Recyclable Keggin Heteropolyacids as an Environmentally Benign Catalyst for the Synthesis of New 2-Benzoylamino-N-phenyl-benzamide Derivatives under Microwave Irradiations at Solvent-Free Conditions and the Evaluation of Biological Activity
Abstract
:1. Introduction
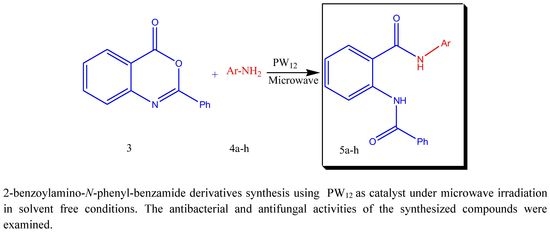
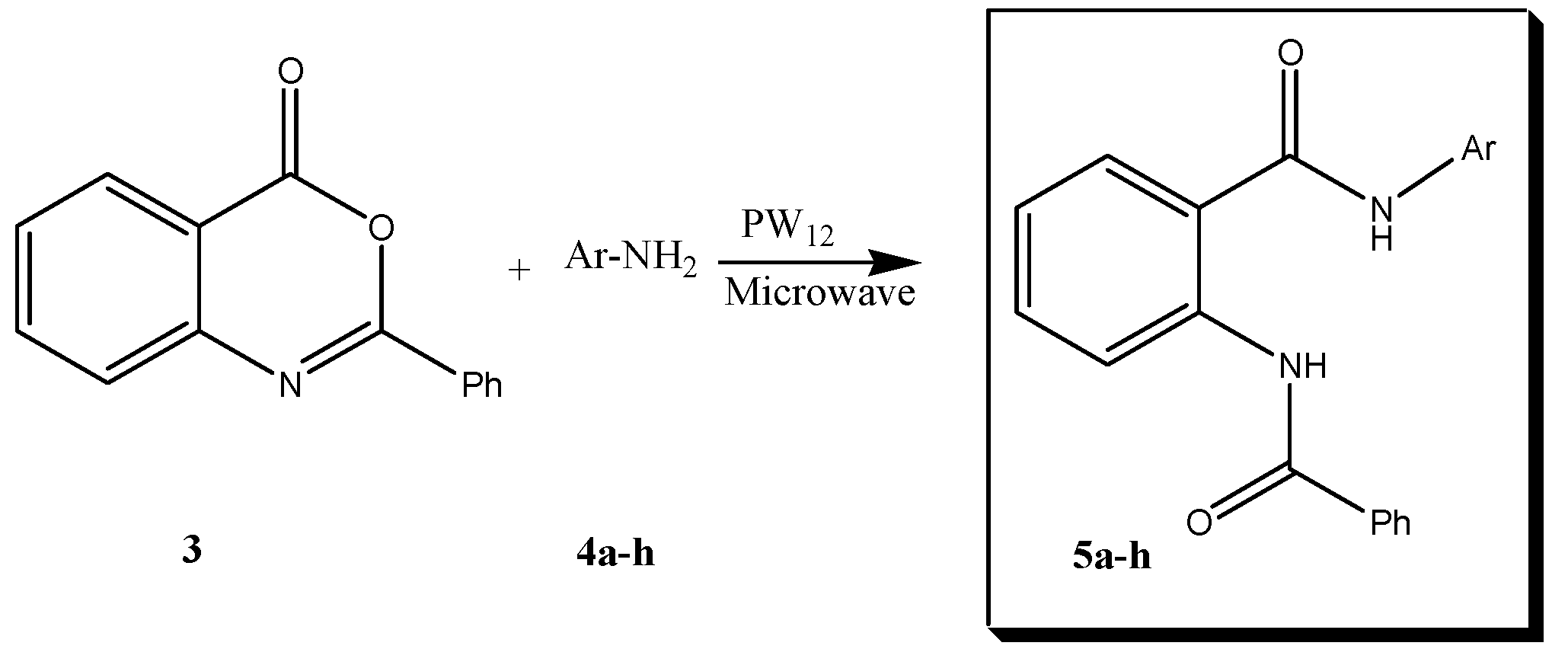
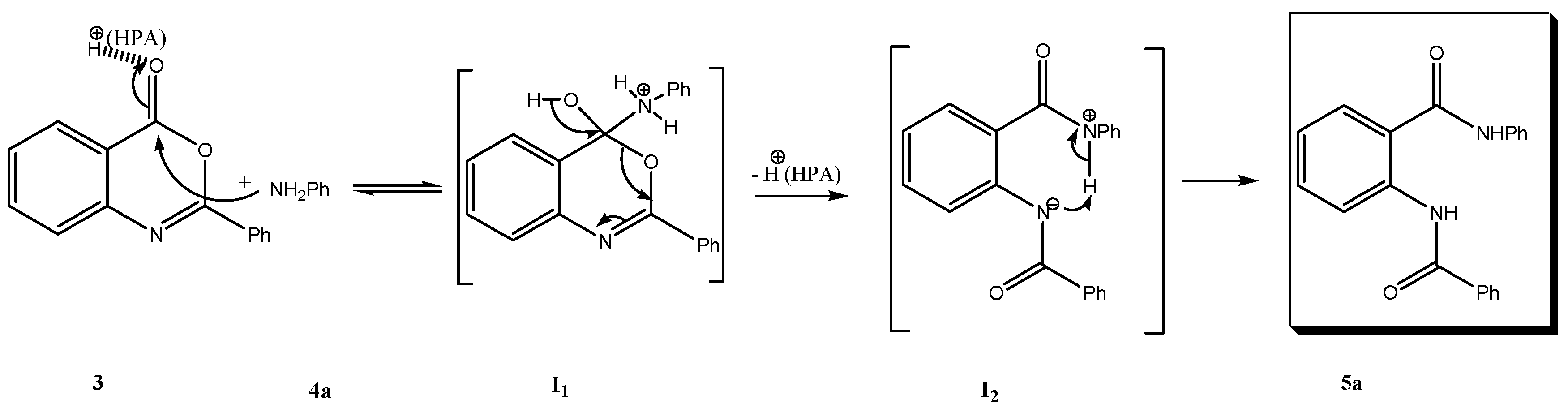
2. Results and Discussion
3. Antibacterial, Antifungal of the Synthesized Compounds
4. Conclusions
5. Experimental Section
5.1. General
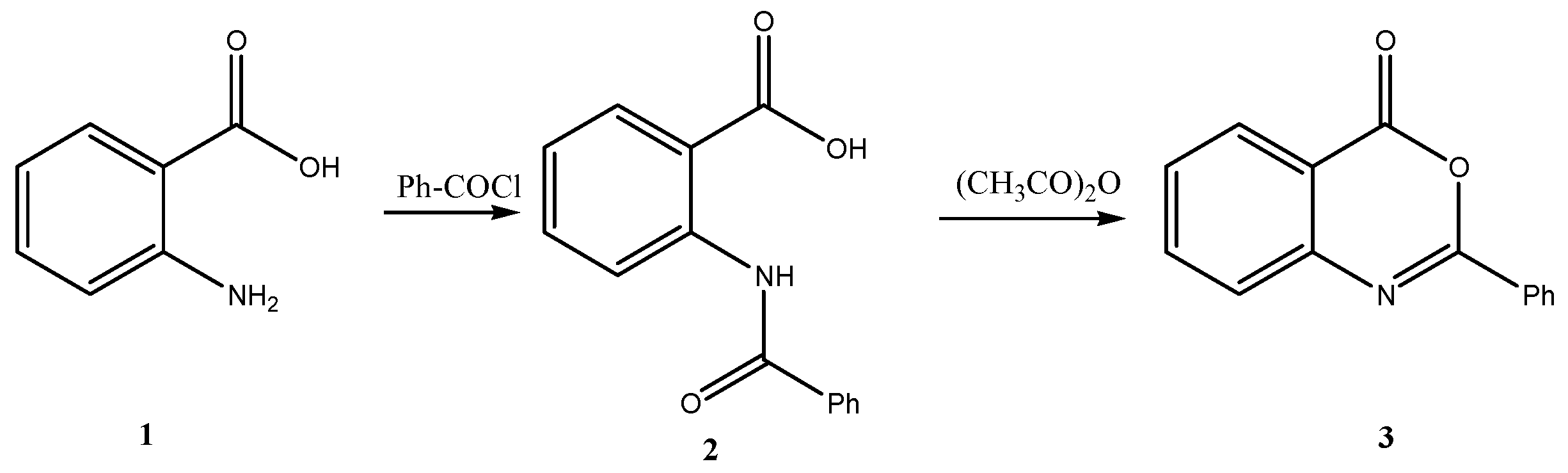
5.2. General Procedure for the Preparation of 2-Phenyl-3,1-(4H)-benzoxazin-4-one 3
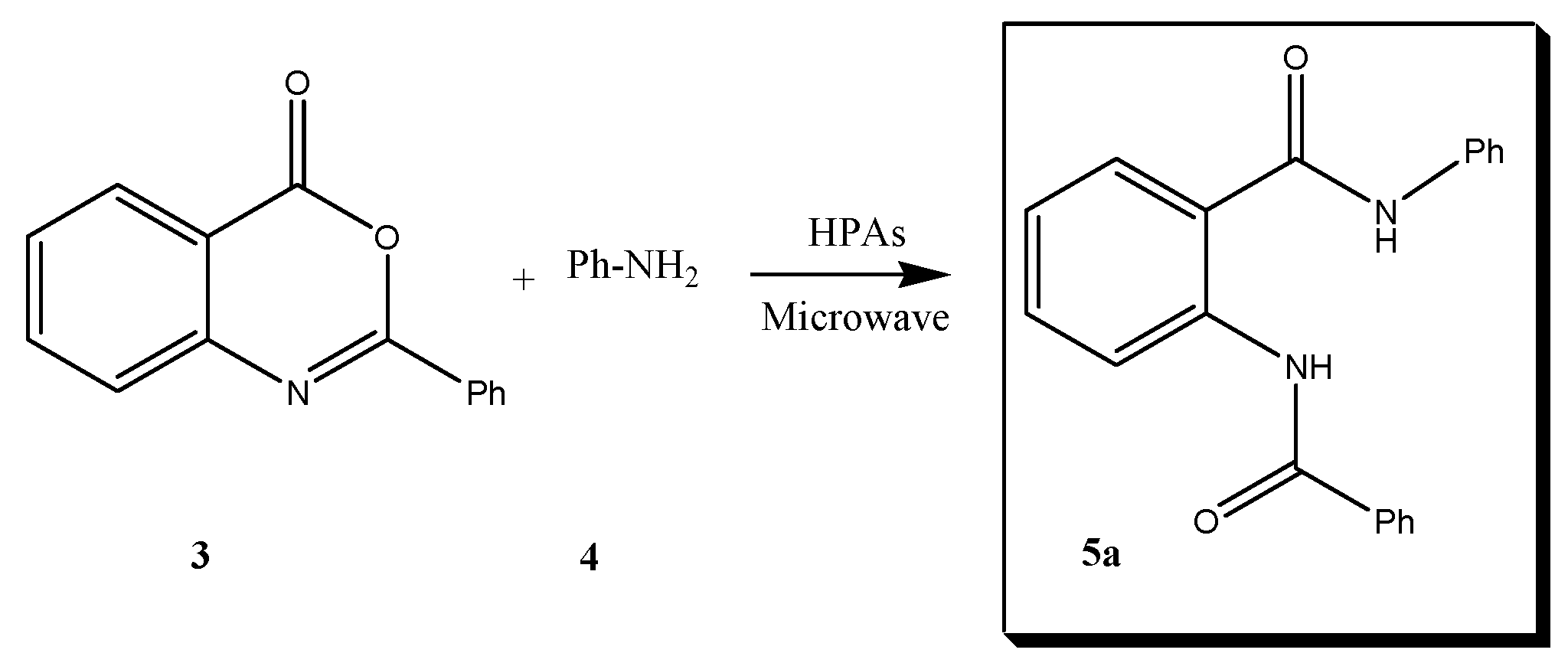
5.3. General Procedure for the Preparation of 2-Benzoylamino-N-phenylbenzamide Derivatives 5a–h
5.4. Screening for Antibacterial Activity by the Agar Diffusion Method for 2-Benzoylamino-N-phenylbenzamide Derivatives 5a–h
5.5. Minimum Inhibitory Concentration Determination of the Compound 5e
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sarma, R.; Prajapati, D.; Boruah, R.C. Green chemistry-A new approach in organic synthesis. Sci. Cult. 2011, 77, 461–465. [Google Scholar]
- Maher, A.E.; Khalid, M.D.; Sameh, A.R.; Fakhry, A.E. The Uses of 2-Ethoxy-(4H)-3,1-benzoxazin-4-onein the Synthesis of Some Quinazolinone Derivatives of Antimicrobial Activity. Pharmaceuticals 2011, 4, 1032–1051. [Google Scholar]
- Saurav, K.; Garima, M.; Pradeep, S.; Jha, K.; Khosa, R.; Gupta, S. Quinazoline-4-one: A highly important the heterocycle with diverse biological activities. Der Chem. Sin. 2011, 2, 36–58. [Google Scholar]
- Parkanyi, C.; Yuan, H.L.; Stromberg, B.H.E.; Evenzahav, A. Synthesis of 5-fluoro-2 -methyl- 3-(2-trifluoro methyl-1,3,4-thiadiazol-5-yl)-4(3H)-quinazolinone and related compounds with potential antiviral and anticancer activities. J. Heterocycl. Chem. 1992, 29, 749–753. [Google Scholar] [CrossRef]
