Immunosuppressive Effect of Geniposide on Mitogen-Activated Protein Kinase Signalling Pathway and Their Cross-Talk in Fibroblast-Like Synoviocytes of Adjuvant Arthritis Rats
Abstract
:1. Introduction
2. Results
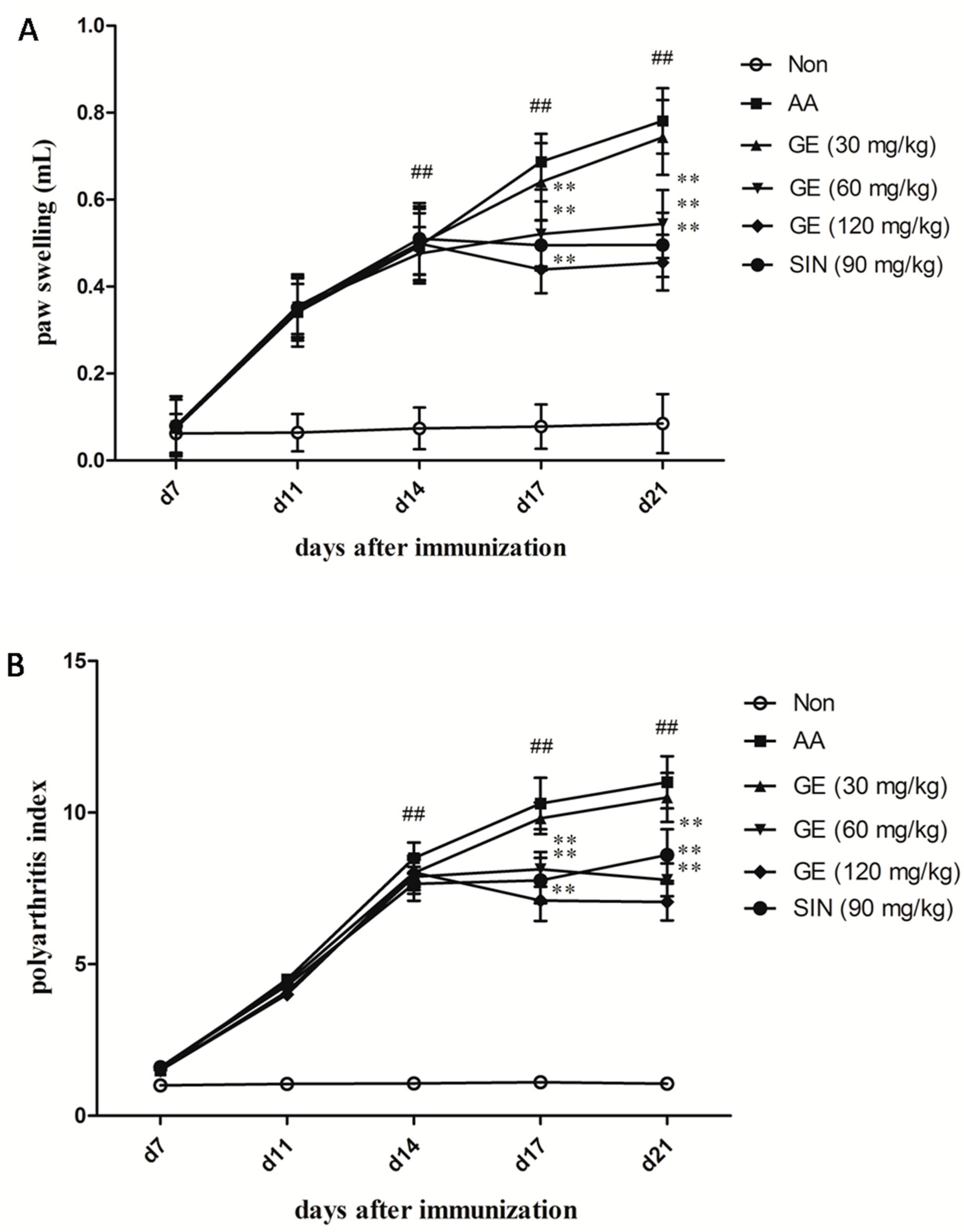
2.1. Evaluation of Arthritis on AA Rats
2.2. The Effects of GE on the Joint Histopathology of AA Rats
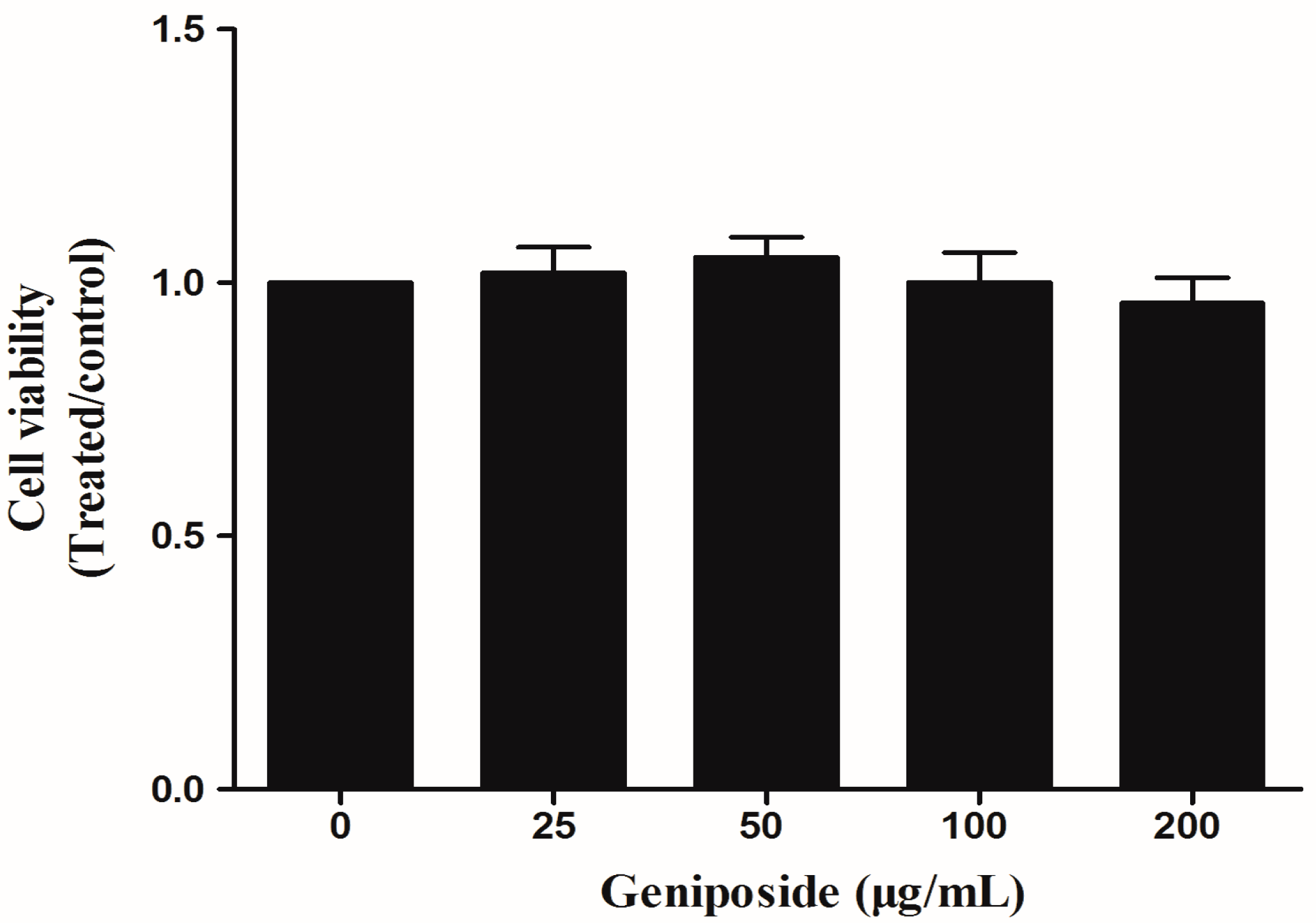
2.3. The Effect of GE on FLS Viability
2.4. Effects of GE on the Proliferation of FLS
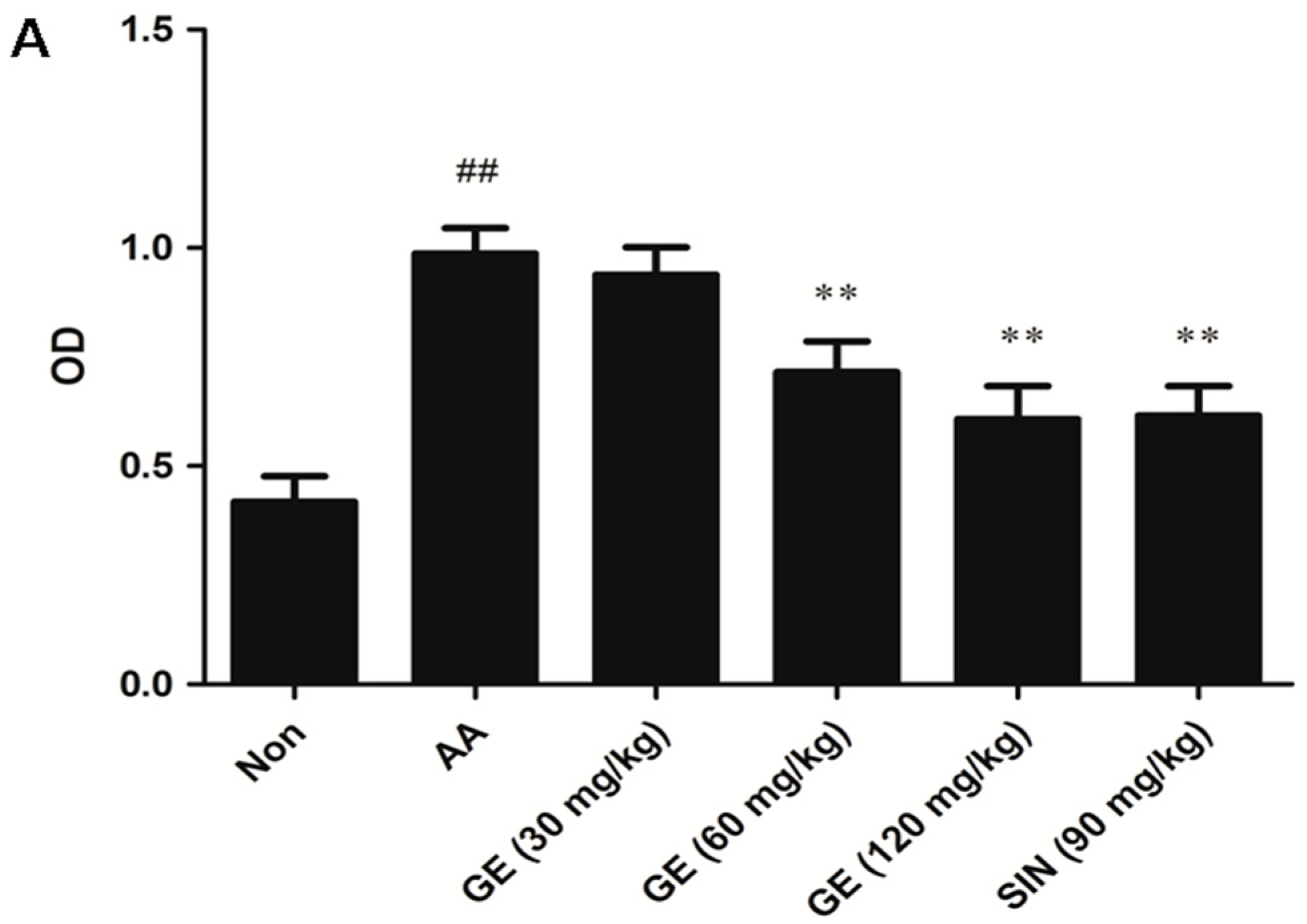
2.4.1. Effects of GE on the Proliferation of FLS In Vivo
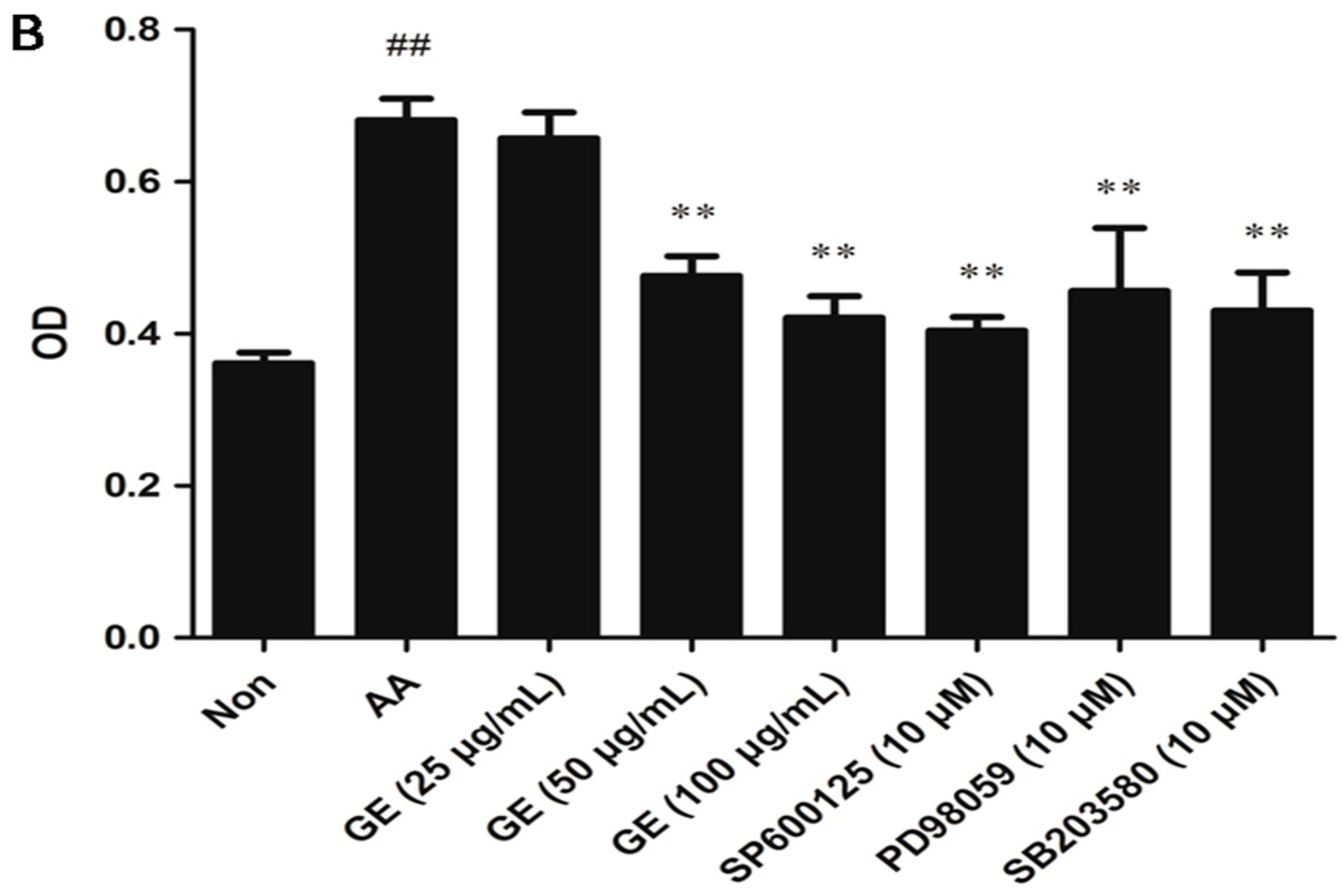
2.4.2. Effects of GE on the Proliferation of FLS In Vitro
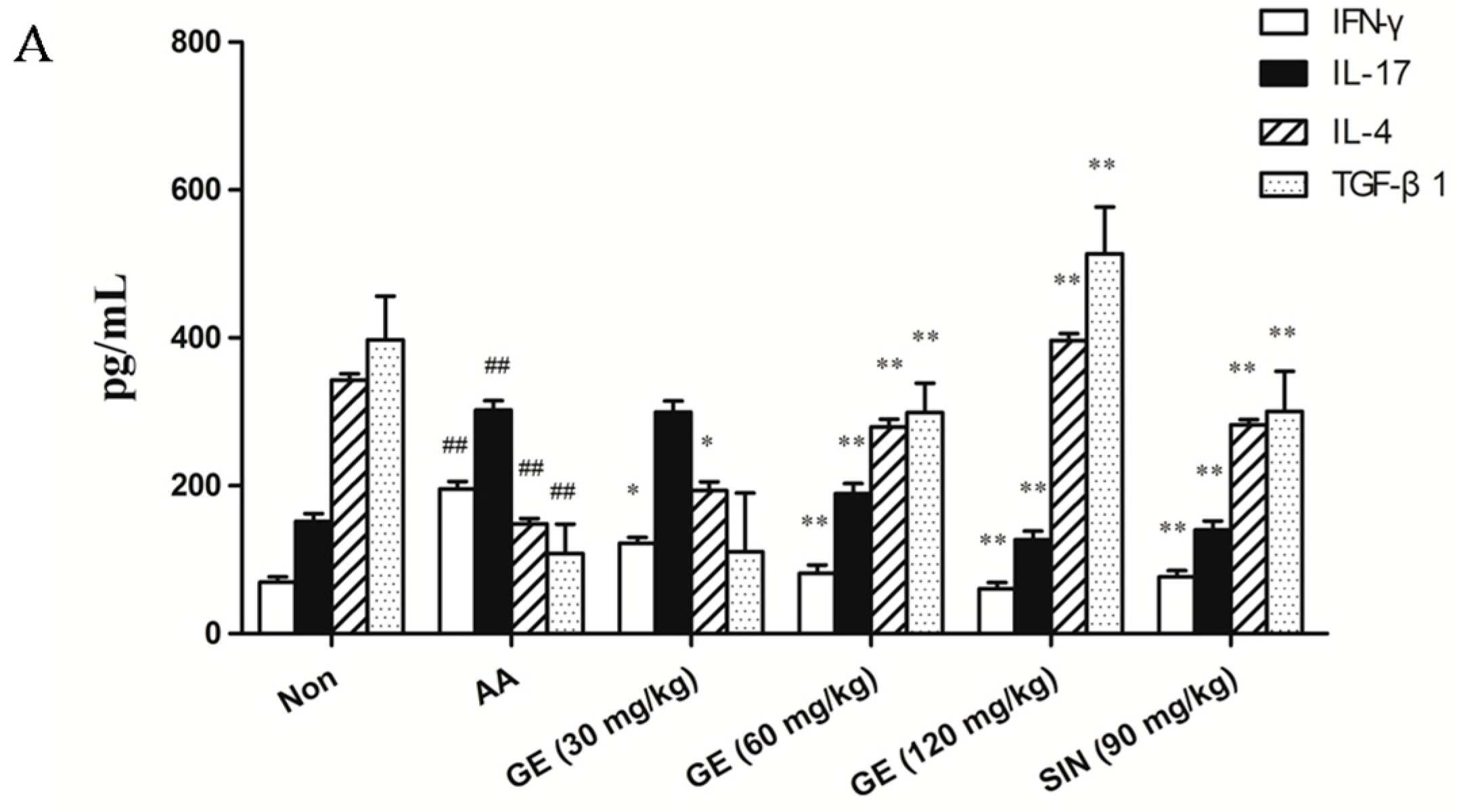
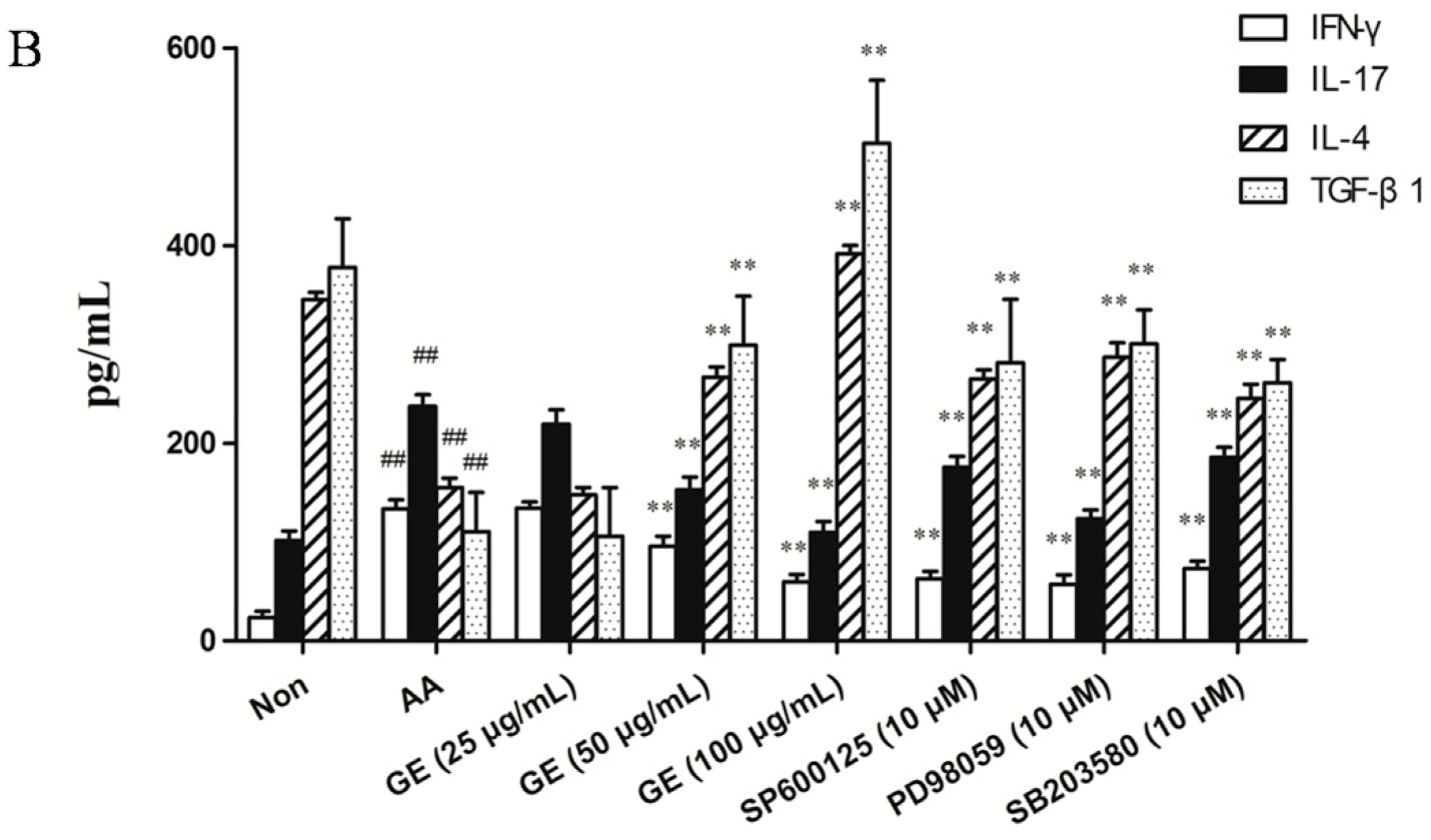
2.5. Effects of GE on Production of IFN-γ, IL-17, IL-4, and TGF-β1 in FLS
2.5.1. Effects of GE on Production of IFN-γ, IL-17, IL-4, and TGF-β1 in FLS In Vivo
2.5.2. Effects of GE on Production of IFN-γ, IL-17, IL-4, and TGF-β1 in FLS In Vitro
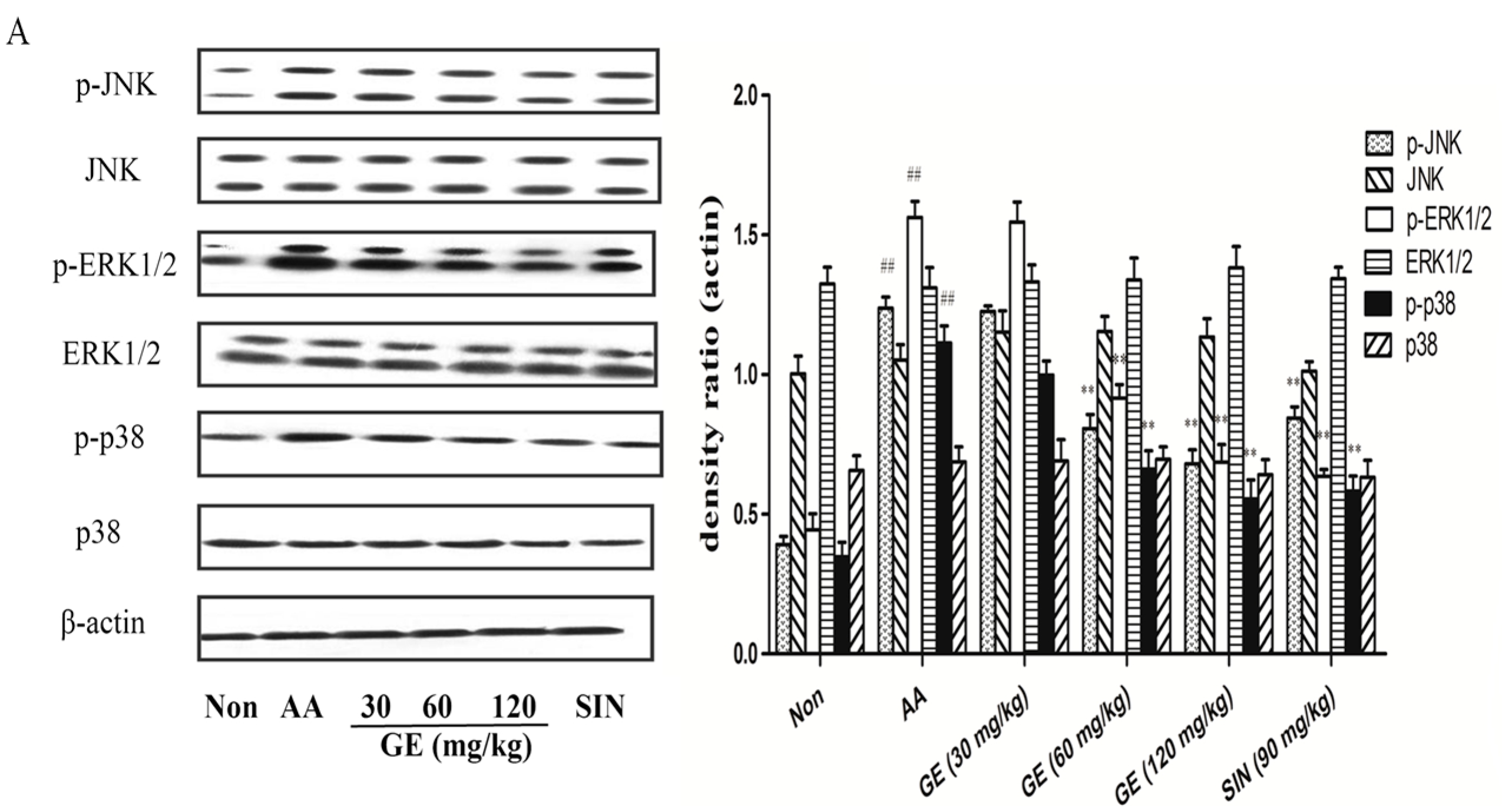
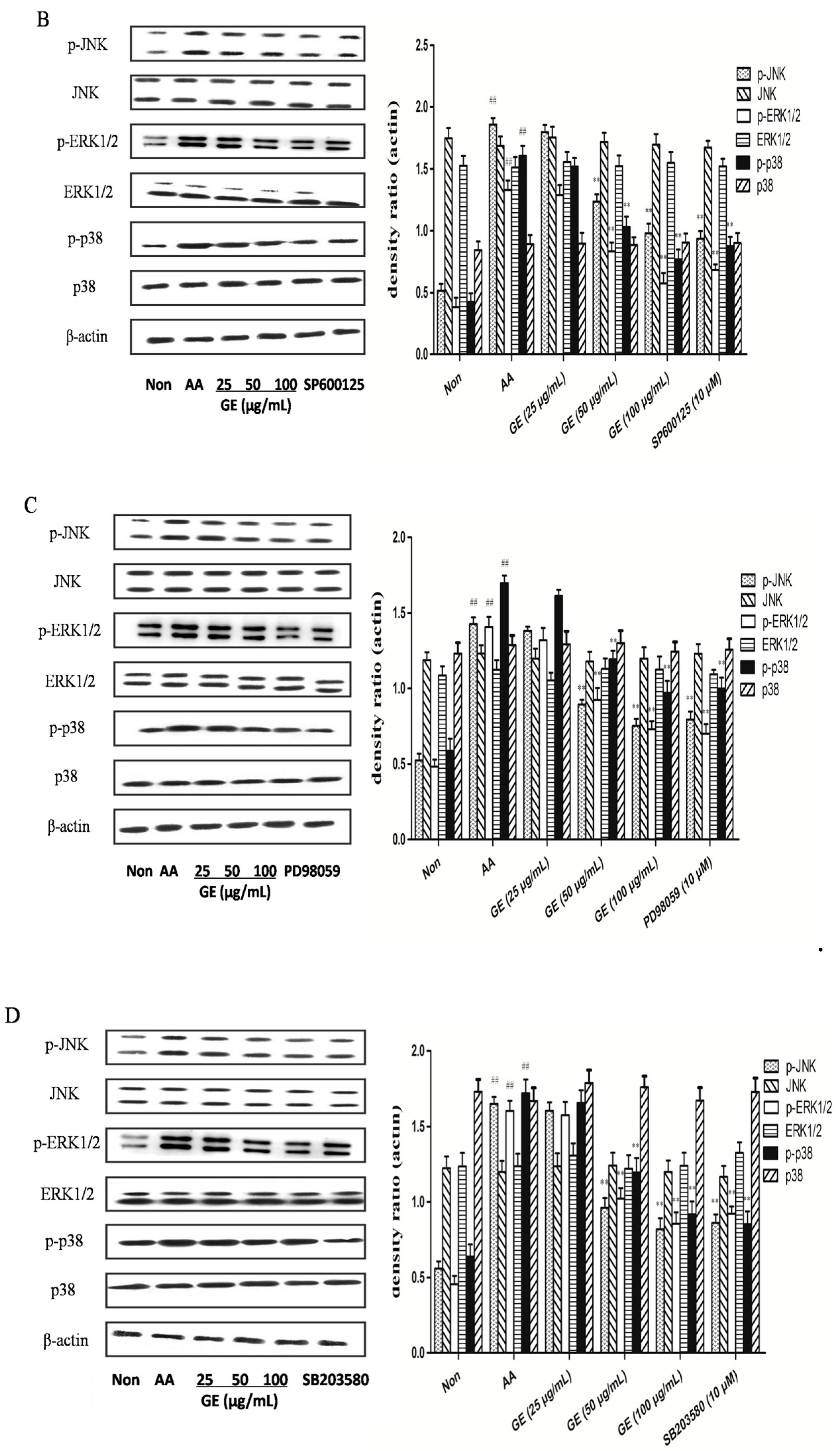
2.6. Effects of GE on MAPK Pathways and Cross-Talk among MAPK Pathways in FLS
