Enhanced Hydrolysis of Cellulose in Ionic Liquid Using Mesoporous ZSM-5
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization Methods
2.3. Preparation of Mesoporous ZSM-5
2.4. Catalytic Reactions
2.5. Analysis Methods
3. Result and Discussion
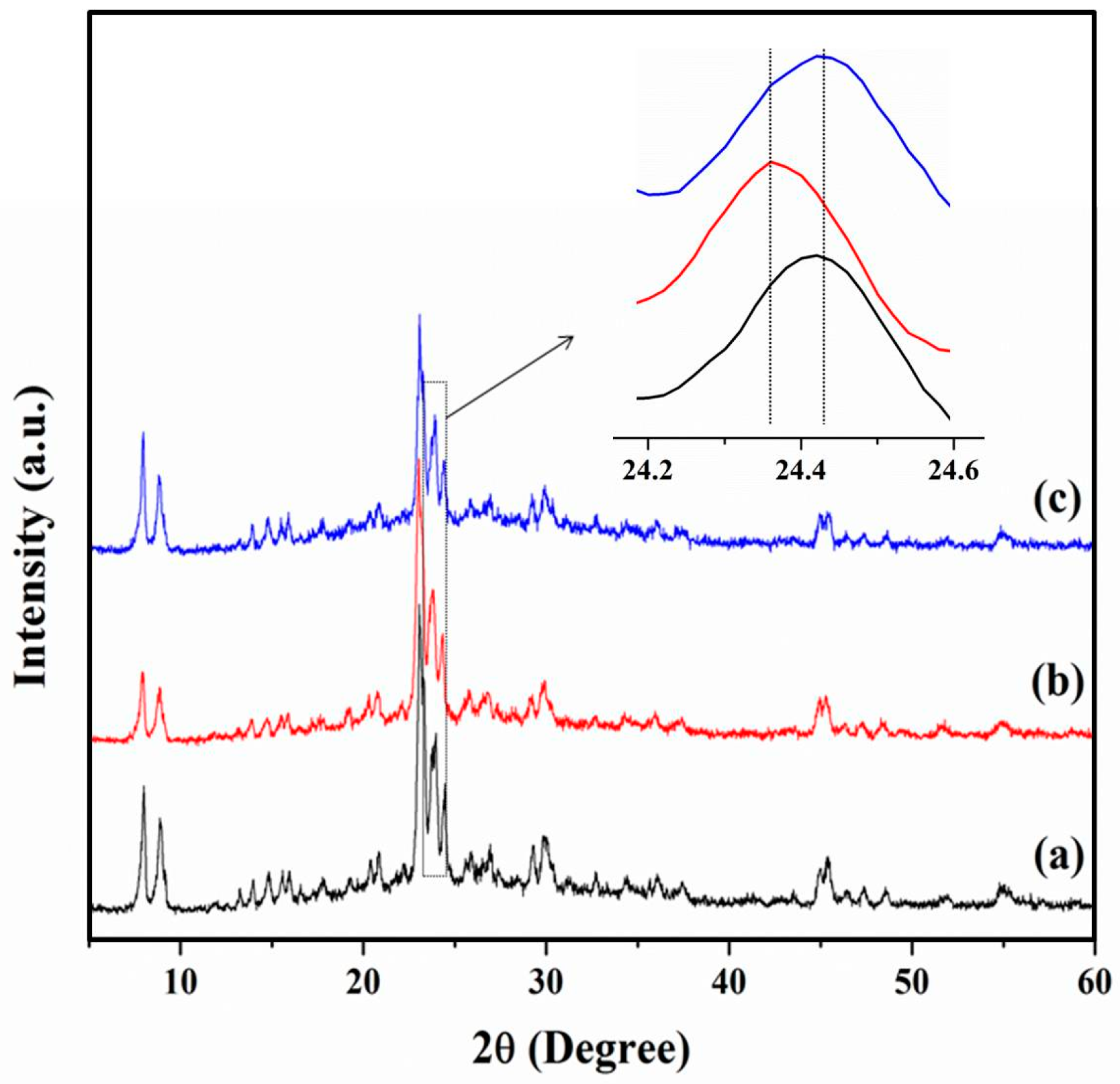
3.1. Chemical and Pore Structures of the Samples
3.2. Catalytic Performance of the Samples
3.2.1. Hydrolysis of Cellulose
3.2.2. Effect of Reaction Conditions on Cellulose Hydrolysis over H-Z-30
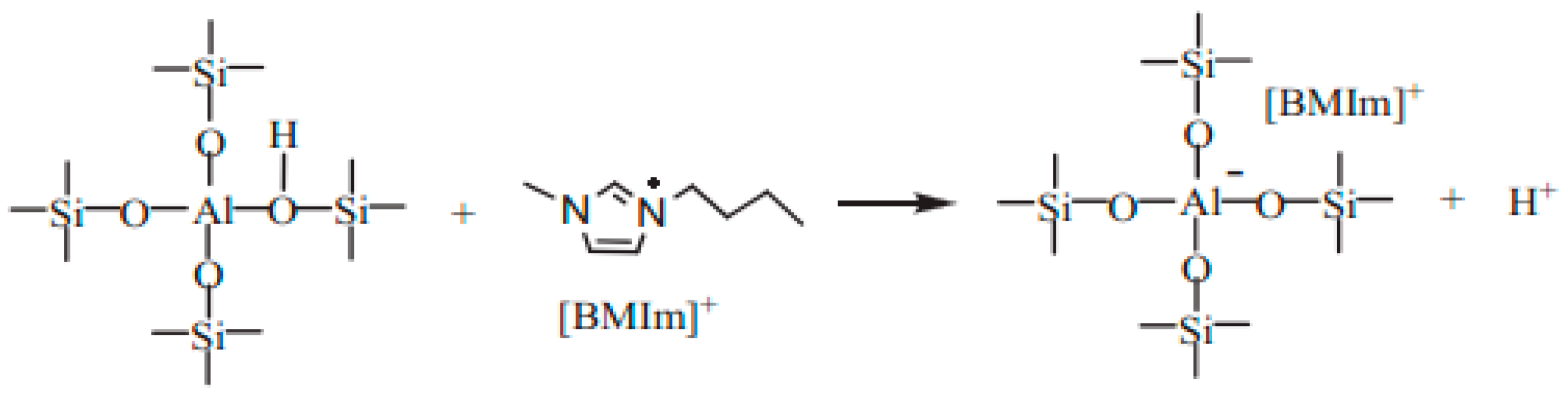
3.2.3. The Role of Mesopores in Zeolites for Ionic Liquid
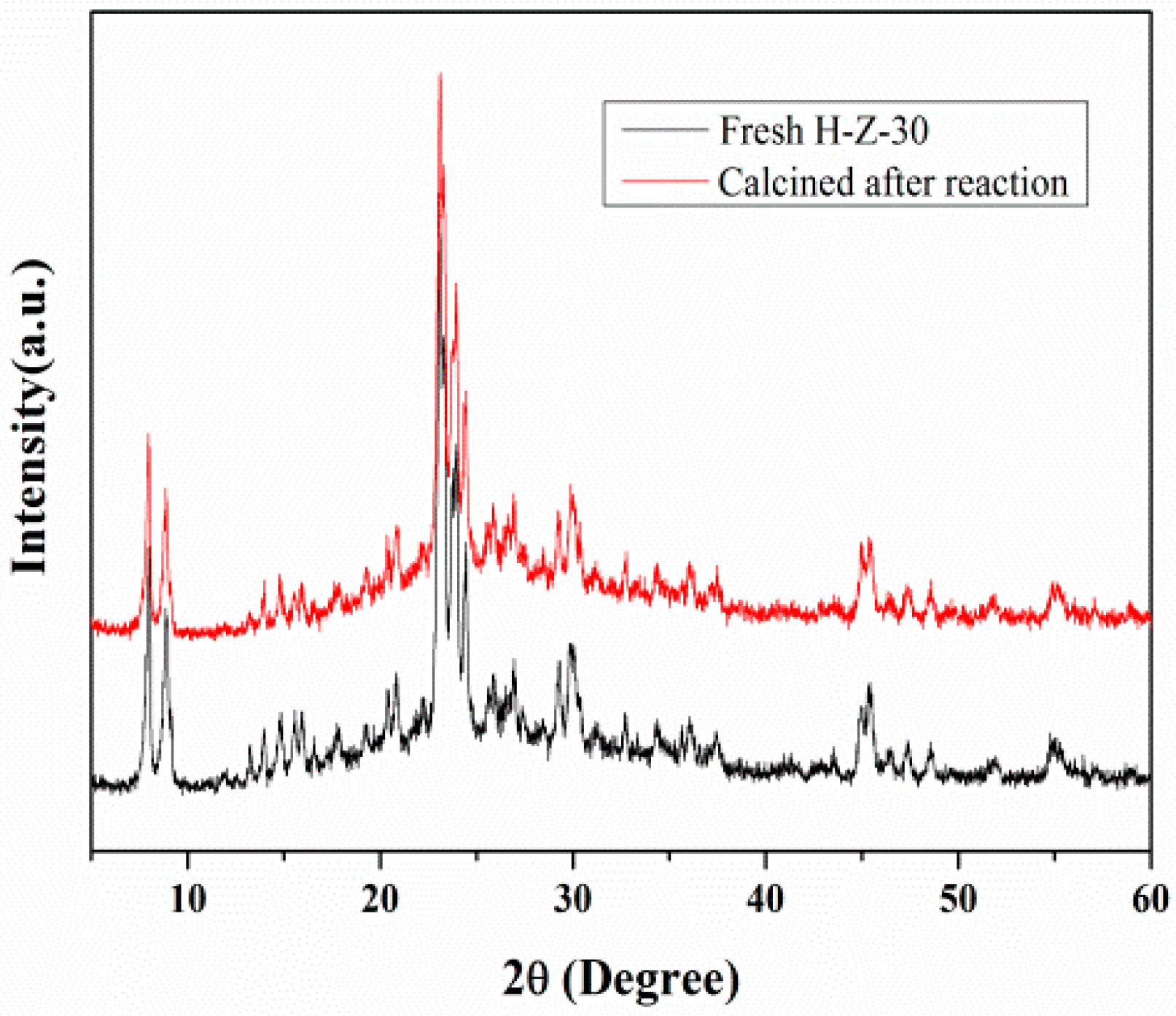
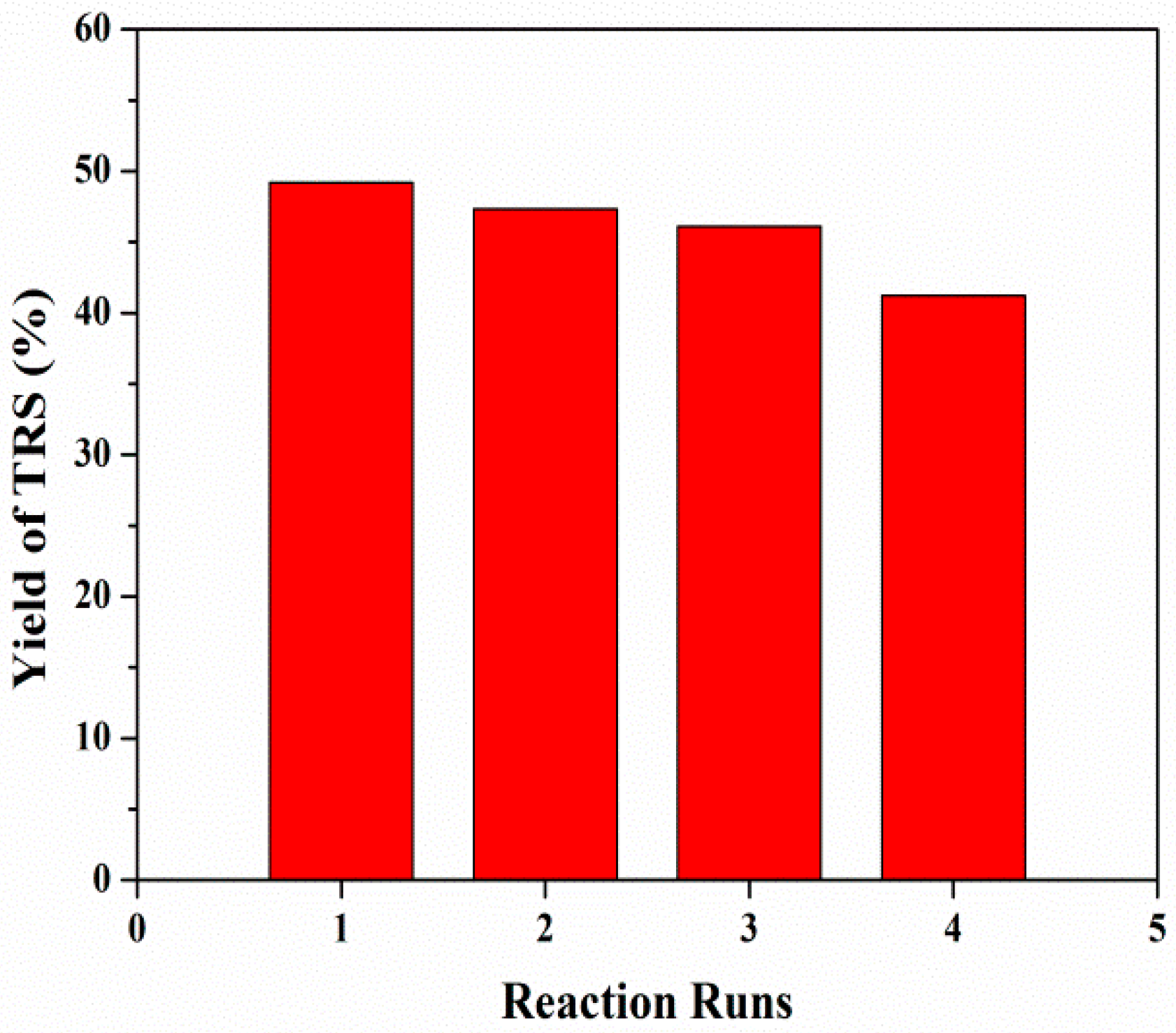
3.2.4. Catalytic Cycling of Mesoporous ZSM-5 in Cellulose Hydrolysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schmidt, L.D.; Dauenhauer, P.J. Chemical engineering: Hybrid routes to biofuels. Nature 2007, 447, 914–915. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Jarvis, M. Chemistry: Cellulose stacks up. Nature 2003, 426, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Antonoplis, R.A.; Blanch, H.W.; Freitas, R.P.; Sciamanna, A.F.; Wilke, C.R. Production of sugars from wood using high-pressure hydrogen chloride. Biotechnol. Bioeng. 