- Abbas, E.S.; Awadallah, M.F.; Ibrahim, A.N.; Said, G.E.; Kamel, M.G. New quinazolinone-pyrimidine hydrids: Synthesis, anti-inflammatory and ulceronicity studies. Eur. J. Med. Chem. 2012, 53, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Sicker, D.; Schulz, M. Benzoxazinones in plants: Occurrence, synthetic access, and biological activity. In Studies of Natural Product Chemistry; Atta-ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Erusalimsky, J.D.; Franklin, R. Is the platelet lowering activity of anagrelide mediated by its major metabolit 2-amino-5,6-dichloro-3,4-dihydroquinazoline (RL603)? Exp. Hematol. 2002, 30, 625–626. [Google Scholar] [CrossRef]
- Farghaly, A.M.; Soliman, R.; Khalil, M.A.; Bekhit, A.A.; El-Din, A. Hioglycolic acid and pyrazole derivatives of 4(3H)-quinazolinone: Synthesis and antimicrobial evaluation. Boll. Chim. Farm. 2002, 141, 372–378. [Google Scholar] [PubMed]
- Blackburn, C.; Lamorche, M.J.; Brown, J. Identification and characterization of amino piperidinequinolones and quinazolinones as MCHr 1antagonists. Bioorg. Med. Chem. Lett. 2006, 16, 2621–2627. [Google Scholar] [CrossRef] [PubMed]
- Sayed, H.M.; Hamed, A.A.; Madkour, H.M.F.; Shiba, S.A. Utility of 3-(4-Methoxy phenyl) and/or (2-Thinyl)-2-cyano-2-propenoyl chloride in heterocyclic synthesis. Sulfur Lett. 2001, 24, 151–179. [Google Scholar]
- Al-Obaid, A.M.; Abdel-Hamide, S.G.; El-Kashef, H.A.; Abdel-Aziz, A.M.; El-Azab, A.S. Substituted quinazolines, Part 3. Synthesis, in vitro antitumor activity and molecular modeling study of certain 2-thieno-4(3H)-quinazolinone analogs. Eur. J. Med. Chem. 2009, 44, 2379–2391. [Google Scholar] [CrossRef] [PubMed]
