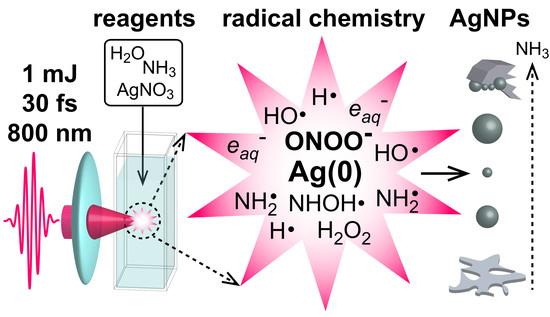
Radical Chemistry in a Femtosecond Laser Plasma: Photochemical Reduction of Ag+ in Liquid Ammonia Solution
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Sample Preparation
2.3. Instrumentation
2.4. Characterization
2.4.1. Quantification of H2O2
2.4.2. Transmission Electron Microscopy (TEM)
2.4.3. Ion Chromatography (IC)
3. Results
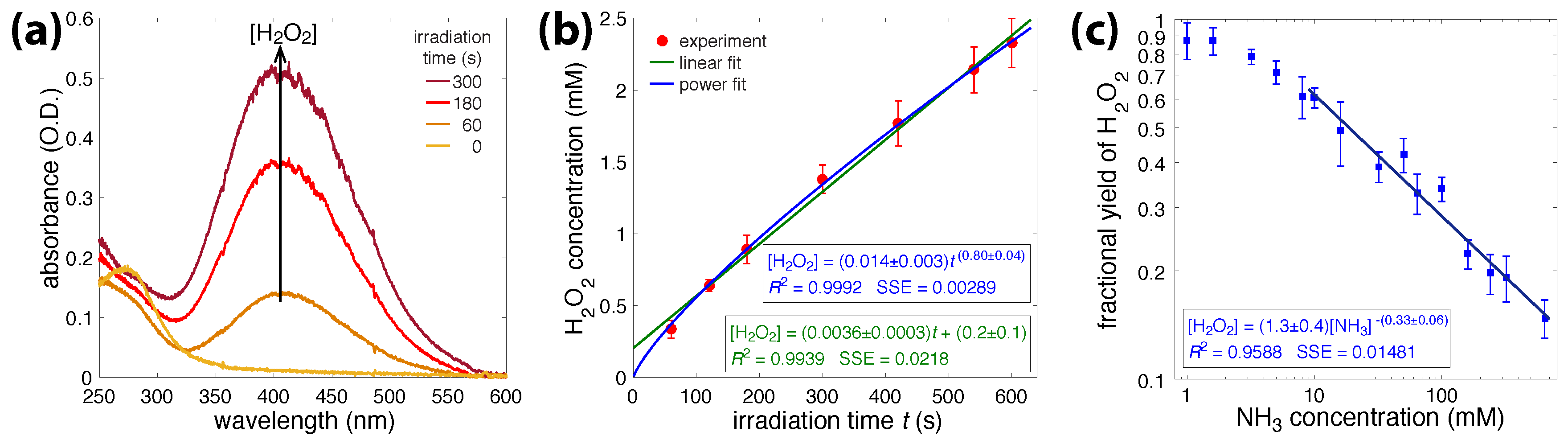
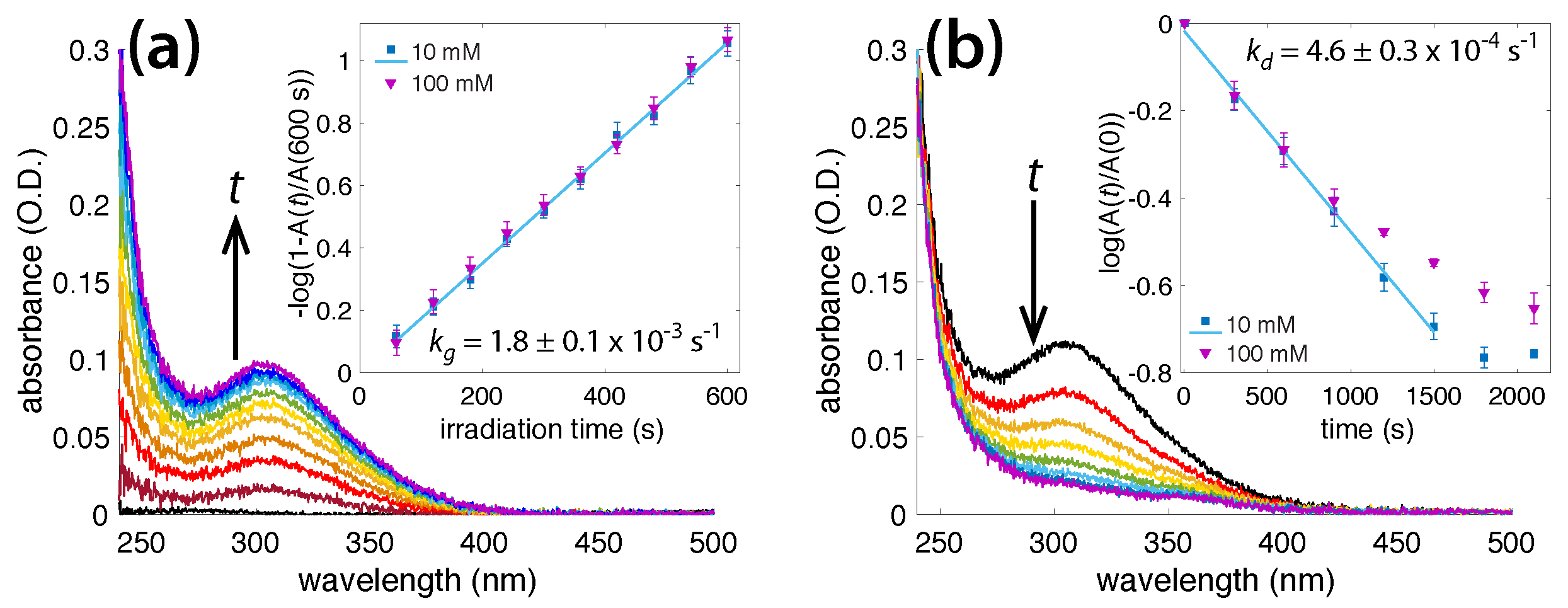
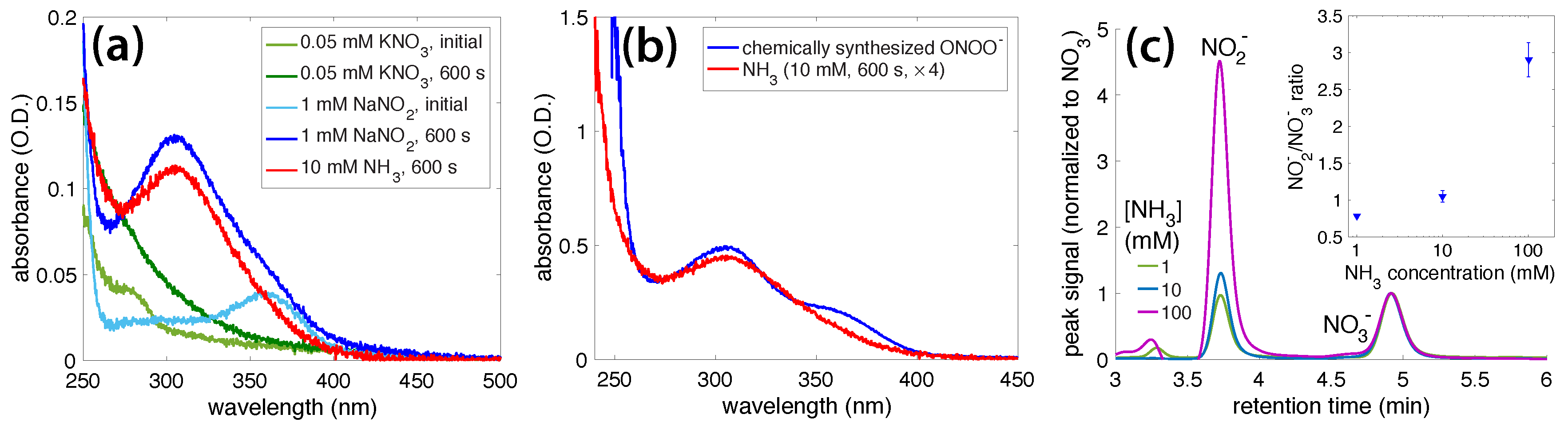
3.1. Irradiation of Aqueous Ammonia Solutions
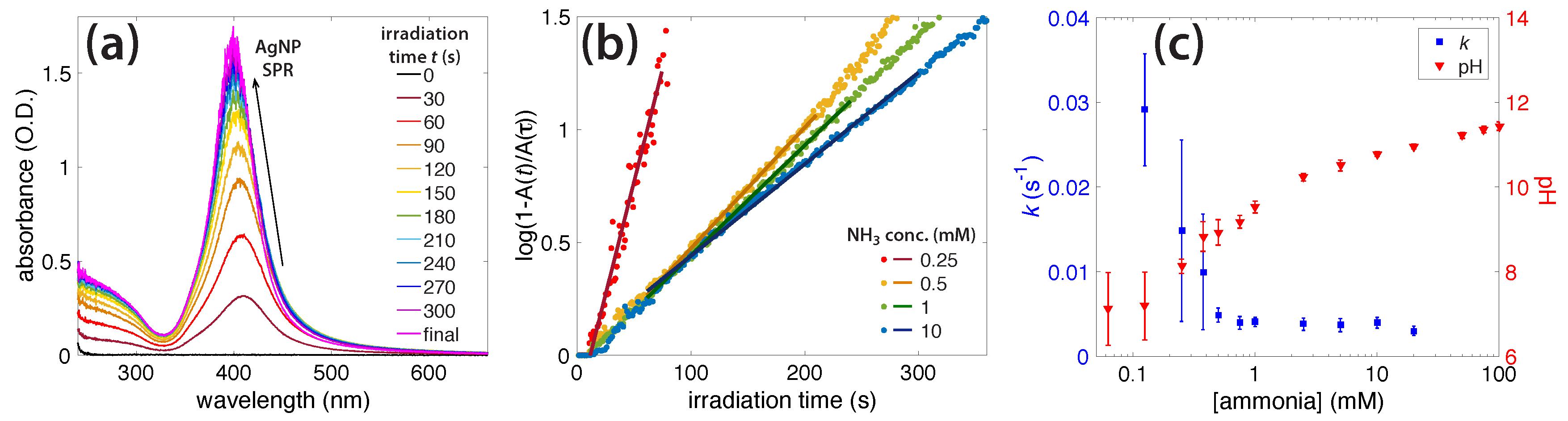
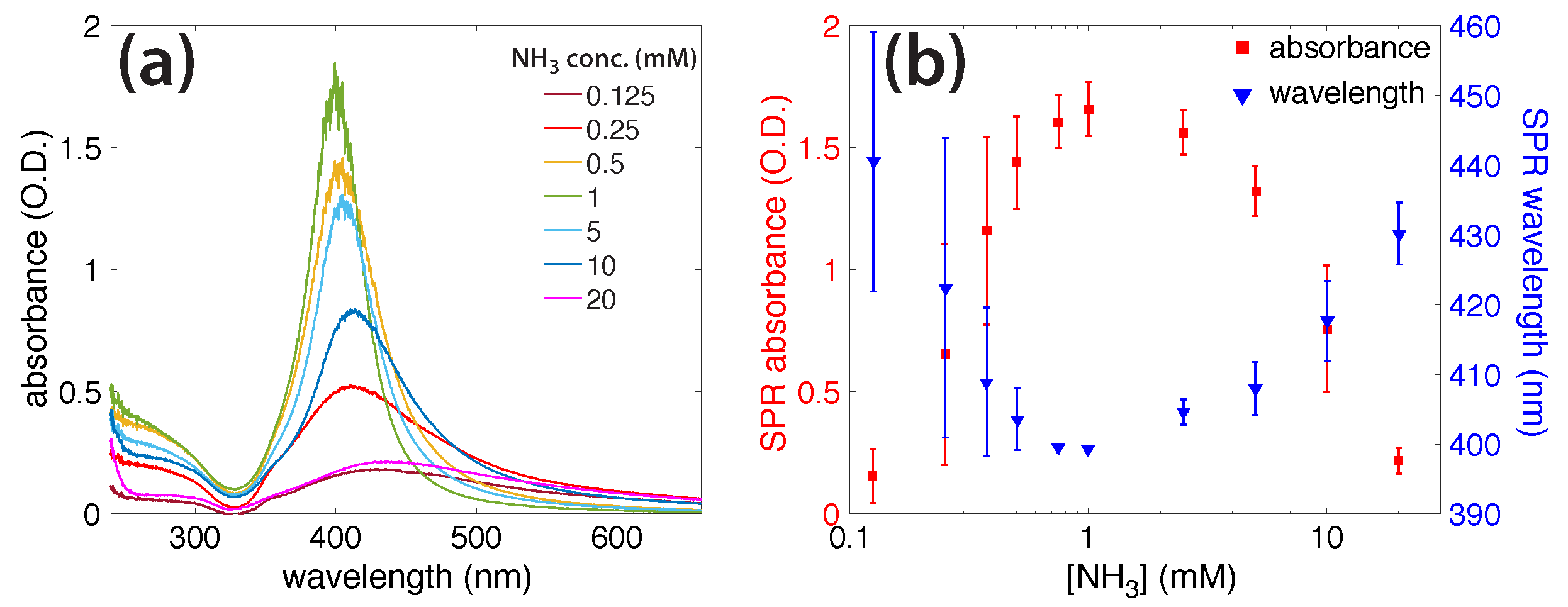
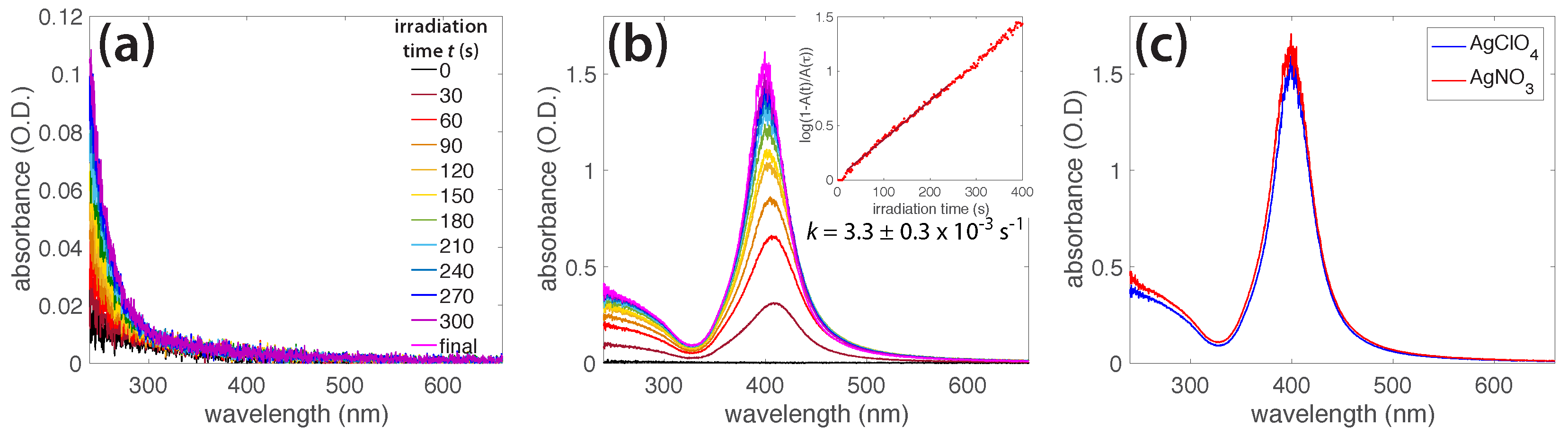
3.2. Photochemical AgNO3 Reduction in Liquid Ammonia Solutions
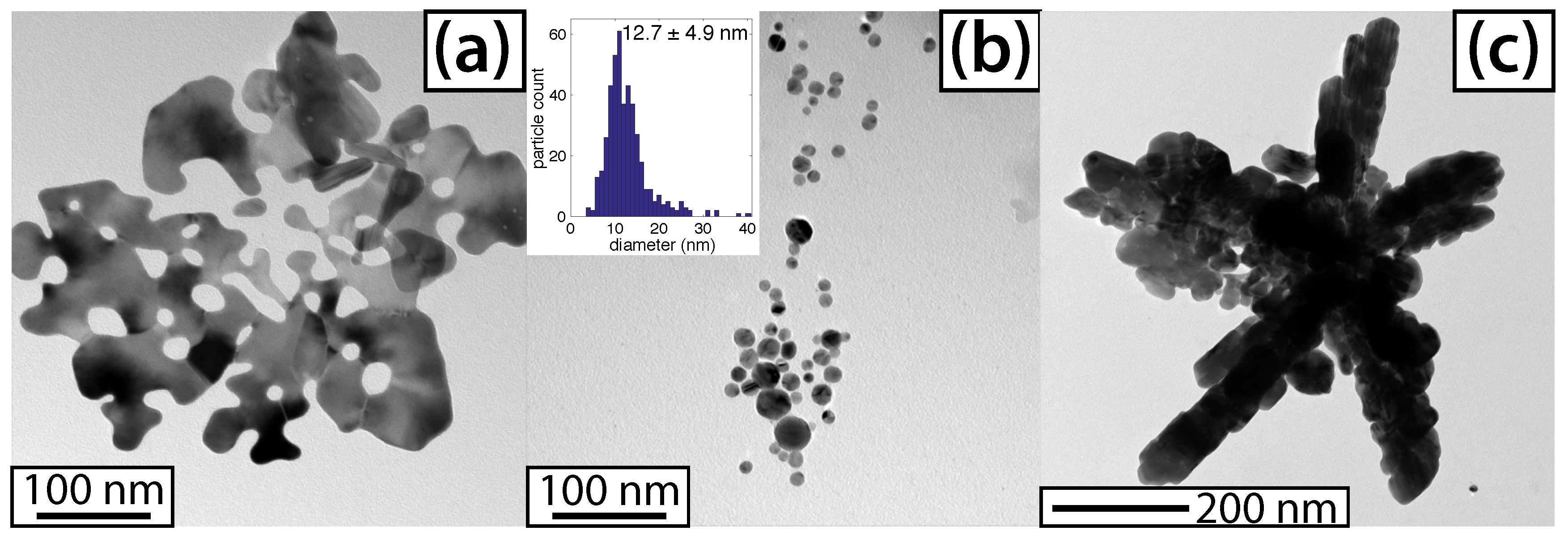
3.3. Characterization of AgNPs
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- LaVerne, J.A.; Pimblott, S.M. Yields of Hydroxyl Radical and Hydrated Electron Scavenging Reactions in Aqueous Solutions of Biological Interest. Radiat. Res. 1993, 135, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Breen, A.P.; Murphy, J.A. Reactions of oxyl radicals with DNA. Free Radic. Biol. Med. 1995, 18, 1033–1077. [Google Scholar] [CrossRef]
- Getoff, N. Radiation-induced degradation of water pollutants—state of the art. Radiat. Phys. Chem. 1996, 47, 581–593. [Google Scholar] [CrossRef]
- Huang, L.; Li, L.; Dong, W.; Liu, Y.; Hou, H. Removal of Ammonia by OH Radical in Aqueous Phase. Environ. Sci. Technol. 2008, 42, 8070–8075. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, M.; Chen, B.; Wang, L.; Zhu, R. Effects of pH and H2O2 on ammonia, nitrite, and nitrate transformations during UV254nm irradiation: Implications to nitrogen removal and analysis. Chemosphere 2017, 184, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xu, X.; Ge, X.; Zhang, Z. Preparation of silver nanocrystals in microemulsion by the γ-radiation method. Radiat. Phys. Chem. 1997, 50, 585–588. [Google Scholar]
- Belloni, J.; Mostafavi, M.; Remita, H.; Marignier, J.L.; Delcourt, M.O. Radiation-induced synthesis of mono- and multi-metallic clusters and nanocolloids. New J. Chem. 1998, 22, 1239–1255. [Google Scholar] [CrossRef]
