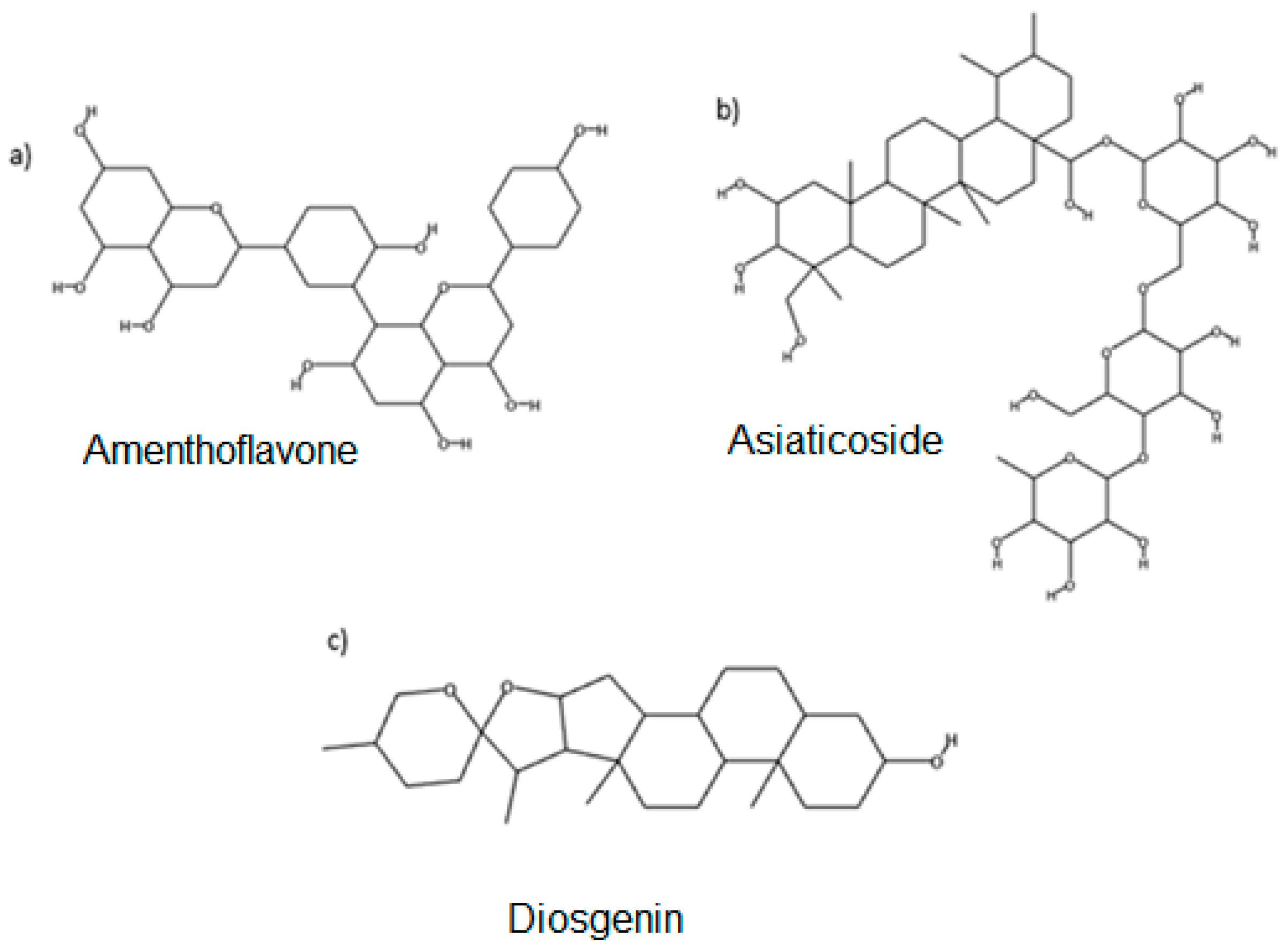


| Rank | Compound Name | Binding Affinity (∆G) (kcal/mol) | Plant |
|---|---|---|---|
| 1 | Α-Viniferin | −11.3 | Shorea gibbosa |
| 2 | Gnetin E | −11.3 | Gnetum gnemonoside |
| 3 | Isorhoifolin | −11.1 | Theobroma cacao |
| 4 | Lupenone | −11.0 | Murraya paniculata |
| 5 | Amenthoflavone | −11.0 | Garcinia prainiana |
| 6 | Diosgenin | −10.8 | Costus speciousus |
| 7 | Echinocyctic acid | −10.7 | Eclipta prostata |
| 8 | Smilagenin | −10.7 | Trigonella foenum |
| 9 | Asiaticoside | −10.7 | Centella asiatica |
| 10 | Friedelin | −10.6 | Lawsonia inermis |
| Compound | Dm (mg/mL) a | m b | r c |
|---|---|---|---|
| Bromelain | 0.0723 | 0.6767 ± 0.0450 | 0.9879 |
| Amenthoflavone | 0.1079 | 0.4480 ± 0.0489 | 0.9968 |
| Asiaticoside | 0.3143 | 0.5258 ± 0.0381 | 0.9846 |
| Diosgenin | 0.9659 | 0.7018 ± 0.0229 | 0.9659 |
| Bromelain (Br) + amenthoflavone (Am) | 0.6093 (Br = 0.2437 mg/mL) (Am = 0.3655mg/mL) | - | - |
| Bromelain (Br) + asiaticoside (As) | 0.311 (Br = 0.020 mg/mL) (As = 0.2911 mg/mL) | - | - |
| Bromelain (Br) + diosgenin (Di) | 3.0603 (Br = 0.1500 mg/mL) (Di = 2.9103 mg/mL) | - | - |
| Compound Combination | DRI Value + | |||
|---|---|---|---|---|
| IC50 | IC75 | IC90 | IC95 | |
| Bromelain (Br) + amenthoflavone (Am) | 0.29 (Br) 0.29 (Am) | 0.48 (Br) 0.44 (Am) | 0.76 (Br) 0.68 (Am) | 1.06 (Br) 0.90 (Am) |
| Bromelain (Br) + asiaticoside (As) | 1.14 (Br) 1.07 (As) | 2.36 (Br) 3.55 (As) | 4.87 (Br) 11.7 (As) | 8.00 (Br) 26.35 (As) |