- Aly, M.M.; Mohamed, Y.A.; El–Bayouki, K.A.M.; Basyaouni, W.M.; Abbas, S.Y. Synthesis of some 4(3H)-quinazolinone-2-carboxaldehyde thiosemicarbazones and their metal complexes and a study on their anticonvulsant, analgesic, cytoxic and antimicrobial activities Part-1. Eur. J. Med. Chem. 2010, 45, 3365–3373. [Google Scholar] [CrossRef] [PubMed]
- Chandrika, P.M.; Yakaiah, T.; Ramrao, A.R.; Rao, J.V. Synthesis of novel 4,6-disubstituted quinazoline derivatives, their anti-inflammatory and anti-cancer activity (cytotoxic) against U937 leukemia cell lines. Eur. J. Med. Chem. 2008, 43, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.P.; Kumar, A.; Sharma, S.; Shukla, P.K.; Nath, M. Aneco-friendly synthesis and antimicrobial activities of dihydro-2H-benzo-and naphtho-1,3-oxazine derivatives. Eur. J. Med. Chem. 2010, 45, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, A.K.; Sayd, A.R.N.; Sohail, A.S.; Nasir, M.; Muhammad, Y.; Ameer, F.Z. Synthesis and Antimicrobial Activity of 2-Aryl-4H-3,1-benzoxazin-4-ones. Asian. J. Chem. 2013, 25, 152–156. [Google Scholar]
- Kaniskan, N.; Kokten, S.; Celik, I. A new protocol for the synthesis of primary, secondary and tertiary anthranilamides utilizing N-(2-aminoarylacyl)benzotriazoles. Arkivok 2012, 8, 198–213. [Google Scholar]
- Alyaa, A.S.; Abdel Momen, A.E.; Shiba, S.A.; Abdel, A.A.E. Synthesis and antifungal activity of some new quinazoline and benzoxazinone derivatives. Arch. Pharm. Med. Chem. 2000, 333, 365–372. [Google Scholar]
- Héctor, R.B.; Waldo, L. Antialga and Antifungal Activity of Natural Hydroxamic Acids and Related Compounds. J. Agric. Food Chem. 1996, 44, 1569–1571. [Google Scholar]
- Héctor, R.B.; Sylvia, V.C.; Waldo, L. Antimicrobial activity of natural 2-benzoxazolinones and related derivatives. J. Agric. Food Chem. 1997, 45, 3255–3257. [Google Scholar]