- Li, T.; Park, H.G.; Choi, S.H. γ-Irradiation-induced preparation of Ag and Au nanoparticles and their characterizations. Mater. Chem. Phys. 2007, 105, 325–330. [Google Scholar] [CrossRef]
- Du, B.D.; Phu, D.V.; Duy, N.N.; Lan, N.T.K.; Lang, V.T.K.; Thanh, N.V.K.; Phong, N.T.P.; Hien, N.Q. Preparation of colloidal silver nanoparticles in poly(N-vinylpyrrolidone) by γ-irradiation. J. Exp. Nanosci. 2008, 3, 207–213. [Google Scholar] [CrossRef]
- Wang, C.H.; Liu, C.J.; Wang, C.L.; Chien, C.C.; Hwu, Y.; Liu, R.S.; Yang, C.S.; Je, J.H.; Lin, H.M.; Margaritondo, G. Intense X-ray induced formation of silver nanoparticles stabilized by biocompatible polymers. Appl. Phys. A 2009, 97, 295–300. [Google Scholar] [CrossRef]
- Noack, J.; Vogel, A. Laser-Induced Plasma Formation in Water at Nanosecond to Femtosecond Time Scales: Calculation of Thresholds, Absorption Coefficients, and Energy Density. IEEE J. Quantum Electron. 1999, 35, 1156–1167. [Google Scholar] [CrossRef]
- Vogel, A.; Noack, J.; Hüttman, G.; Paltauf, G. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl. Phys. B 2005, 81, 1015–1047. [Google Scholar] [CrossRef]
- Kurihara, K.; Kizling, J.; Stenius, P.; Fendler, J.H. Laser and Pulse Radiolytically Induced Colloidal Gold Formation in Water and in Water-in-Oil Microemulsions. J. Am. Chem. Soc. 1983, 105, 2574–2579. [Google Scholar] [CrossRef]
- Crowell, R.A.; Bartels, D.M. Multiphoton Ionization of Liquid Water with 3.0–5.0 eV Photons. J. Phys. Chem. 1996, 100, 17940–17949. [Google Scholar] [CrossRef]
- Chin, S.L.; Lagacé, S. Generation of H2, O2, and H2O2 from Water by the Use of Intense Femtosecond Laser Pulses and the Possibility of Laser Sterilization. Appl. Opt. 1996, 35, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Reuther, A.; Laubereau, A.; Nikogosyan, D.N. Primary Photochemical Processes in Water. J. Phys. Chem. 1996, 100, 16794–16800. [Google Scholar] [CrossRef]
- Pommeret, S.; Gobert, F.; Mostafavi, M.; Lampre, I.; Mialocq, J.C. Femtochemistry of the Hydrated Electron at Decimolar Concentration. J. Phys. Chem. A 2001, 105, 11400–11406. [Google Scholar] [CrossRef]