1983, 25, 2757–2773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.P.; Lynd, L.R. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol. Bioeng. 2004, 88, 797–824. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Fang, Z.; Fukushima, Y.; Adschiri, T.; Arai, K. Dissolution and hydrolysis of cellulose in subcritical and supercritical water. Ind. Eng. Chem. Res. 2000, 39, 2883–2890. [Google Scholar] [CrossRef]
- Huang, Y.B.; Fu, Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 2013, 15, 1095–1111. [Google Scholar] [CrossRef]
- Van de Vyver, S.; Geboers, J.; Jacobs, P.A.; Sels, B.F. Recent advances in the catalytic conversion of cellulose. ChemCatChem 2011, 3, 82–94. [Google Scholar] [CrossRef]
- Shuai, L.; Pan, X. Hydrolysis of cellulose by cellulase-mimetic solid catalyst. Energy Environ. Sci. 2012, 5, 6889–6894. [Google Scholar] [CrossRef]
- Suganuma, S.; Nakajima, K.; Kitano, M.; Yamaguchi, D.; Kato, H.; Hayashi, S.; Hara, M. Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups. J. Am. Chem. Soc. 2008, 130, 12787–12793. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Furukawa, H.; Kobayashi, N.; Itaya, Y.; Satsuma, A. Effects of Brønsted and Lewis acidities on activity and selectivity of heteropolyacid-based catalysts for hydrolysis of cellobiose and cellulose. Green Chem. 2009, 11, 1627–1632. [Google Scholar] [CrossRef]
- Kobayashi, H.; Komanoya, T.; Hara, K.; Fukuoka, A. Water-Tolerant Mesoporous-Carbon-Supported Ruthenium Catalysts for the Hydrolysis of Cellulose to Glucose. ChemSusChem 2010, 3, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Wang, A.; Zheng, M.; Zhang, T. Hydrolysis of cellulose into glucose over carbons sulfonated at elevated temperatures. Chem. Commun. 2010, 46, 6935–6937. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Deng, L.; Li, J.; Liao, B.; Guo, Q.; Fu, Y. Hydrolysis of cellulose into glucose by magnetic solid acid. ChemSusChem 2011, 4, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, C.; Wang, A.; Xu, G.; Zhang, T. Zeolite-promoted hydrolysis of cellulose in ionic liquid, insight into the mutual behavior of zeolite, cellulose and ionic liquid. Appl. Catal. B 2012, 123, 333–338. [Google Scholar] [CrossRef]