- Arfan, M.; Khan, R.; Imran, M.; Khan, H.; Mehmood, J. One-pot synthesis and antimicrobial activities of some 2-aryl/alkyl, 3-aminoquinazolin-4(3H)-ones. J. Chem. Soc. Pak. 2008, 30, 229–305. [Google Scholar]
- Hsieh, P.; Chong, F.; Chang, C.; Zheng, F.; Lin, K.H. 2-Substituted benzoxazinone analogues as anti-human Corona virus (anti-HcoV) and ICAM-1 expression inhibition agents. Bioorg. Med. Chem. Lett. 2004, 14, 4751–4754. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, G.R.; Chakrabarti, R.; Reddy, K.A.; Rajesh, B.M.; Rao, P.B.; Rajagopalan, R.; Iqbal, J. Dual PPAR-α and -γ activators derived from novel benzoxazinone containing thiazolidinediones having antidiabetic and hypolipidemic potential. Bioorg. Med. Chem. 2006, 14, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Nachiket, S.D.; Pankaj, S.S.; Ravindra, B.L.; Santosh, B.D.; Deepak, S.M. Design, synthesis and evaluation of acute toxicity studies and anti-depressant activities of some new derivatives of 1,3-benzoxazin-4-one. Int. J. Pharm. Chem. 2015, 5, 158–165. [Google Scholar]
- Colson, E.; Wallach, J.; Hauteville, M. Biochimie, Synthesis and anti-elastase properties of 6-amino-2-phenyl-4H-3,1-benzoxazin-4-one aminoacyl and dipeptidyl derivatives. Biochimie 2005, 87, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Pei-Wen, H.; Tsong-Long, H.; Chin-Chung, W.; Fang-Rong, C.; Tsai-Wei, W.; Yang-Chang, W. The evaluation of 2,8-disubstituted benzoxazinone derivatives as anti-inflammatory and anti-platelet aggregation agents. Bioorg. Med. Chem. Lett. 2005, 15, 2786–2789. [Google Scholar]
- Habib, O.M. O.; Hassan, H.M.; El-Mekabaty, A. Novel quinazolinone derivatives: Synthesis and anti-microbial activity. Med. Chem. Res. 2013, 22, 507–519. [Google Scholar] [CrossRef]