- Nakashima, N.; Yamanaka, K.; Saeki, M.; Ohba, H.; Taniguchi, S.; Yatsuhashi, T. Metal Ion Reductions by Femtosecond Laser Pulses with Micro-Joule Energy and their Efficiencies. J. Photochem. Photobiol. A 2016, 319–320, 70–77. [Google Scholar] [CrossRef]
- Nakamura, T.; Mochidzuki, Y.; Sato, S. Fabrication of Gold Nanoparticles in Intense Optical field by femtosecond laser irradiation of aqueous solution. J. Mater. Res. 2008, 23, 968–974. [Google Scholar] [CrossRef]
- Nakamura, T.; Magara, H.; Herbani, Y.; Sato, S. Fabrication of silver nanoparticles by highly intense laser irradiation of aqueous solution. Appl. Phys. A 2011, 104, 1021. [Google Scholar] [CrossRef]
- Islam Sarker, M.S.; Nakamura, T.; Sato, S. Composition-controlled ternary Rh–Pd–Pt solid-solution alloy nanoparticles by laser irradiation of mixed solution of metallic ions. J. Mater. Res. 2014, 29, 856–864. [Google Scholar] [CrossRef]
- Belmouaddine, H.; Shi, M.; Karsenti, P.L.; Meesat, R.; Sanche, L.; Houde, D. Dense Ionization and Subsequent Non-Homogeneous Radical-Mediated Chemistry of Femtosecond Laser-Induced Low Density Plasma in Aqueous Solutions: Synthesis of Colloidal Gold. Phys. Chem. Chem. Phys. 2017, 19, 7897–7909. [Google Scholar] [CrossRef] [PubMed]
- Herbani, Y.; Nakamura, T.; Sato, S. Spectroscopic monitoring on irradiation-induced formation of AuAg alloy nanoparticles by femtosecond laser. AIP Conf. Proc. 2016, 1711, 030005. [Google Scholar]
- Herbani, Y.; Nakamura, T.; Sato, S. Silver nanoparticle formation by femtosecond laser induced reduction of ammonia-containing AgNO3 solution. J. Phys. Conf. Ser. 2017, 817, 012048. [Google Scholar] [CrossRef]
- Uwada, T.; Wang, S.F.; Liu, T.H.; Masuhara, H. Preparation and micropatterning of gold nanoparticles by femtosecond laser-induced optical breakdown. J. Photochem. Photobiol. A 2017, 346, 177–186. [Google Scholar] [CrossRef]
- Odhner, J.H.; Moore Tibbetts, K.; Tangeysh, B.; Wayland, B.B.; Levis, R.J. Mechanism of Improved Au Nanoparticle Size Distributions Using Simultaneous Spatial and Temporal Focusing for Femtosecond Laser Irradiation of Aqueous KAuCl4. J. Phys. Chem. C 2014, 118, 23986–23995. [Google Scholar] [CrossRef]