| Bromelain (Br) + diosgenin (Di) | 0.84 (Br) 0.70 (Di) | 0.77 (Br) 2.58 (Di) | 1.22 (Br) 9.42 (Di) | 1.68 (Br) 22.73 (Di) |
| Compounds | Concentration (mg/mL) | ||||
|---|---|---|---|---|---|
| 2.5 IC50 | 2 IC50 | 1 IC50 | 0.5 IC50 | 0.2 IC50 | |
| Bromelain | 0.1808 | 0.1446 | 0.0723 | 0.03615 | 0.01445 |
| Amenthoflavone | 0.2698 | 0.2158 | 0.1079 | 0.0540 | 0.0216 |
| Asiaticoside | 0.7858 | 0.6286 | 0.3143 | 0.1572 | 0.0628 |
| Diosgenin | 2.4148 | 1.9308 | 0.9659 | 0.4829 | 0.1932 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed Tap, F.; Abd Majid, F.A.; Ismail, H.F.; Wong, T.S.; Shameli, K.; Miyake, M.; Ahmad Khairudin, N.B. In Silico and In Vitro Study of the Bromelain-Phytochemical Complex Inhibition of Phospholipase A2 (Pla2). Molecules 2018, 23, 73. https://doi.org/10.3390/molecules23010073
Mohamed Tap F, Abd Majid FA, Ismail HF, Wong TS, Shameli K, Miyake M, Ahmad Khairudin NB. In Silico and In Vitro Study of the Bromelain-Phytochemical Complex Inhibition of Phospholipase A2 (Pla2). Molecules. 2018; 23(1):73. https://doi.org/10.3390/molecules23010073
Chicago/Turabian StyleMohamed Tap, Fatahiya, Fadzilah Adibah Abd Majid, Hassan Fahmi Ismail, Tet Soon Wong, Kamyar Shameli, Mikio Miyake, and Nurul Bahiyah Ahmad Khairudin. 2018. "In Silico and In Vitro Study of the Bromelain-Phytochemical Complex Inhibition of Phospholipase A2 (Pla2)" Molecules 23, no. 1: 73. https://doi.org/10.3390/molecules23010073