- Abou-Yousef, H.; Hassan, E.B. A novel approach to enhance the activity of H-form zeolite catalyst for production of hydroxymethylfurfural from cellulose. J. Ind. Eng. Chem. 2014, 20, 1952–1957. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.K. Solid acid and microwave-assisted hydrolysis of cellulose in ionic liquid. Carbohydr. Res. 2009, 344, 2069–2072. [Google Scholar] [CrossRef] [PubMed]
- Perego, C.; Bosetti, A. Biomass to fuels: The role of zeolite and mesoporous materials. Microporous Mesoporous Mater. 2011, 144, 28–39. [Google Scholar] [CrossRef]
- Onda, A.; Ochi, T.; Yanagisawa, K. Selective hydrolysis of cellulose into glucose over solid acid catalysts. Green Chem. 2008, 10, 1033–1037. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Abrams, L.; Kaneko, K. Mesopore-modified zeolites: Preparation, characterization, and applications. Chem. Rev. 2006, 106, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Zhou, G.; Wang, W.; Wang, C.; Komarneni, S.; Wang, Y. Catalytic fast pyrolysis of biomass with mesoporous ZSM-5 zeolites prepared by desilication with NaOH solutions. Appl. Catal. 2014, 470, 115–122. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Galande, N.D.; Thakur, P.; Sawant, S.D.; Zambre, V.P.; Bokade, V.V. One-Pot Synthesis of 5-Hydroxymethylfurfural by Cellulose Hydrolysis over Highly Active Bimodal Micro/Mesoporous H-ZSM-5 Catalyst. ACS Sustain. Chem. Eng. 2014, 2, 1928–1932. [Google Scholar] [CrossRef]