- Tangeysh, B.; Moore Tibbetts, K.; Odhner, J.H.; Wayland, B.B.; Levis, R.J. Gold Nanoparticle Synthesis Using Spatially and Temporally Shaped Femtosecond Laser Pulses: Post-Irradiation Auto-Reduction of Aqueous [AuCl4]−. J. Phys. Chem. C 2013, 117, 18719–18727. [Google Scholar] [CrossRef]
- Tibbetts, K.M.; Tangeysh, B.; Odhner, J.H.; Levis, R.J. Elucidating Strong Field Photochemical Reduction Mechanisms of Aqueous [AuCl4]−: Kinetics of Multiphoton Photolysis and Radical-Mediated Reduction. J. Phys. Chem. A 2016, 120, 3562–3569. [Google Scholar] [CrossRef] [PubMed]
- Meader, V.K.; John, M.G.; Rodrigues, C.J.; Tibbetts, K.M. Roles of Free Electrons and H2O2 in the Optical Breakdown-Induced Photochemical Reduction of Aqueous [AuCl4]−. J. Phys. Chem. A 2017, 121, 6742–6754. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.C.; Braun, A.M. VUV photolysis of aqueous solutions of nitrate and nitrite. Res. Chem. Intermed. 1995, 21, 837–859. [Google Scholar] [CrossRef]
- Besner, S.; Meunier, M. Femtosecond Laser Synthesis of AuAg Nanoalloys: Photoinduced Oxidation and Ions Release. J. Phys. Chem. C 2010, 114, 10403–10409. [Google Scholar] [CrossRef]
- He, D.; Garg, S.; Waite, T.D. H2O2-Mediated Oxidation of Zero-Valent Silver and Resultant Interactions among Silver Nanoparticles, Silver Ions, and Reactive Oxygen Species. Langmuir 2012, 28, 10266–10275. [Google Scholar] [CrossRef] [PubMed]
- Remita, S.; Mostafavi, M.; Delcourt, M.O. Stabilization, growth, and reactivity of silver aggregates produced by radiolysis in the presence of EDTA. New J. Chem. 1994, 18, 581–588. [Google Scholar]
- Zeng, H.; Zhao, C.; Qiu, J.; Yang, Y.; Chen, G. Preparation and optical properties of silver nanoparticles induced by a femtosecond laser irradiation. J. Cryst. Growth 2007, 300, 519–522. [Google Scholar] [CrossRef]
- Xu, B.B.; Ma, Z.C.; Wang, L.; Zhang, R.; Niu, L.G.; Yang, Z.; Zhang, Y.L.; Zheng, W.H.; Zhao, B.; Xu, Y.; et al. Localized flexible integration of high-efficiency surface enhanced Raman scattering (SERS) monitors into microfluidic channels. Lab Chip 2011, 11, 3347–3351. [Google Scholar] [CrossRef] [PubMed]