- Habib, O.M.O.; Hassan, H.M.; El-Mekabaty, A. Studies on Some Benzoxazine-4-one Derivatives with Potential Biological Activity. Am. J. Org. Chem. 2012, 2, 45–51. [Google Scholar] [CrossRef]
- Misono, M. Heterogeneous Catalysis by Heteropoly Compounds of Molybdenum and Tungsten. Catal. Rev. 1987, 29, 269–321. [Google Scholar] [CrossRef]
- Kaoua, R.; Bennamane, N.; Bakhta, S.; Benadji, S.; Rabia, C.; Nedjar-Kolli, B. Synthesis of substituted 1,4-diazepines and 1,5-benzodiazepines using an efficient heteropolyacid-catalyzed procedure. Molecules 2011, 16, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Ighilahriz, K.; Boutemeur, B.; Chami, F.; Rabia, C.; Hamdi, M.; Hamdi, S.M. A microwave-assisted and heteropolyacids-catalysed cyclocondensation reaction for the synthesis of 4(3H)-quinazolinones. Molecules 2008, 13, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Hedidi, M.; Hamdi, S.M.; Mazari, T.; Boutemeur, B.; Rabia, C.; Chemat, F.; Hamdi, M. Microwave-assisted synthesis of calix [4] resorcinarenes. Tetrahedron 2006, 62, 5652–5655. [Google Scholar] [CrossRef]
- Saher, L.; Makhloufi-Chebli, M.; Dermeche, L.; Boutemeur-Khedis, B.; Rabia, C.; Silva, A.M.S.; Hamdi, M. Keggin and dawson-type polyoxometalates and efficient catalysts for the synthesis 3,4-dihydropyrimidinones: Experimental and theoretical studies. Tetrahedron Lett. 2016, 57, 1492–1496. [Google Scholar] [CrossRef]
- Shariat, M.; Samsudin, M.W.; Zakaria, Z. One-pot synthesis of 2-substituted 4H-3,1-benzoxazin-4-one derivatives under mild conditions using iminium cation from cyanuric chloride/dimethylformamide as a cyclizing agent. Chem. Cent. J. 2013, 1–6, 7–58. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, M.N. Acid catalysis by heteropoly acids. Appl. Catal. A 2003, 256, 19–35. [Google Scholar] [CrossRef]
