Preparation of Starch-Hard Carbon Spherules from Ginkgo Seeds and Their Phenol-Adsorption Characteristics
Abstract
:1. Introduction
2. Results
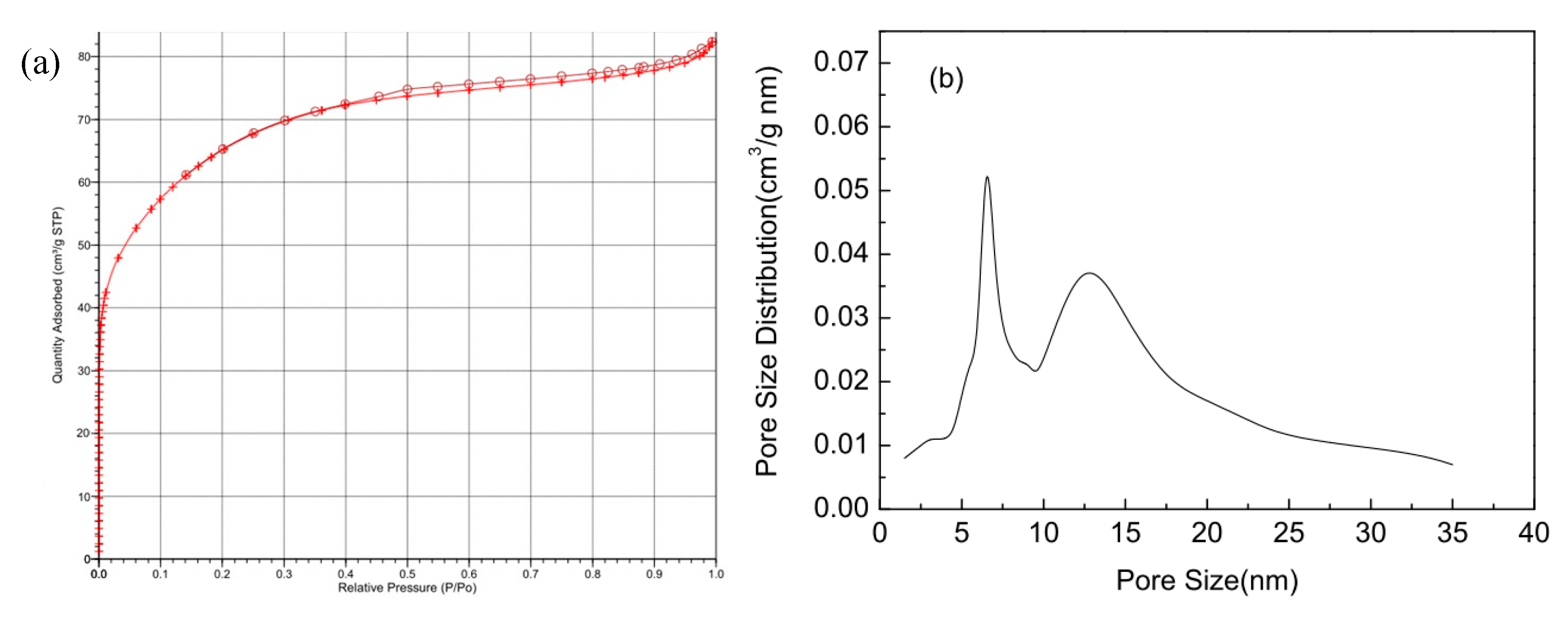
2.1. Specific Surface Area and Pore Structure
2.2. Elemental Analysis
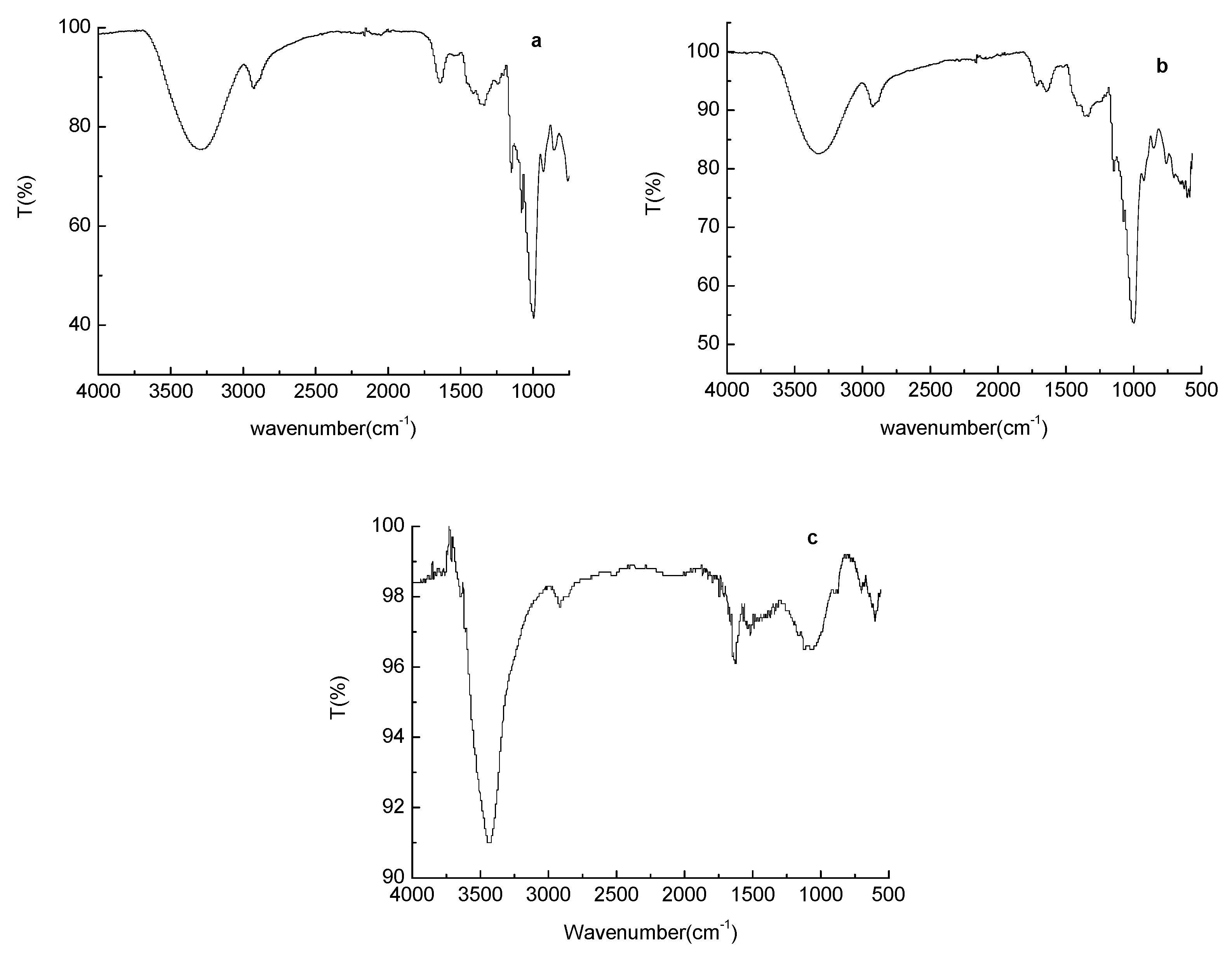
2.3. Fourier-Transform Infrared (FT–IR) Spectroscopy
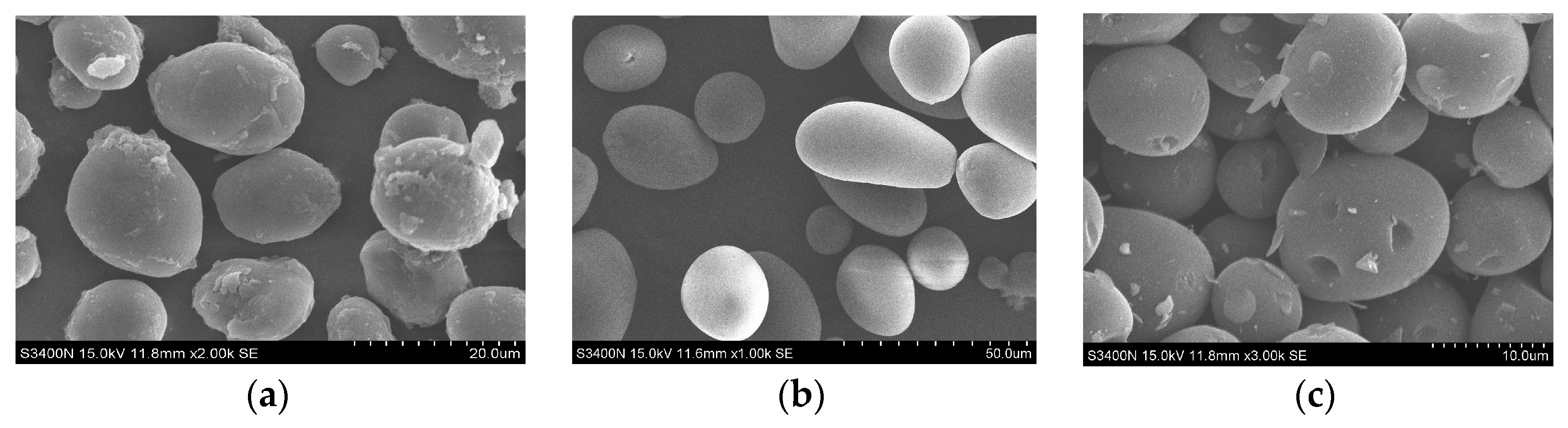
2.4. Scanning-Electron Micrograph (SEM) Observation
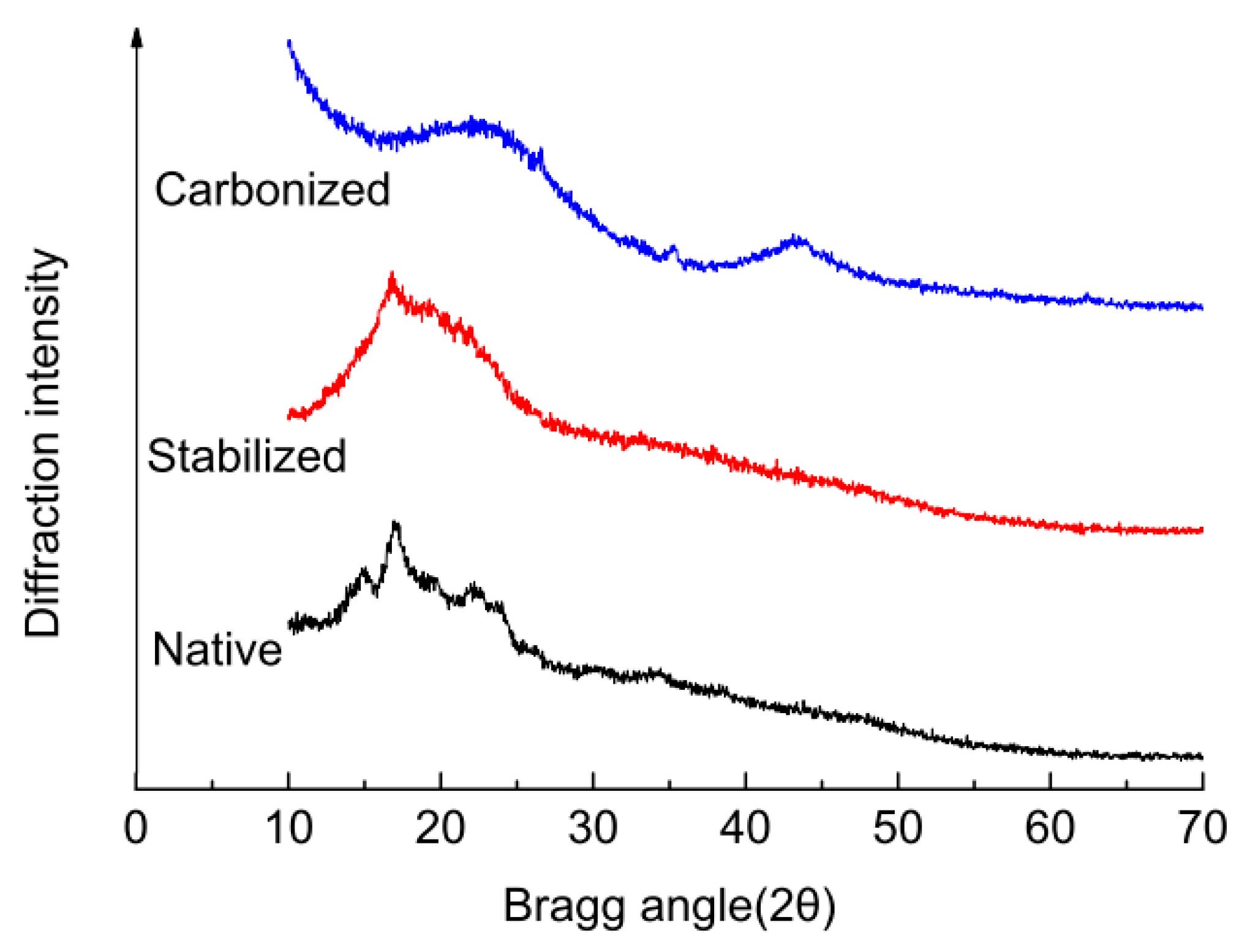
2.5. X-ray Diffraction (XRD) Characterization
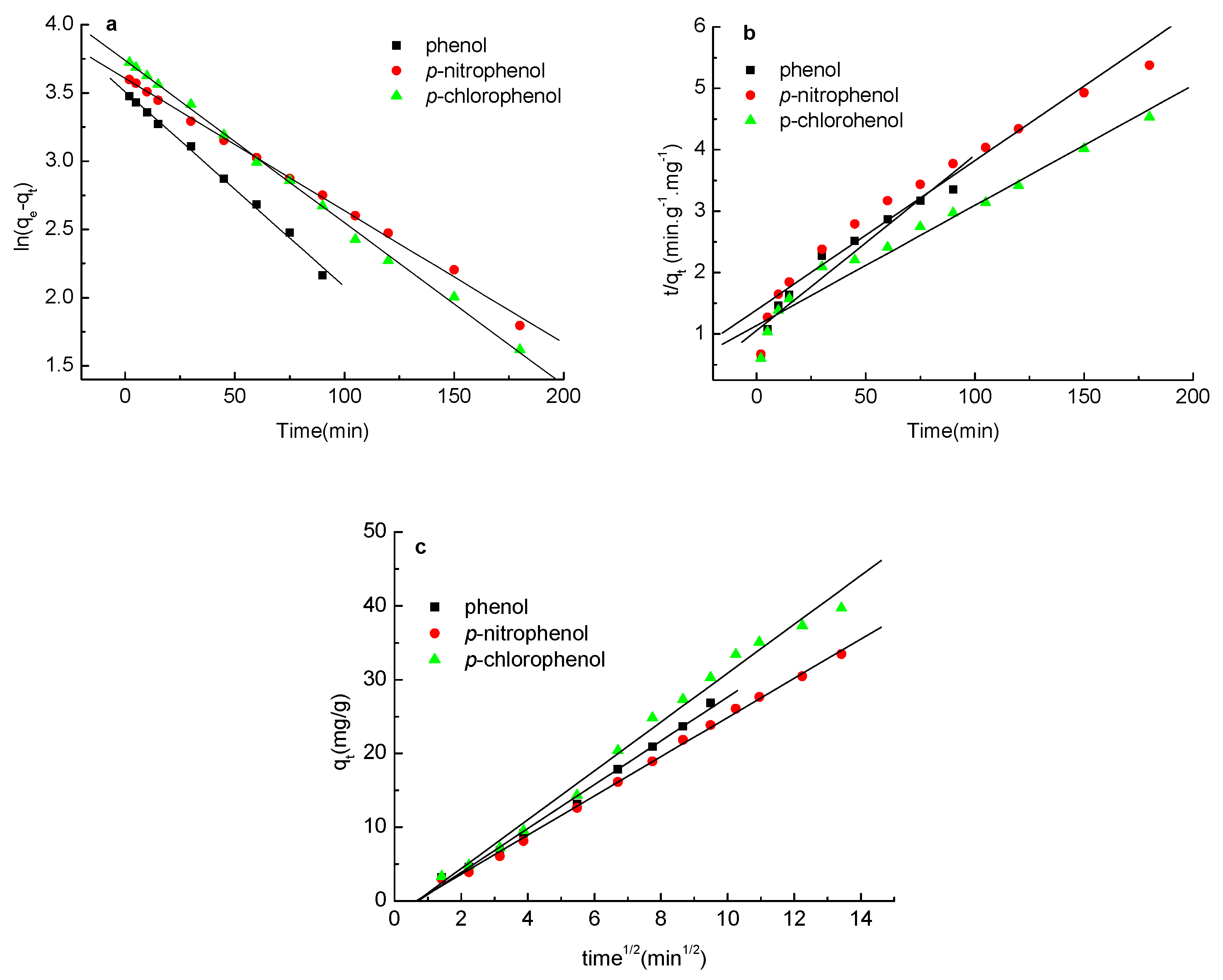
2.6. Adsorption Kinetics of Phenols on the Carbon Spherules
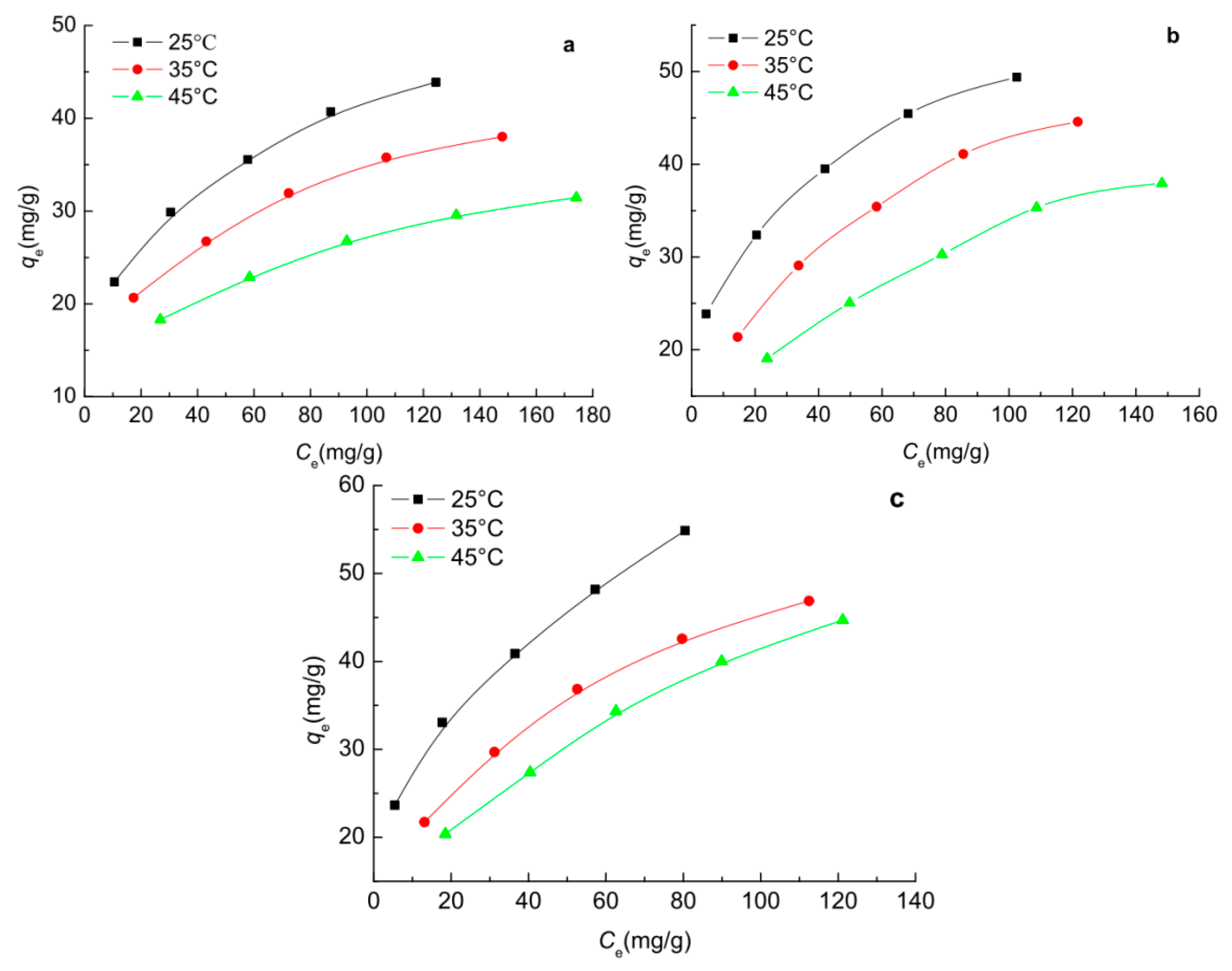
2.7. Adsorption Isotherms of Phenols on the Carbon Spherules
2.8. Adsorption Thermodynamics of Phenols on the Carbon Spherules
3. Materials and Methods
3.1. Materials
3.2. Starch Preparation
3.3. Starch-Hard Carbon Spherule Preparation
3.4. Structural Characterization
3.5. Static Adsorption Experiments
3.6. Adsorption Kinetics