- Abid, J.P.; Wark, A.W.; Brevet, P.F.; Girault, H.H. Preparation of silver nanoparticles in solution from a silver salt by laser irradiation. Chem. Commun. 2002, 792–793. [Google Scholar] [CrossRef]
- Šišková, K.; Vlčková, B.; Turpin, P.Y.; Fayet, C. Ion-Specific Effects on Laser Ablation of Silver in Aqueous Electrolyte Solutions. J. Phys. Chem. C 2008, 112, 4435–4443. [Google Scholar] [CrossRef]
- Zepp, R.G.; Hoigne, J.; Bader, H. Nitrate-induced photooxidation of trace organic chemicals in water. Environ. Sci. Technol. 1987, 21, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Rémita, S.; Mostafavi, M.; Delcourt, M.O. EDTA and CN- Complexing Effect on the Kinetics, Spectral Properties, and Redox Properties of Ag10 and Ag12+ in Aqueous Solution. J. Phys. Chem. 1996, 100, 10187–10193. [Google Scholar] [CrossRef]
- Texier, I.; Rémita, S.; Archirel, P.; Mostafavi, M. Reduction of AgI1(NH3)2+ to Ag01(NH3)2 in Solution. Redox Potential and Spectral Study. J. Phys. Chem. 1996, 100, 12472–12476. [Google Scholar] [CrossRef]
- Ershov, B.G.; Henglein, A. Reduction of Ag+ on Polyacrylate Chains in Aqueous Solution. J. Phys. Chem. B 1998, 102, 10663–10666. [Google Scholar] [CrossRef]
- Goia, D.V. Preparation and formation mechanisms of uniform metallic particles in homogeneous solutions. J. Mater. Chem. 2004, 14, 451–458. [Google Scholar] [CrossRef]
- Chopra, I. The increasing use of silver-based products as antimicrobial agents: A useful development or a cause for concern? J. Antimicrob. Chemother. 2007, 59, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Lee, C.; Yang, M.S.; Jeong, S.; Kim, D.; Kang, T. Two-dimensional Hyper-branched Gold Nanoparticles Synthesized on a Two-dimensional Oil/Water Interface. Sci. Rep. 2014, 4, 6119. [Google Scholar] [CrossRef] [PubMed]
- Papée, H.M.; Petriconi, G.L. Formation and Decomposition of Alkaline ‘Pernitrite’. Nature 1964, 204, 142. [Google Scholar] [CrossRef]
- Eisenberg, G. Colorimetric Determination of Hydrogen Peroxide. Ind. Eng. Chem. Anal. Ed. 1943, 15, 327–328. [Google Scholar] [CrossRef]