- Zhou, L.; Shi, M.; Cai, Q.; Wu, L.; Hu, X.; Yang, X.; Chen, C.; Xu, J. Hydrolysis of hemicellulose catalyzed by hierarchical H-USY zeolites–The role of acidity and pore structure. Microporous Mesoporous Mater. 2013, 169, 54–59. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Z.; Shi, M.; Du, S.; Su, Y.; Yang, X.; Xu, J. Sulfonated hierarchical H-USY zeolite for efficient hydrolysis of hemicellulose/cellulose. Carbohydr. Polym. 2013, 98, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Ogura, M.; Shinomiya, S.; Tateno, J.; Nara, Y.; Nomura, M.; Kikuchi, E.; Matsukata, M. Alkali-treatment technique—New method for modification of structural and acid-catalytic properties of ZSM-5 zeolites. Appl. Catal. 2001, 219, 33–43. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Kaneko, K. Developments and structures of mesopores in alkaline-treated ZSM-5 zeolites. Adsorption 2006, 12, 309–316. [Google Scholar] [CrossRef]
- Groen, J.C.; Jansen, J.C.; Moulijn, J.A.; Pérez-Ramírez, J. Optimal aluminum-assisted mesoporosity development in MFI zeolites by desilication. J. Phys. Chem. B 2004, 108, 13062–13065. [Google Scholar] [CrossRef]