- Doley, P.; Jha, D.K. Antimicrobial activity of bacterial endophytes from medicinal endemic plant Garcinia Lancifolia Roxb. Ann. Plant Sci. 2016, 4, 1243–1247. [Google Scholar]
- Jorgensen, J.H.; Turnidge, J.D. Susceptibility test methods: Dilution and disk diffusion methods. In Manual of Clinical Microbiology; ASM Press: Washington, DC, USA, 2015. [Google Scholar]
- Nascimento, G.G.F.; Locatelli, J.; Freitas, P.C.; Silva, G.L. Antibacterial activity of plant extracts and phytochemicals on antibiotic- resistant bacteria. Br. J. Microbiol. 2000, 31, 247–256. [Google Scholar] [CrossRef]
- Pope, M.T. Heteropoly and isopolyoxometallates. In Inorganic Chemistry Concepts; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]
- Nüchter, M.; Ondruschka, B.; Bonrath, W.; Gum, A. Microwave assisted synthesis—A critical technology overview. Green Chem. 2004, 6, 128–141. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 5a–h are available from the authors. |
| Catalysts | PW12 | SiW12 | PMo12 | SiMo12 |
|---|---|---|---|---|
| Yields (%) | 80 | 72 | 65 | 56 |
| Products | ArNH2 (4a–h) | Yield (%) | M.p. (°C) | T (°C) a |
|---|---|---|---|---|
| 5a | C6H5 | 80 | 281–282 | 151 |
| 5b | 4-Me-C6H4 | 85 | 123–124 | 155 |
| 5c | 4-OH-C6H4 | 91 | 160–163 | 155 |
| 5d | 4-Cl-C6H4 | 77 | 161–162 | 160 |
| 5e | 2,4-Cl2-C6H3 | 73 | 140–142 | 106 |
| 5f | 2,5-Cl2-C6H3 | 67 | 167–168 | 105 |
| 5g | 2,6-Cl2-C6H3 | 65 | 162–164 | 121 |
| 5h | 3,4-Cl2-C6H3 | 70 | 192–193 | 124 |
| Compounds | Bacteria | Fungi | ||||
|---|---|---|---|---|---|---|
| E. coli | S. aureus | P. aeruginosa | B. subtilis | C. albicans | A. brasiliensis | |
| 5a | ++ | - | +++ | + | +++ | +++ |
| 5b | + | + | ++ | ++ | +++ | +++ |
| 5c | ++ | ++ | ++ | ++ | +++ | +++ |
| 5d | ++ | +++ | ++ | +++ | +++ | +++ |
| 5e | ++ | +++ | +++ | +++ | +++ | +++ |
| 5f | ++ | + | ++ | - | +++ | +++ |