- Huckaba, C.E.; Keyes, F.G. The Accuracy of Estimation of Hydrogen Peroxide by Potassium Permanganate Titration. J. Am. Chem. Soc. 1948, 70, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- McBride, R.S. The Standardization of Potassium Permanganate Solution by Sodium oxalate. J. Am. Chem. Soc. 1912, 34, 393–416. [Google Scholar] [CrossRef]
- Hughes, M.N.; Nicklin, H.G. The chemistry of pernitrites. Part I. Kinetics of decomposition of pernitrous acid. J. Chem. Soc. A 1968, 450–452. [Google Scholar] [CrossRef]
- Edwards, J.O.; Plumb, R.C. Progress in Inorganic Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1994; Chapter The Chemistry of Peroxonitrites; pp. 599–635. [Google Scholar]
- Plumb, R.C.; Edwards, J.O. Color centers in UV-irradiated nitrates. J. Phys. Chem. 1992, 96, 3245–3247. [Google Scholar] [CrossRef]
- Mahoney, L.R. Evidence for the formation of hydroxyl radicals in the isomerization of pernitrous acid to nitric acid in aqueous solution. J. Am. Chem. Soc. 1970, 92, 5262–5263. [Google Scholar] [CrossRef]
- Leopold, N.; Lendl, B. A New Method for Fast Preparation of Highly Surface-Enhanced Raman Scattering (SERS) Active Silver Colloids at Room Temperature by Reduction of Silver Nitrate with Hydroxylamine Hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Ong, B.S. Facile Synthesis of Silver Nanoparticles Useful for Fabrication of High-Conductivity Elements for Printed Electronics. J. Am. Chem. Soc. 2005, 127, 3266–3267. [Google Scholar] [CrossRef] [PubMed]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 1991, 266, 4244. [Google Scholar] [PubMed]
- Goldstein, S.; Lind, J.; Merényi, G. Chemistry of Peroxynitrites as Compared to Peroxynitrates. Chem. Rev. 2005, 105, 2457–2470. [Google Scholar] [CrossRef] [PubMed]
- Pagsberg, P. Investigation of the NH2 radical produced by pulse radiolysis of ammonia in aqueous solution. In Aspects of Research at Risø; Aspects of Research at Risø: Roskilde, Denmark, 1972; pp. 209–222. [Google Scholar]
- Dey, G. Nitrogen compounds’ formation in aqueous solutions under high ionizing radiation: An overview. Radiat. Phys. Chem. 2011, 80, 394–402. [Google Scholar] [CrossRef]
