Modification and Characterization of Fe3O4 Nanoparticles for Use in Adsorption of Alkaloids
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Physical Measurements
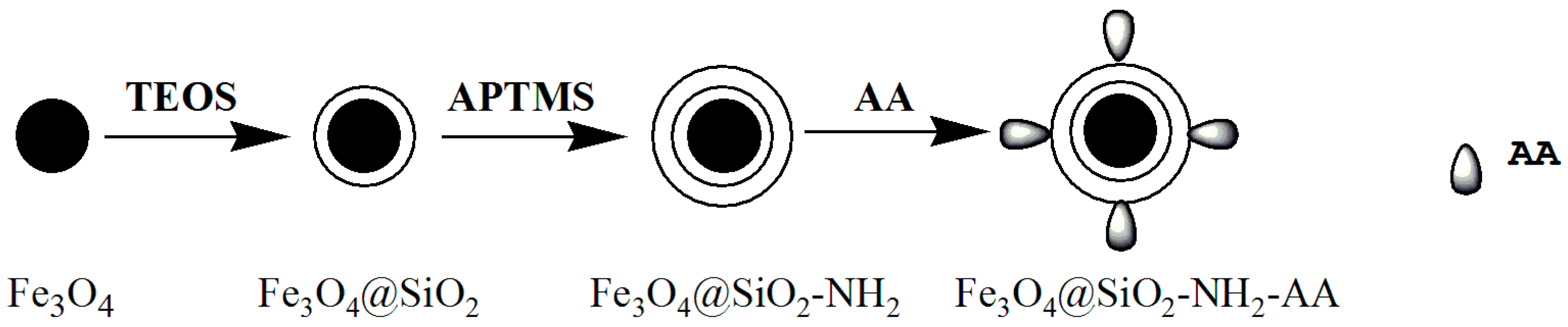
2.2. Preparation and Modification of Fe3O4 Nanoparticles
2.2.1. Preparation of Fe3O4 Nanoparticles
2.2.2. Preparation of Fe3O4@SiO2
2.2.3. Preparation of Fe3O4@SiO2-NH2
2.2.4. Preparation of Fe3O4@SiO2-NH2-AA
2.3. Alkaloid Adsorption Test
2.3.1. Preparation of Calibration Standards
2.3.2. Influence from pH
2.3.3. Influence from Adsorption Time
3. Results and Discussion
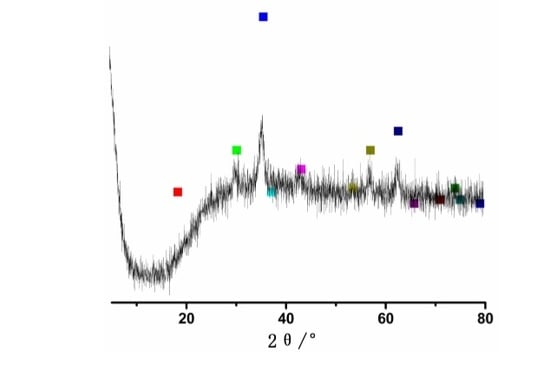
3.1. XRD Analysis of Fe3O4 Nanoparticles
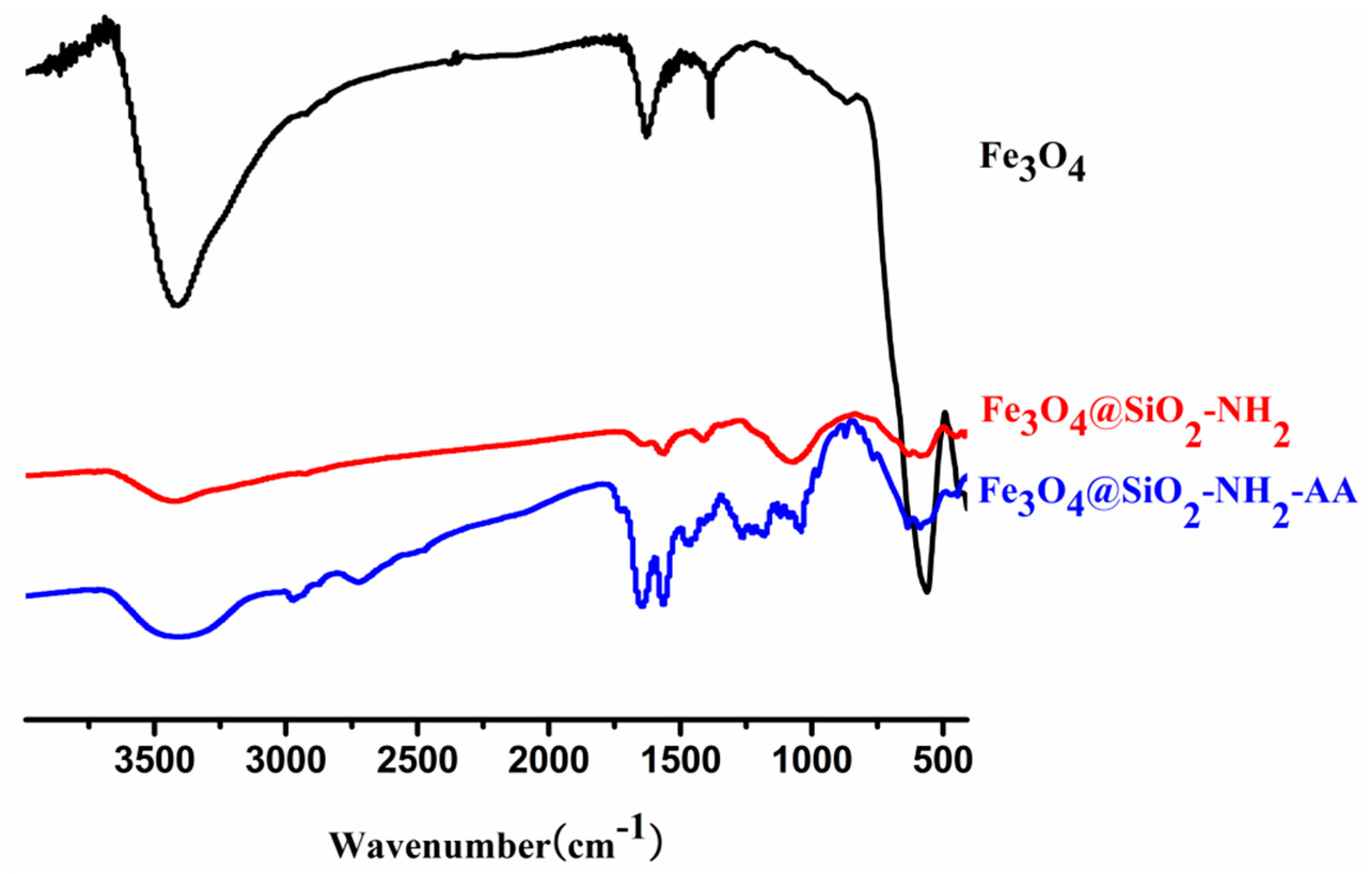
3.2. FTIR Spectra Analysis of Nanoparticles
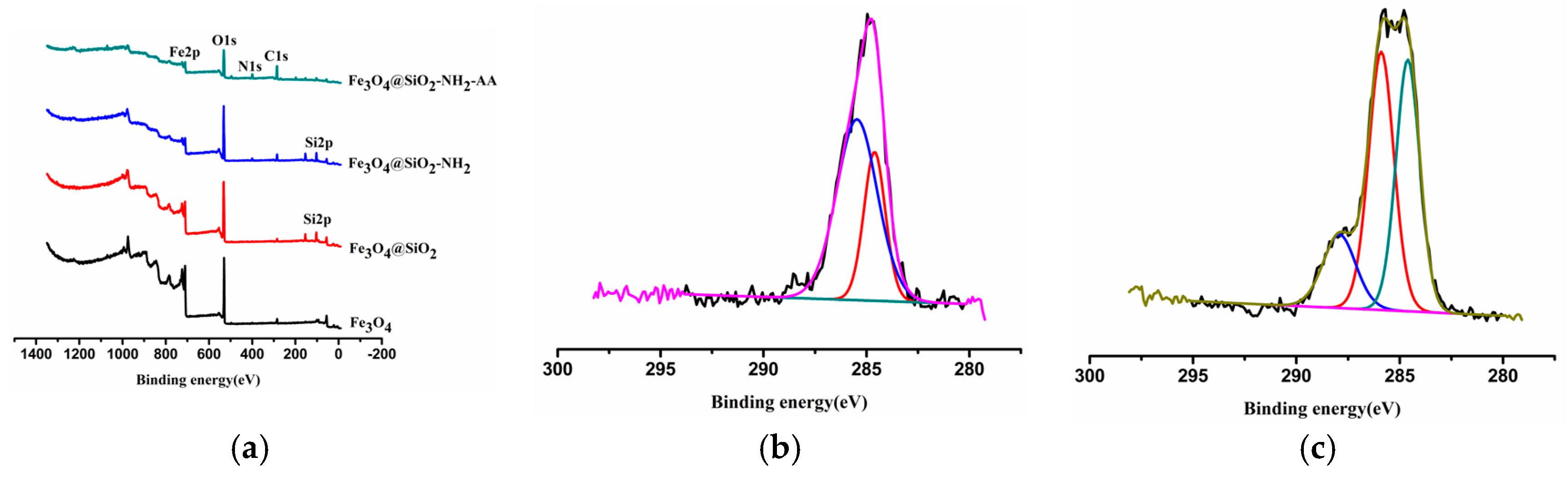
3.3. XPS Analysis of Nanoparticles
3.4. SEM Analysis of Nanoparticles
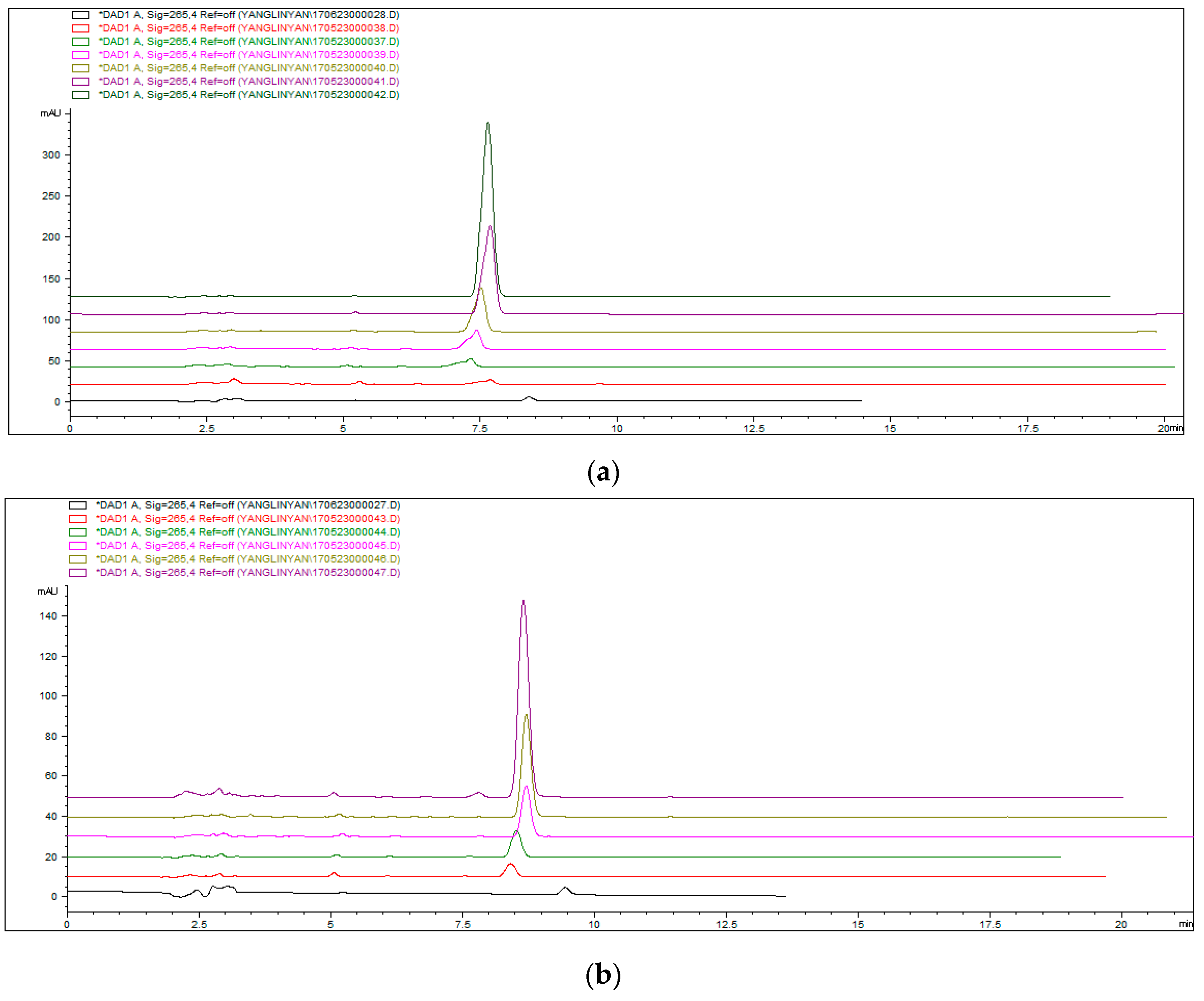
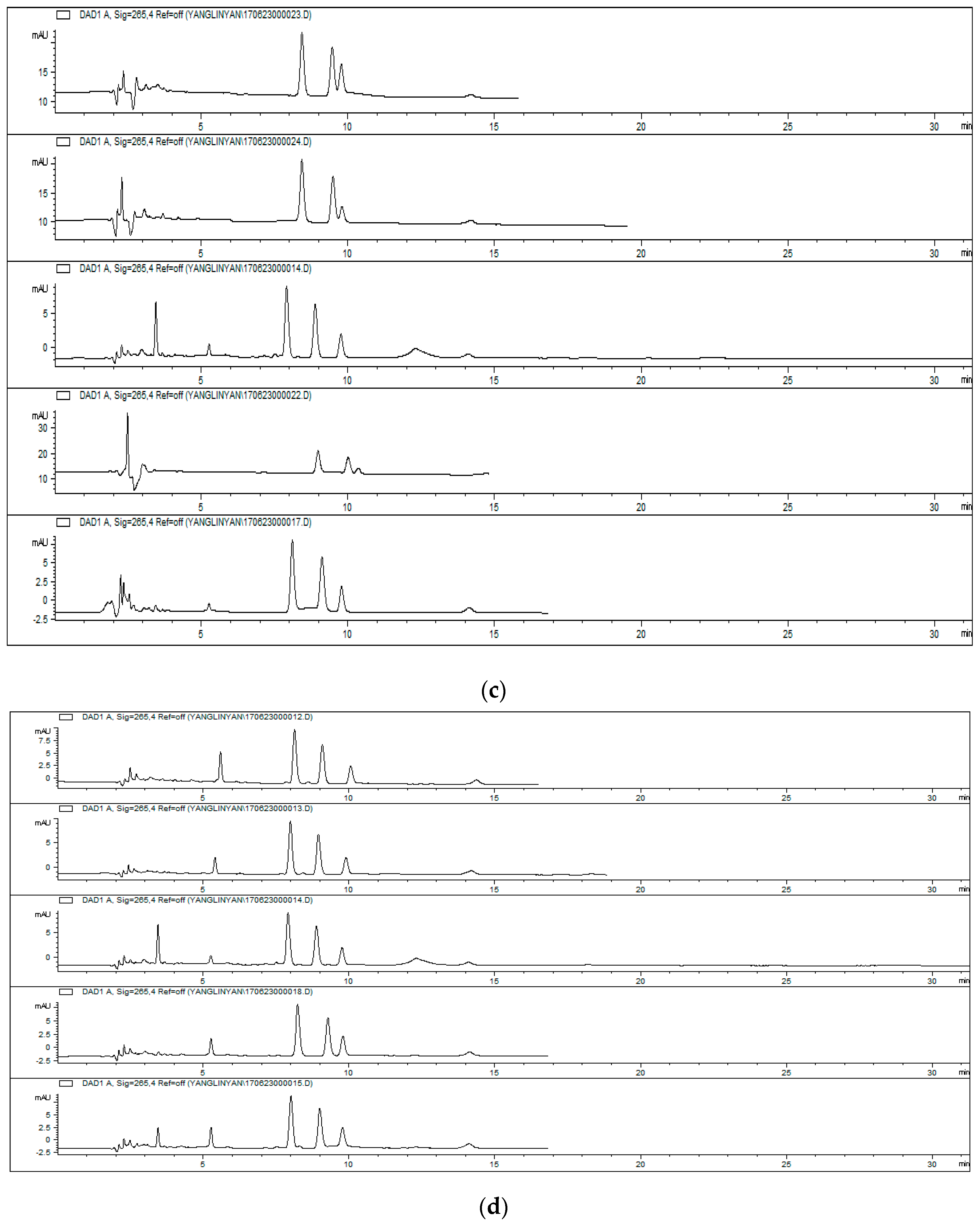
3.5. Analysis of Alkaloid Adsorption Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–4. [Google Scholar] [CrossRef]
- Xu, X.; Friedman, G.; Humfeld, K.D.; Majetich, S.A.; Asher, S.A. Synthesis and utilization of monodisperse superparamagnetic colloidal particles for magnetically controllable photonic crystals. Chem. Mater. 2002, 14, 1249–1256. [Google Scholar] [CrossRef]