- Su, L.; Liu, L.; Zhuang, J.; Wang, H.; Li, Y.; Shen, W.; Xu, Y.; Bao, X. Creating mesopores in ZSM-5 zeolite by alkali treatment: A new way to enhance the catalytic performance of methane dehydroaromatization on Mo/HZSM-5 catalysts. Catal. Lett. 2003, 91, 155–167. [Google Scholar] [CrossRef]
- Salman, N.; Rüscher, C.H.; Buhl, J.C.; Lutz, W.; Toufar, H.; Stöcker, M. Effect of temperature and time in the hydrothermal treatment of HY zeolite. Microporous Mesoporous Mater. 2006, 90, 339–346. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wu, Y.; Chen, Q.; Yu, Z.; Wang, C.; Jin, S.; Ding, Y.; Wu, G. Dissolution of cellulose with ionic liquids and its application: A mini-review. Green Chem. 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X.; Wu, Q.; Zhang, C.; Yu, Y.; Lan, M.; Wei, X.; Ying, Z.; Liu, T.; Liang, G.; Zhao, F. Synthesis of Ni/mesoporous ZSM-5 for direct catalytic conversion of cellulose to hexitols: Modulating the pore structure and acidic sites via a nanocrystalline cellulose template. Green Chem. 2016, 18, 3315–3323. [Google Scholar] [CrossRef]