3.7. Adsorption Isotherm
3.8. High-Performance Liquid Chromatography (HPLC) Analysis of Phenols
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mahady, G.B. Global harmonization of herbal health claims. J. Nutr. 2001, 131, 1120–1123. [Google Scholar]
- Yang, J.T.; Wu, C.E. Advance in allergic composition and mechanism of allergy of Ginkgo biloba seeds. Food Sci. Technol. 2009, 6, 282–286. [Google Scholar]
- Miao, M.; Jiang, H.; Jiang, B. Structure and functional properties of starches from Chinese ginkgo (Ginkgo biloba L.) nuts. Food Res. Int. 2012, 49, 303–310. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Karaman, S.; Kayacier, A. Mathematical approach for two component modeling of salep-starch mixtures using central composite rotatable design: Part I. Physicochemical and steady shear properties. Food Hydrocoll. 2013, 31, 49–60. [Google Scholar] [CrossRef]
- Angellier, H.; Molinaboisseau, S.; Dole, P. Thermoplastic starch-waxy maize starch nanocrystals nanocomposites. Biomacromolecules 2006, 7, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ai, F.; Chang, P.R. Structure and Properties of Starch Nanocrystal-Reinforced Soy Protein Plastics. Polym. Compos. 2010, 30, 474–480. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, C.Y.; Chen, M.M.; Shi, Z.Q.; Liu, N. Preparation of carbon spheres from potato starch and its stabiliztion mechanism. New Carbon Mater. 2010, 25, 438–443. [Google Scholar] [CrossRef]
- Shu, J.; Shui, M.; Xu, D. Preparation of nano-sized hard carbon spherule and its electrochemical property. J. Electroanal. Chem. 2011, 657, 187–191. [Google Scholar] [CrossRef]
- Khezami, L.; Capart, R. Removal of chromium(VI) from aqueoussolution by activated carbons: Kinetic and equilibrium studies. J. Hazard. Mater. 2005, 123, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Lorenc-Grabowska, E.; Gryglewicz, G.; Diez, M.A. Kinetics and equilibrium study of phenol adsorption on nitrogen-enriched activated carbons. Fuel 2013, 114, 235–243. [Google Scholar] [CrossRef]