- Ge, J.; Hu, Y.; Biasini, M.; Beyermann, W.P.; Yin, Y. Superparamagnetic magnetite colloidal nanocrystal clusters. Angew. Chem. Int. Ed. 2007, 46, 4342–4345. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Hu, Y.; Yin, Y. Highly tunable superparamagnetic colloidal photonic crystals. Angew. Chem. Int. Ed. 2007, 46, 7428–7431. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zheng, L.L.; Lu, F.H.; Hong, R.J.; Chen, M.Z.Q.; Zhuang, L. Facile synthesis and characterization of magnetochromatic Fe3O4 nanoparticles. AIP Adv. 2017, 7, 056317. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Soliman, E.M. Silica coated magnetic particles using microwave synthesis for removal of dyes from natural water samples: Synthesis, characterization, equilibrium, isotherm and kinetics studies. Appl. Surf. Sci. 2013, 284, 23–32. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, J.C.; You, C.X.; Song, Z.W.; Yu, B.W.; Shen, Y. Surface modification of Fe3O4 nanoparticles and their magnetic properties. Int. J. Miner. Metall. Mater. 2009, 16, 226–229. [Google Scholar] [CrossRef]
- Zhang, L.; Shao, H.P.; Zheng, H.; Lin, T.; Guo, Z.M. Synthesis and characterization of Fe3O4@SiO2 magnetic compositenanoparticles by a one-pot process. Int. J. Miner. Metall. Mater. 2016, 23, 1112–1118. [Google Scholar] [CrossRef]