2.6.1. Effects of GE on MAPK Pathways and Cross-Talk among MAPK Pathways in FLS In Vivo
2.6.2. Effects of GE on MAPK Pathways and Cross-Talk among MAPK Pathways in FLS In Vitro
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Reagents
4.3. Experimental Design
4.4. Arthritis Assessment
4.5. Histopathological Analysis
4.6. CCK8 Assay for FLS Viability
4.7. Proliferation Assay by MTT
4.8. Measurement of IFN-γ, IL-17, IL-4, and TGF-β1
4.9. Western Blot Analysis
4.9.1. Separation of Key Proteins from FLS In Vivo and In Vitro
4.9.2. Western Blotting
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhalekar, M.R.; Upadhaya, P.G.; Madgulkar, A.R. Fabrication and efficacy evaluation of chloroquine nanoparticles in CFA-induced arthritic rats using TNF-α ELISA. Eur. J. Pharm. Sci. 2016, 84, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jennifer, N. Treatment of rheumatoid arthritis: A review of recommendations and emerging therapy. Formulary 2011, 46, 532–545. [Google Scholar]
- Filer, A. The fibroblast as a therapeutic target in rheumatoid arthritis. Curr. Opin. Pharmacol. 2013, 13, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Chen, G. Immunotherapy of Rheumatoid Arthritis Targeting Inflammatory Cytokines and Autoreactive T Cells. Arch. Immunol. Ther. Exp. 2010, 58, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Scian, R.; Barrionuevo, P.; Giambartolomei, G.H.; De Simone, E.A.; Vanzulli, S.I.; Fossati, C.A.; Baldi, P.C.; Delpino, M.V. Potential role of fibroblast-like synoviocytes in joint damage induced by Brucella abortus infection through production and induction of matrix metalloproteinases. Infect. Immunity 2011, 79, 3619–3632. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yao, Y.; Ding, C.Z. A combination of Sinomenine and Methotrexate reduces joint damage of collagen induced arthritis in rats by modulating osteoclast-related cytokines. Int. Immunopharmacol. 2014, 18, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Cai, Q.R.; He, J.P.; Chu, X.; Wei, M.M.; Feng, X.R.; Xie, X.X.; Huo, M.X.; Liu, J.; Wei, J.Y.; et al. Geniposide, an Iridoid Glucoside Derived from Gardenia jasminoides, Protects against Lipopolysaccharide-Induced Acute Lung Injury in Mice. Planta Medica 2012, 78, 557–564. [Google Scholar]