- Rinaldi, R.; Meine, N.; vom Stein, J.; Palkovits, R.; Schüth, F. Which controls the depolymerization of cellulose in ionic liquids: The solid acid catalyst or cellulose? ChemSusChem 2010, 3, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Wu, Y.; Shen, Q.; Li, X.; Chen, Y.; Meng, C. Effect of Si/Al ratio on mesopore formation for zeolite beta via NaOH treatment and the catalytic performance in α-pinene isomerization and benzoylation of naphthalene. Microporous Mesoporous Mater. 2013, 173, 129–138. [Google Scholar] [CrossRef]
- Milina, M.; Mitchell, S.; Michels, N.L.; Kenvin, J.; Pérez-Ramírez, J. Interdependence between porosity, acidity, and catalytic performance in hierarchical ZSM-5 zeolites prepared by post-synthetic modification. J. Catal. 2013, 308, 398–407. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Amin, N.A.S. Catalytic hydrolysis of cellulose and oil palm biomass in ionic liquid to reducing sugar for levulinic acid production. Fuel Process. Technol. 2014, 128, 490–498. [Google Scholar] [CrossRef]
- Hu, L.; Wu, Z.; Xu, J.; Sun, Y.; Lin, L.; Liu, S. Zeolite-promoted transformation of glucose into 5-hydroxymethylfurfural in ionic liquid. Chem. Eng. J. 2014, 244, 137–144. [Google Scholar] [CrossRef]