- Bao, S.G.; Tang, L.H.; Li, K.; Ning, P.; Peng, J.H.; Guo, H.B.; Zhu, T.T.; Liu, Y. Highly selective removal of Zn(II) ion from hot-dip galvanizing pickling waste with amino-functionalized Fe3O4@SiO2 magnetic nano-adsorbent. J. Colloid Interface Sci. 2016, 462, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.G.; Li, K.; Ning, P.; Peng, J.H.; Jin, X.; Tang, L.H. Highly selective removal of mercury and lead ions from wastewater by mercaptoamine-functionalised silica-coated magnetic nano-adsorbents: Behaviours and mechanisms. Appl. Surf. Sci. 2017, 393, 457–466. [Google Scholar] [CrossRef]
- Lim, S.F.; Zheng, Y.M.; Zou, S.W.; Chen, J.P. Uptake of arsenate by an alginate-encapsulated magnetic sorbent: Process performance and characterization of adsorption chemistry. J. Colloid Interface Sci. 2009, 333, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Daemi, H.; Barikani, M. Fast removal of malachite green dye using novel superparamagnetic sodium alginate-coated Fe3O4 nanoparticles. Int. J. Biol. Macromol. 2014, 69, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Solanki, S.; Chaudhari, R.; Bahadur, D.; Aslam, M.; Srivastava, R. Multifunctional alginate microspheres for biosensing, drug delivery and magnetic resonance imaging. Acta Biomater. 2011, 7, 3955–3963. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Ren, L.; Wang, X.Q.; Wang, Q.; Wan, Z.F.; Li, L.; Liu, W.M.; Wang, X.M.; Li, M.L.; Tong, D.W.; et al. Superparamagnetic microsphere-assisted fluoroimmunoassay for rapid assessment of acute myocardial infarction. Biosens. Bioelectr. 2009, 24, 3097–3102. [Google Scholar] [CrossRef] [PubMed]
- Rezayan, A.H.; Mousavi, M.; Kheirjou, S.; Amoabediny, G.; Ardestani, M.S.; Mohammadnejad, J. Monodisperse magnetite (Fe3O4) nanoparticles modified with water soluble polymers for the diagnosis of breast cancer by MRI method. J. Magn. Magn. Mater. 2016, 420, 210–217. [Google Scholar] [CrossRef]
- Biradar, A.V.; Patil, V.S.; Chandra, P.; Doke, D.S.; Asefa, T. A trifunctional mesoporous silica-based, highly active catalyst for one-pot, three-step cascade reactions. Chem. Commun. 2015, 51, 8496–8499. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ding, W.; Meng, F. Fabrication and in vitro evaluation of folate-modified iron ferrite nanoparticles with high doxorubicin loading for receptors-magnetic-guided drug delivery. Nano Brief Rep. Rev. 2014, 9, 1450021–1450028. [Google Scholar] [CrossRef]
- Cai, Y.; Yuan, F.; Wang, X.; Sun, Z.; Chen, Y.; Liu, Z.; Wang, X.; Yang, S.; Wang, S. Synthesis of core-shell structured Fe3O4@carboxymethylcellulose magnetic composite for highly efficient removal of Eu(III). Cellulose 2017, 24, 175–190. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.B.; Song, Y.; Yang, J.; Wu, L.J.; Yang, B.Y.; Wang, Q.H.; Wang, L.Q.; Wang, R.X.; Yang, C.J. Simultaneous determination of eight alkaloids in rat plasma by UHPLC-MS/MS after oral administration of Coptisdeltoidea C.Y. Cheng et Hsiao and Coptischinensis Franch. Molecules 2016, 21, 913–915. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Long, Y.; Yao, L.; Ye, M.; Xu, L. C18-COOH silica: preparation, characterization and its application in purification of quaternary ammonium alkaloids from Coptischinensis. Phytochem. Anal. 2017, 28, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.H.; Liu, X.D.; Chang, Y.X.; An, M.R.; Wang, S.L.; Xu, J.J.; Peng, L.Q. Analysis of isoquinoline alkaloids using chitosan-assisted liquid-solid extration followed by microemulsion liquid chromatography employing a sub-2-micron particles stationary phase. Electrophoresis 2016, 37, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wu, J.; Shen, Y.; Tian, R.; Zhou, S.; Jiang, W. Synthesis and characterization of doxorubicin loaded pH-sensitive magnetic core-shell nanocomposites for targeted drug delivery applications. Nano Brief Rep. Rev. 2016, 11, 1650127–1650140. [Google Scholar] [CrossRef]