- Li, L.; Quinlivan, P.A.; Knappe, D.R.U. Effects of activated carbon surface chemistry and pore structure on the adsorption of organic contaminants from aqueous solution. Carbon 2002, 40, 2085–2100. [Google Scholar] [CrossRef]
- Lu, A.H.; Zheng, J.T. Study of Microstructure of High-Surface-Area Polyacrylonitrile Activated Carbon Fibers. J. Colloid Interface Sci. 2001, 236, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Gibaud, A.; Xue, J.S.; Dahn, J.R. A small angle X-ray scattering study of carbons made from pyrolyzed sugar. Carbon 1996, 34, 499–503. [Google Scholar] [CrossRef]
- Liu, Y.; Di, D.; Bai, Q.; Li, J.; Chen, Z.; Lou, S.; Ye, H. Preparative separation and purification of rebaudioside a from steviol glycosides using mixed-mode macroporous adsorption resins. J. Agric. Food Chem. 2011, 59, 9629–9636. [Google Scholar] [CrossRef] [PubMed]
- Anandkumar, J.; Mandal, B. Removal of Cr(VI) from aqueous solution using Bael fruit (Aegle marmelos correa) shell as an adsorbent. J. Hazard. Mater. 2009, 168, 633–640. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Guang, W.; Li, G.H.; Mi, N.; Xue, H.X.; Tian, H.J.; Lu, C.W.; Gao, X.D. Adsorptive behaviors of phenolic compounds in sediments of the yellow river. J. Agro-Environ. Sci. 2005, 24, 480–485. [Google Scholar]
- Liu, Y.F.; Bai, Q.Q.; Lou, S.; Di, D.L.; Li, J.T.; Guo, M. Adsorption characteristics of (–)-epigallocatechin gallate and caffeine in the extract of waste tea on macroporous adsorption resins functionalized with chloromethyl, amino, and phenylamino groups. Agric. Food Chem. 2012, 60, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.R.; Ibrahim, S.M.; El-Molla, S.A. Textile dye removal from aqueous solutions using cheap MgO nanomaterials: Adsorption kinetics, isotherm studies and thermodynamics. Adv. Powder Technol. 2016, 27, 223–231. [Google Scholar] [CrossRef]