- Fu, Y.H.; Liu, B.; Liu, J.H.; Liu, Z.C.; Liang, D.J.; Li, F.Y.; Li, D.P.; Cao, Y.G.; Zhang, X.C.; Zhang, N.S.; et al. Geniposide, from Gardenia jasminoides Ellis, inhibits the inflammatory response in the primary mouse macrophages and mouse models. Int. Immunopharmacol. 2012, 14, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.F.; Wu, S.Y.; Xu, W.; Jin, H.; Zhu, Z.G.; Li, Z.H.; Tian, Y.X.; Zhang, J.J.; Rao, J.J.; Wu, S.G. Geniposide inhibits high glucose-induced cell adhesion through the NF-κB signaling pathway in human umbilical vein endothelial cells. Acta Pharmacol. Sin. 2010, 31, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Teramto, H.; Gutkind, J.S. Mitogen-Activated Protein Kinase Family. Encycl. Biol. Chem. 2004, 2, 737–742. [Google Scholar]
- Liu, R.; Molkentin, J.D. Regulation of cardiac hypertrophy and remodeling through the dual-specificity MAPK phosphatases (DUSPs). J. Mol. Cell. Cardiol. 2016, 101, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Zwerina, J.; Firestein, G.S. The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann. Rheum. Dis. 2008, 67, 909–9016. [Google Scholar] [CrossRef] [PubMed]