- Jung, Y.C.; Muramatsu, H.; Fujisawa, K.; Kim, J.H.; Hayashi, T.; Kim, Y.A.; Endo, M.; Terrones, M.; Dresselhaus, M.S. Optically and biologically active mussel protein-coated double-walled carbon nanotubes. Small 2011, 7, 3292–3297. [Google Scholar] [CrossRef] [PubMed]
- Andhariya, N.; Upadhyay, R.; Mehta, R.; Chudasama, B. Folic acid conjugated magnetic drug delivery system for controlled release of doxorubicin. J. Nanopart. Res. 2013, 15, 1–12. [Google Scholar] [CrossRef]
- Chen, X.L.; Zhao, T.T.; Zou, J.L. A novel mimetic peroxidase catalyst by using magnetite-containing silica nanoparticles as carriers. Microchim. Acta 2009, 164, 93–99. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Tian, J.; Meng, J.; Zhao, R.; Li, C.; Ma, J.; Jin, T. Modification and Characterization of Fe3O4 Nanoparticles for Use in Adsorption of Alkaloids. Molecules 2018, 23, 562. https://doi.org/10.3390/molecules23030562
Yang L, Tian J, Meng J, Zhao R, Li C, Ma J, Jin T. Modification and Characterization of Fe3O4 Nanoparticles for Use in Adsorption of Alkaloids. Molecules. 2018; 23(3):562. https://doi.org/10.3390/molecules23030562
Chicago/Turabian StyleYang, Linyan, Jing Tian, Jiali Meng, Ruili Zhao, Cun Li, Jifei Ma, and Tianming Jin. 2018. "Modification and Characterization of Fe3O4 Nanoparticles for Use in Adsorption of Alkaloids" Molecules 23, no. 3: 562. https://doi.org/10.3390/molecules23030562