- Wang, D.W.; Liu, H.C.; Song, C.C.; Wei, C.G.; Liu, T.T. Ultrasonic-assisted extraction and characterization of potato starch. Food Sci. 2013, 34, 17–22. [Google Scholar]
Sample Availability: Samples of the compounds Starch-Hard Carbon Spherules are available from the authors. |

| Kind | C (Weight%) | H (Weight%) | O (Weight%) |
|---|---|---|---|
| Ginkgo starch | 44.79 | 6.18 | 48.76 |
| Stabilized ginkgo starch | 72.24 | 6.12 | 21.29 |
| Carbonized ginkgo seed starch | 93.58 | 4.34 | 0.85 |
| Adsorbate | Pseudo-First Order Equation | Pseudo-Second Order Equation | Weber–Morris | |||||
|---|---|---|---|---|---|---|---|---|
| k1 (1/min) | qe (mg/g) | R12 | k2 (g/mg·min) | qe (mg/g) | R22 | kp (mg/min1/2/g) | Rp2 | |
| Phenol | 0.014 | 33.41 | 0.995 | 7.42 × 10−4 | 35.71 | 0.926 | 2.971 | 0.994 |
| p-Nitrophenol | 0.009 | 36.86 | 0.997 | 4.13 × 10−4 | 41.67 | 0.952 | 2.656 | 0.997 |
| p-Chlorophenol | 0.011 | 42.01 | 0.998 | 3.18 × 10−4 | 52.63 | 0.960 | 3.310 | 0.989 |