- Gharagozloo, M.; Jafari, S.; Esmaeil, N.; Javid, E.N.; Bagherpour, B.; Rezaei, A. Immunosuppressive Effect of Silymarin on Mitogen-Activated Protein Kinase Signalling Pathway: The Impact on T Cell Proliferation and Cytokine Production. Basic Clin. Pharmacol. Toxicol. 2013, 113, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, H.; Dai, M.M.; Li, H.; Chen, J.Y.; Hu, S.L. Identification and distribution of four metabolites of geniposide in rats with adjuvant arthritis. Fitoterapia 2014, 97, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, H.; Xu, G.B.; Dai, M.M.; Hu, S.L.; Sun, L.L.; Wang, W.; Wang, R.; Li, S.P.; Li, G.Q. Determination of geniposide in adjuvant arthritis rat plasma by ultra-high performance liquid chromatography tandem mass spectrometry method and its application to oral bioavailability and plasma protein binding ability studies. J. Pharm. Biomed. Anal. 2015, 108, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Wu, H.; Li, H.; Hu, S.L.; Dai, M.M.; Chen, J. Anti-inflammatory effects and pharmacokinetics study of geniposide on rats with adjuvant arthritis. Int. Immunopharmacol. 2015, 24, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, H.; Shen, C.; Chen, J.Y.; Hu, S.L.; Wu, H. Comparative Pharmacokinetics Study after Oral Administration of Geniposide in Normal Rats and Adjuvant-induced Arthritis Rats by UPLC-MS/MS. Basic Clin. Pharmacol. Toxicol. 2013, 113, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.M.; Wu, H.; Li, H.; Chen, J.; Chen, J.Y.; Hu, S.L.; Shen, C. Effects and mechanisms of Geniposide on rats with adjuvant arthritis. Int. Immunopharmacol. 2014, 20, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lü, S.; Wang, Q.; Li, G.; Sun, S.; Guo, Y.; Kuang, H. The treatment of rheumatoid arthritis using Chinese medicinal plants: From pharmacology to potential molecular mechanisms. J. Ethnopharmacol. 2015, 176, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Kiener, H.P.; Watts, G.F.; Cui, Y.; Wright, J.; Thornhill, T.S.; Sköld, M.; Behar, S.M.; Niederreiter, B.; Lu, J.; Cernadas, M.; et al. Synovial fibroblasts self-direct multicellular lining architecture and synthetic function in three-dimensional organ culture. Arthritis Rheumat. 2010, 62, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.D.; He, J.; Bazan, H.E. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: Evidence of cross-talk activation between MAP kinase cascades. J. Biol. Chem. 2003, 278, 21989–21997. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.H.; Godlewski, J.; Zhu, J.; Sathyanarayana, P.; Leaner, V.; Birrer, M.J.; Rana, A.; Tzivion, G. Cross-talk between JNK/SAPK and ERK/MAPK pathways: Sustained activation of JNK blocks ERK activation by mitogenic factors. J. Biol. Chem. 2003, 278, 26715–26721. [Google Scholar] [CrossRef] [PubMed]
- Perdiguero, E.; Ruiz-Bonilla, V.; Serrano, A.L.; Muñoz-Cánoves, P. Genetic deficiency of p38α reveals its critical role in myoblast cell cycle exit: The p38α-JNK connection. Cell Cycle 2007, 6, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, B.C.; Kim, S.J.; Choi, K.S. Role of MAP Kinases and Their Cross-Talk in TGF-β1-Induced Apoptosis in FaO Rat Hepatoma Cell Line. Hepatology 2002, 35, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wei, W.; Song, L.; Zhang, L.; Chen, Y.; Hu, X. Paeoniflorin induced immunetolerance of mesenteric lymph node lymphocytes via enhancing beta 2-adrenergicreceptor desensitization in rats with adjuvant arthritis. Int. Immunopharmacol. 