- Rigg, T.; Scholes, G.; Weiss, J. 580. Chemical actions of ionising radiations in solutions. Part X. The action of X-rays on ammonia in aqueous solution. J. Chem. Soc. 1952, 3034–3038. [Google Scholar] [CrossRef]
- Maurer, P.; Thomas, C.F.; Kissner, R.; Rüegger, H.; Greter, O.; Röthlisberger, U.; Koppenol, W.H. Oxidation of Nitrite by Peroxynitrous Acid. J. Phys. Chem. A 2003, 107, 1763–1769. [Google Scholar] [CrossRef]
- Merényi, G.; Lind, J.; Goldstein, S.; Czapski, G. Mechanism and Thermochemistry of Peroxynitrite Decomposition in Water. J. Phys. Chem. A 1999, 103, 5685–5691. [Google Scholar] [CrossRef]
- Thøgersen, J.; Kissner, R.; Nauser, T.; Koppenol, W.H.; Richter, B.; Jensen, F.; Keiding, S.R.; Jensen, S.J.K. Primary photochemistry of peroxynitrite in aqueous solution. Chem. Phys. Lett. 2015, 641, 187–192. [Google Scholar] [CrossRef]
- Goldstein, S.; Rabani, J. Mechanism of Nitrite Formation by Nitrate Photolysis in Aqueous Solutions: The Role of Peroxynitrite, Nitrogen Dioxide, and Hydroxyl Radical. J. Am. Chem. Soc. 2007, 129, 10597–10601. [Google Scholar] [CrossRef] [PubMed]
- Staehelin, J.; Hoigne, J. Decomposition of ozone in water: Rate of initiation by hydroxide ions and hydrogen peroxide. Environ. Sci. Technol. 1982, 16, 676–681. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Hu, S.; Hand, D.W.; Green, S.A. A kinetic model for H2O2/UV process in a completely mixed batch reactor. Water Res. 1999, 33, 2315–2328. [Google Scholar] [CrossRef]
- Barker, G.C.; Fowles, P.; Stringer, B. Pulse radiolytic induced transient electrical conductance in liquid solutions. Part 2.-Radiolysis of aqueous solutions of NO, NO and Fe(CN). Trans. Faraday Soc. 1970, 66, 1509–1519. [Google Scholar] [CrossRef]
- Zhu, X.; Castleberry, S.R.; Nanny, M.A.; Butler, E.C. Effects of pH and Catalyst Concentration on Photocatalytic Oxidation of Aqueous Ammonia and Nitrite in Titanium Dioxide Suspensions. Environ. Sci. Technol. 2005, 39, 3784–3791. [Google Scholar] [CrossRef] [PubMed]