| Adsorbate | T (°C) | Langmuir | Freundlich | Temkin-Pyzhev | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RL2 | qm (mg/g) | KL (L/mg) | RF2 | n | KF (mg/g) | RT2 | bT (L/mg) | A′ | ||
| Phenol | 25 | 0.993 | 50.00 | 17.6 | 0.998 | 3.623 | 11.64 | 0.988 | 282.26 | 1.107 |
| 35 | 0.996 | 45.45 | 24.68 | 0.996 | 3.425 | 8.97 | 0.992 | 308.19 | 0.651 | |
| 45 | 0.996 | 37.34 | 31.89 | 0.997 | 3.390 | 6.92 | 0.994 | 369.48 | 0.457 | |
| p-Nitrophenol | 25 | 0.990 | 55.56 | 11.00 | 0.994 | 4.219 | 16.368 | 0.971 | 301.45 | 3.357 |
| 35 | 0.994 | 55.56 | 26.83 | 0.997 | 2.841 | 8.375 | 0.992 | 230.60 | 0.442 | |
| 45 | 0.991 | 50.00 | 43.40 | 0.995 | 2.577 | 5.546 | 0.986 | 248.83 | 0.234 | |
| p-Chlorophenol | 25 | 0.979 | 62.50 | 13.81 | 0.994 | 3.257 | 13.836 | 0.962 | 221.13 | 1.290 |
| 35 | 0.993 | 58.82 | 26.29 | 0.997 | 2.747 | 8.531 | 0.991 | 215.83 | 0.440 | |
| 45 | 0.987 | 58.82 | 41.06 | 0.997 | 2.347 | 5.808 | 0.981 | 202.07 | 0.232 | |
| Thermodynamic Parameters | ∆G (kJ/mol) | ∆H (kJ/mol) | ∆S (J/(mol·K)) | ||
|---|---|---|---|---|---|