2007, 7, 662–673. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Dai, M.; Wu, H.; Deng, R.; Fu, J.; Zhang, Z.; Dai, L.; Wang, W.; Dai, X.; Zhan, X.; et al. Immunosuppressive Effect of Geniposide on Mitogen-Activated Protein Kinase Signalling Pathway and Their Cross-Talk in Fibroblast-Like Synoviocytes of Adjuvant Arthritis Rats. Molecules 2018, 23, 91. https://doi.org/10.3390/molecules23010091
Li F, Dai M, Wu H, Deng R, Fu J, Zhang Z, Dai L, Wang W, Dai X, Zhan X, et al. Immunosuppressive Effect of Geniposide on Mitogen-Activated Protein Kinase Signalling Pathway and Their Cross-Talk in Fibroblast-Like Synoviocytes of Adjuvant Arthritis Rats. Molecules. 2018; 23(1):91. https://doi.org/10.3390/molecules23010091
Chicago/Turabian StyleLi, Feng, Miaomiao Dai, Hong Wu, Ran Deng, Jun Fu, Zhengrong Zhang, Li Dai, Wenyu Wang, Xuejing Dai, Xiang Zhan, and et al. 2018. "Immunosuppressive Effect of Geniposide on Mitogen-Activated Protein Kinase Signalling Pathway and Their Cross-Talk in Fibroblast-Like Synoviocytes of Adjuvant Arthritis Rats" Molecules 23, no. 1: 91. https://doi.org/10.3390/molecules23010091