- Mack, J.; Bolton, J.R. Photochemistry of nitrite and nitrate in aqueous solution: A review. J. Photochem. Photobiol. A Chem. 1999, 128, 1–13. [Google Scholar] [CrossRef]
- Hughes, M.N.; Nicklin, H.G.; Sackrule, W.A.C. The chemistry of peroxonitrites. Part III. The reaction of peroxonitrite with nucleophiles in alkali, and other nitrite producing reactions. J. Chem. Soc. A 1971, 3722–3725. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Reaction No. | Equation | Rate Constant (M−1 s−1) | Ref. |
|---|---|---|---|
| 1 | NH3 + OH· → NH2· + H2O | 1 × 108 | [59] |
| 2 | NH2· + OH· → NH2OH | 9.5 × 109 | [59] |
| 3 | 2NH2· → N2H4 | [60] | |
| 4 | NH2· + H2O2 → ·NHOH + H2O | 9 × 107 | [59] |
| 5 | NH2· + O2 → NH2O2· | [61] | |
| 6 | NH2O2· + OH· → HNO2 + H2O | [61] | |
| 7 | NO2− + OH· → NO2· + OH− | [62] | |
| 8 | NO2· + OH· → NO3− + H+ | 1 × 1010 | [62] |
| 9 | NO3− + eaq− → NO32− | [30] | |
| 10 | NO2− + OH· → ONOOH | 4.5 × 109 | [63] |
| 11 | NO2− + H2O2 → ONOO− + H2O | [47] | |
| 12 | NO3− + → ONOO− | [64] | |
| 13 | ONOO− + → NO3− | 0.9 s−1 | [65] |
| 13’ | ONOO− → NO3− | ~10−5 s−1 | [63] |
| 14 | ONOO− → NO2− + O2 | [52] | |
| 15 | H2O2 → HO2− + H+ | pKa = 11.6 | [66] |
| 16 | HO2− + OH· → OH− + O2H· | 7.5 × 109 | [67] |
| 17 | HO2− + NH2· → NH2O2· + H+ | [4] | |
| 18 | NH2O2· → NO· + H2O | [60] | |
| 19 | NO· + OH· → HNO2 | 8.9 × 109 | [68] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meader, V.K.; John, M.G.; Frias Batista, L.M.; Ahsan, S.; Tibbetts, K.M. Radical Chemistry in a Femtosecond Laser Plasma: Photochemical Reduction of Ag+ in Liquid Ammonia Solution. Molecules 2018, 23, 532. https://doi.org/10.3390/molecules23030532
Meader VK, John MG, Frias Batista LM, Ahsan S, Tibbetts KM. Radical Chemistry in a Femtosecond Laser Plasma: Photochemical Reduction of Ag+ in Liquid Ammonia Solution. Molecules. 2018; 23(3):532. https://doi.org/10.3390/molecules23030532
Chicago/Turabian StyleMeader, Victoria Kathryn, Mallory G. John, Laysa M. Frias Batista, Syeda Ahsan, and Katharine Moore Tibbetts. 2018. "Radical Chemistry in a Femtosecond Laser Plasma: Photochemical Reduction of Ag+ in Liquid Ammonia Solution" Molecules 23, no. 3: 532. https://doi.org/10.3390/molecules23030532