| 25 °C | 35 °C | 45 °C | |||
| Phenol | −4.234 | −3.510 | −2.945 | −23.445 | −64.519 |
| p-Nitrophenol | −6.289 | −4.216 | −3.081 | −54.249 | −161.366 |
| p-Chlorophenol | −5.530 | −4.066 | −3.019 | −43.050 | −126.038 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, C.; Ye, J.; Zhou, H.; Tao, R.; Li, W. Preparation of Starch-Hard Carbon Spherules from Ginkgo Seeds and Their Phenol-Adsorption Characteristics. Molecules 2018, 23, 96. https://doi.org/10.3390/molecules23010096
Chen H, Wang C, Ye J, Zhou H, Tao R, Li W. Preparation of Starch-Hard Carbon Spherules from Ginkgo Seeds and Their Phenol-Adsorption Characteristics. Molecules. 2018; 23(1):96. https://doi.org/10.3390/molecules23010096
Chicago/Turabian StyleChen, Hongxia, Chengzhang Wang, Jianzhong Ye, Hao Zhou, Ran Tao, and Wenjun Li. 2018. "Preparation of Starch-Hard Carbon Spherules from Ginkgo Seeds and Their Phenol-Adsorption Characteristics" Molecules 23, no. 1: 96. https://doi.org/10.3390/molecules23010096