Sample Availability: Samples of the alkaline-treated ZSM-5 and the products are available from the authors. |
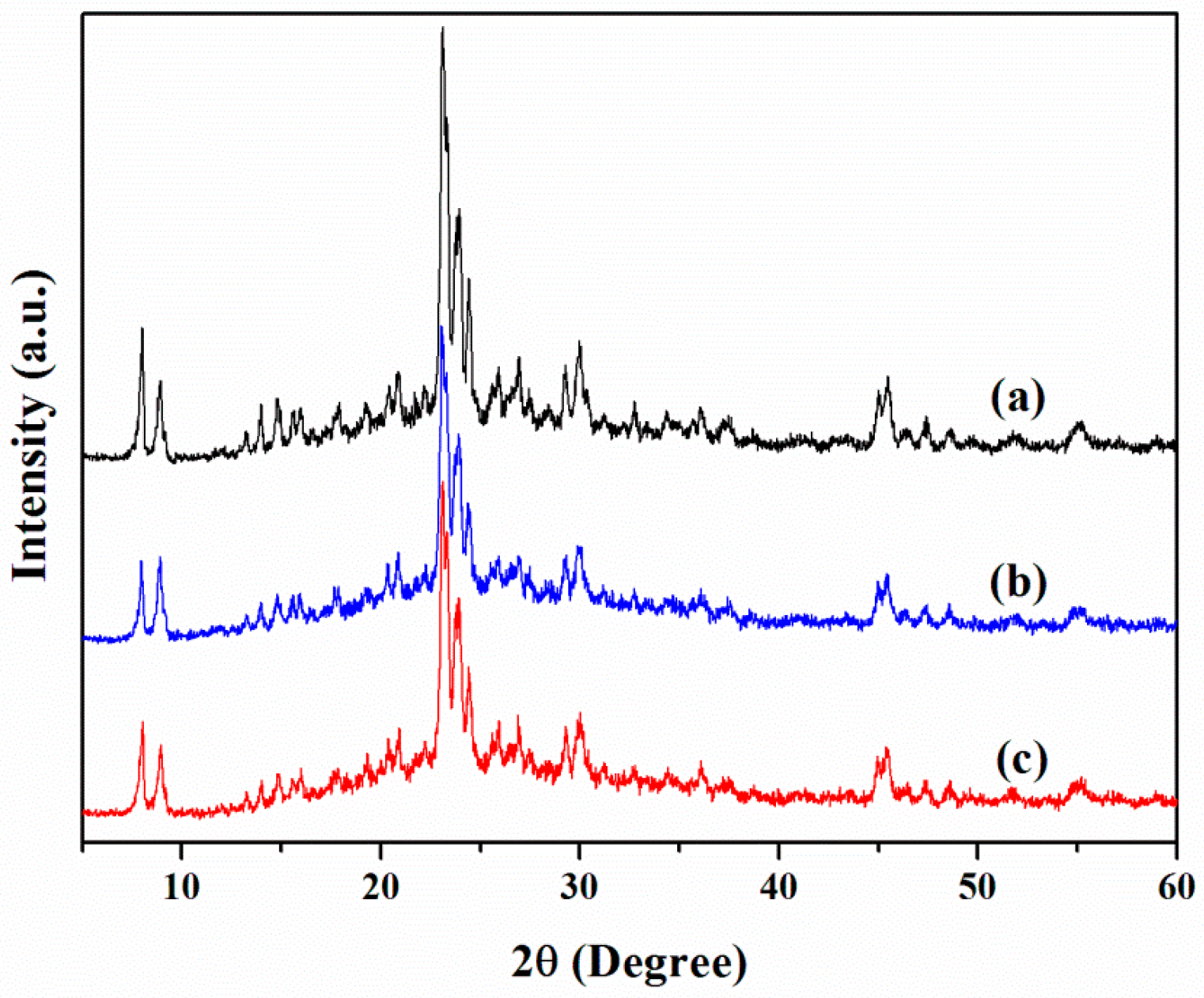
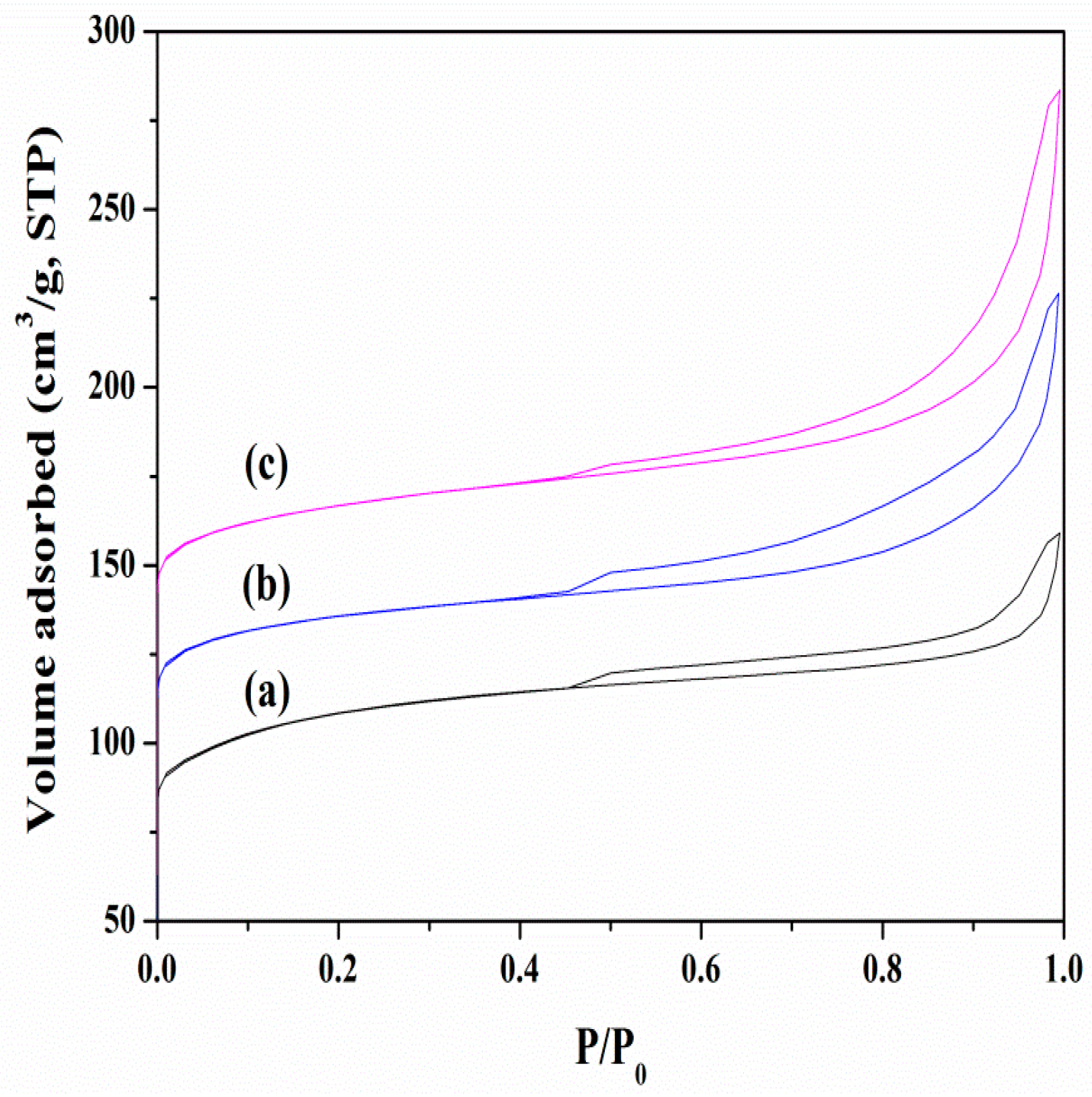

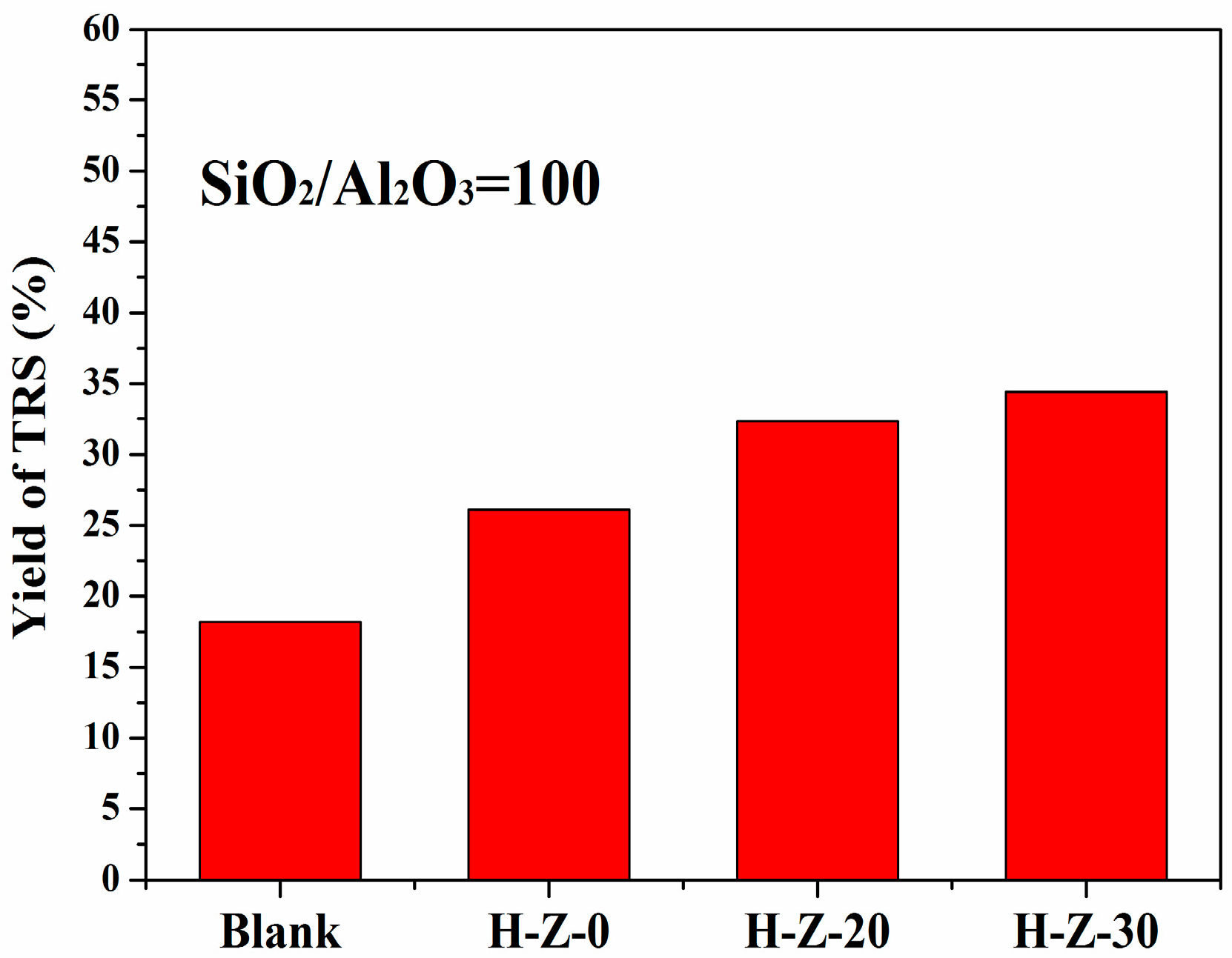
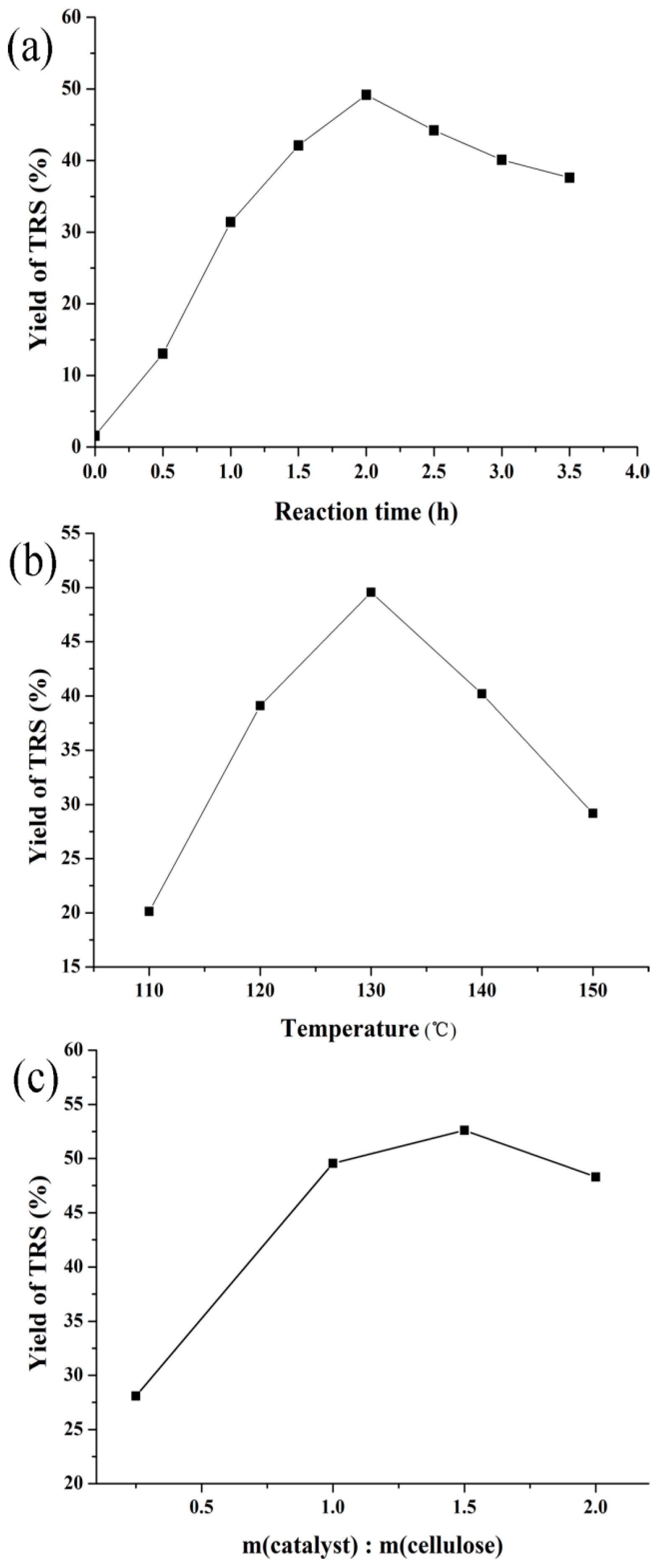
| Catalyst Sample | Al (wt %) | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Acid Sites (mmol/g) | ||
|---|---|---|---|---|---|---|
| Vmeso | Vmicro | Vtotal | ||||
| H-Z-0 | 1.95 | 365 | 0.13 | 0.13 | 0.26 | 0.43 |
| H-Z-20 | 2.03 | 340 | 0.18 | 0.13 | 0.31 | 0.45 |
| H-Z-30 | 2.36 | 350 | 0.22 | 0.12 | 0.34 | 0.51 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Xiong, C.; Tao, Y. Enhanced Hydrolysis of Cellulose in Ionic Liquid Using Mesoporous ZSM-5. Molecules 2018, 23, 529. https://doi.org/10.3390/molecules23030529
Chen T, Xiong C, Tao Y. Enhanced Hydrolysis of Cellulose in Ionic Liquid Using Mesoporous ZSM-5. Molecules. 2018; 23(3):529. https://doi.org/10.3390/molecules23030529
Chicago/Turabian StyleChen, Tianlu, Chunrong Xiong, and Yousheng Tao. 2018. "Enhanced Hydrolysis of Cellulose in Ionic Liquid Using Mesoporous ZSM-5" Molecules 23, no. 3: 529. https://doi.org/10.3390/molecules23030529