| 5g | ++ | ++ | ++ | ++ | +++ | +++ |
| 5h | ++ | + | ++ | ++ | +++ | +++ |
| Concentration (µg/mL) | Bacteria | Fungi | ||||
|---|---|---|---|---|---|---|
| E. coli | S. aureus | P. aeruginosa | B. subtilis | C. albicans | A. brasiliensis | |
| 600 | ++ | + | + | ++ | +++ | +++ |
| 400 | + | + | + | ++ | +++ | + |
| 300 | + | + | + | + | +++ | - |
| 200 | + | + | + | + | ++ | - |
| 100 | + | - | + | + | ++ | - |
| MIC | ≤100 | 100–200 | ≤100 | ≤100 | ≤100 | 300–400 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ighilahriz-Boubchir, K.; Boutemeur-Kheddis, B.; Rabia, C.; Makhloufi-Chebli, M.; Hamdi, M.; Silva, A.M.S. Recyclable Keggin Heteropolyacids as an Environmentally Benign Catalyst for the Synthesis of New 2-Benzoylamino-N-phenyl-benzamide Derivatives under Microwave Irradiations at Solvent-Free Conditions and the Evaluation of Biological Activity. Molecules 2018, 23, 8. https://doi.org/10.3390/molecules23010008
Ighilahriz-Boubchir K, Boutemeur-Kheddis B, Rabia C, Makhloufi-Chebli M, Hamdi M, Silva AMS. Recyclable Keggin Heteropolyacids as an Environmentally Benign Catalyst for the Synthesis of New 2-Benzoylamino-N-phenyl-benzamide Derivatives under Microwave Irradiations at Solvent-Free Conditions and the Evaluation of Biological Activity. Molecules. 2018; 23(1):8. https://doi.org/10.3390/molecules23010008
Chicago/Turabian StyleIghilahriz-Boubchir, Karima, Baya Boutemeur-Kheddis, Cherifa Rabia, Malika Makhloufi-Chebli, Maamar Hamdi, and Artur M. S. Silva. 2018. "Recyclable Keggin Heteropolyacids as an Environmentally Benign Catalyst for the Synthesis of New 2-Benzoylamino-N-phenyl-benzamide Derivatives under Microwave Irradiations at Solvent-Free Conditions and the Evaluation of Biological Activity" Molecules 23, no. 1: 8. https://doi.org/10.3390/molecules23010008