Steroidal Saponins from Vernonia amygdalina Del. and Their Biological Activity
Abstract
:1. Introduction
2. Results and Discussion
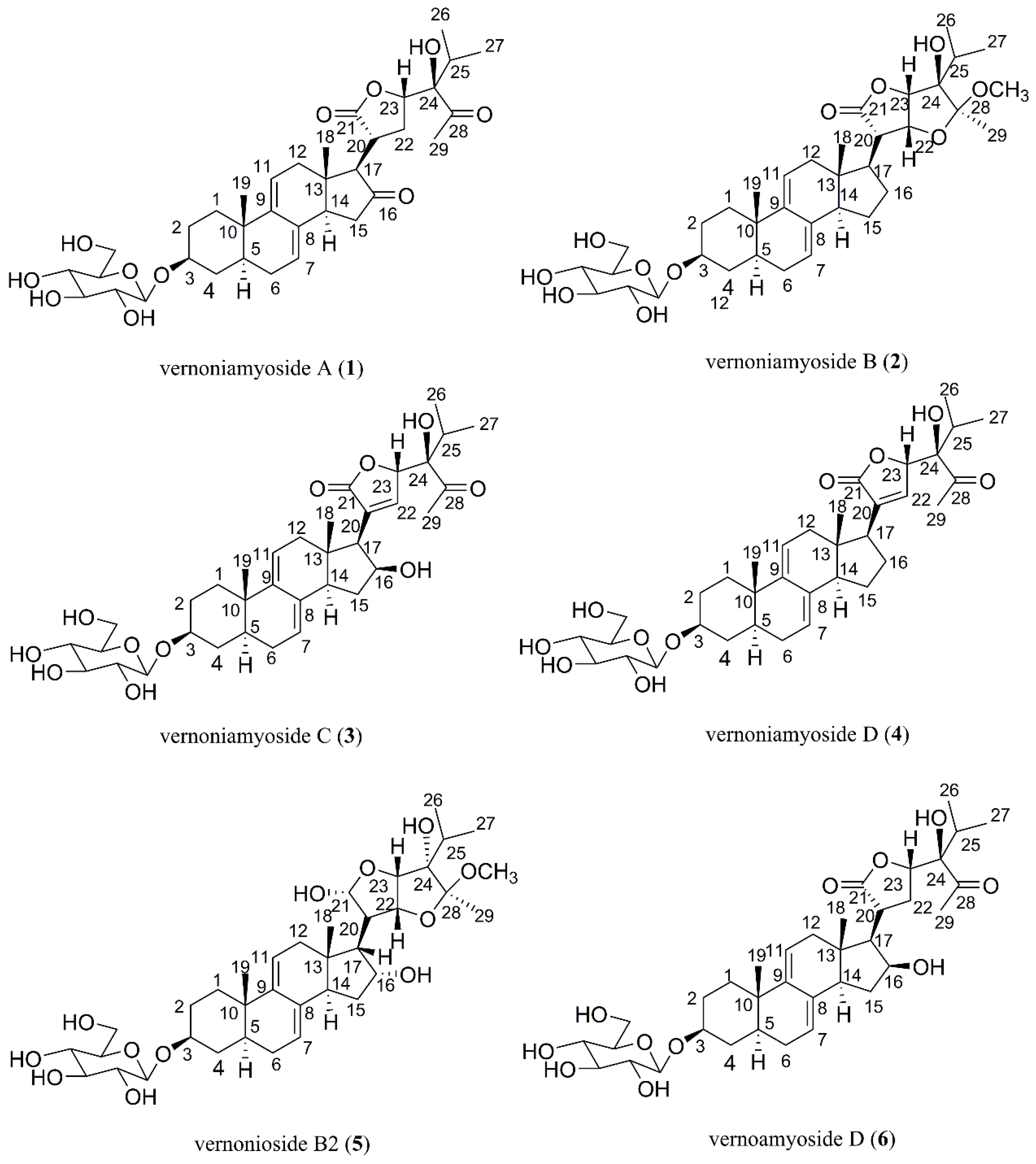
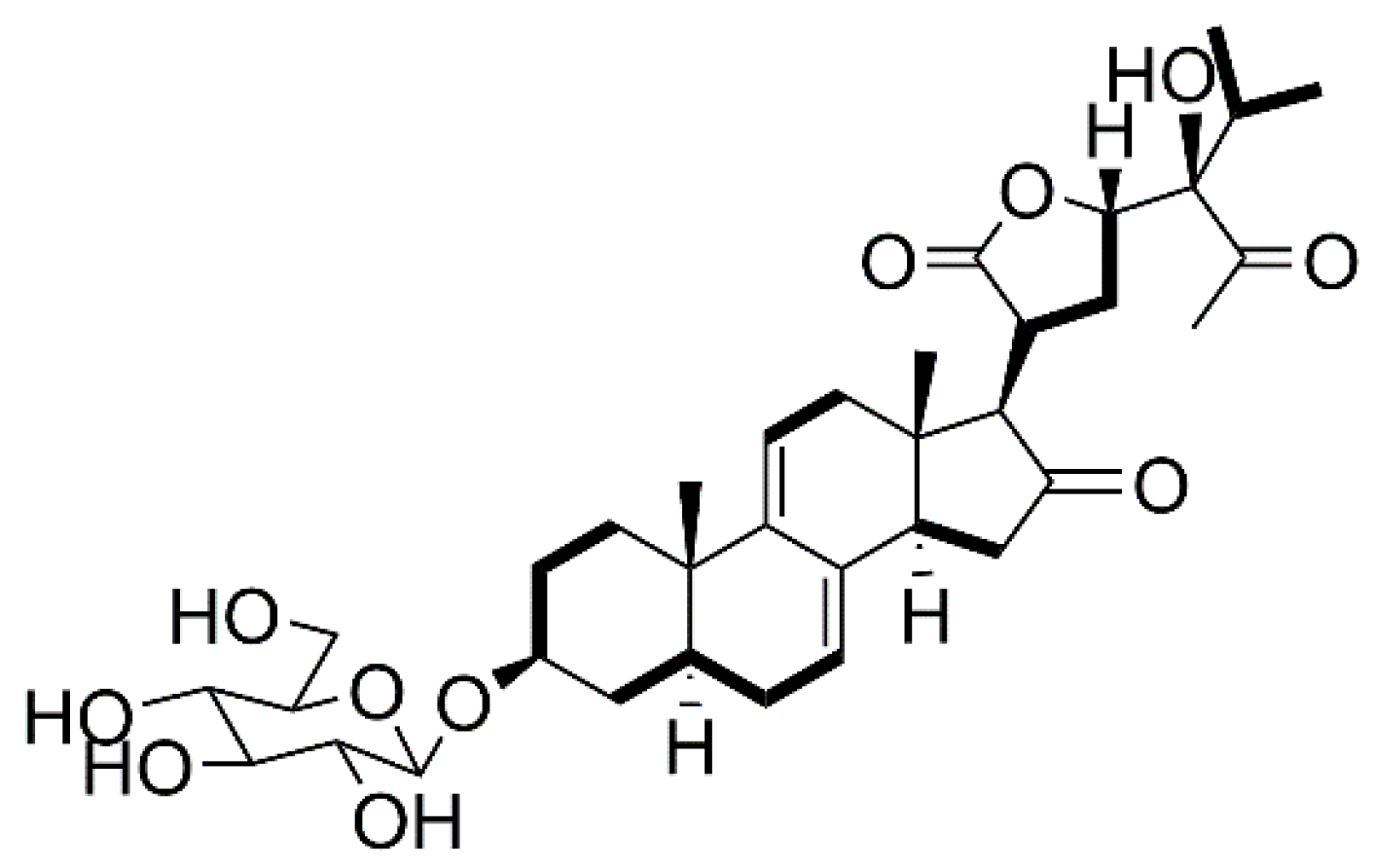
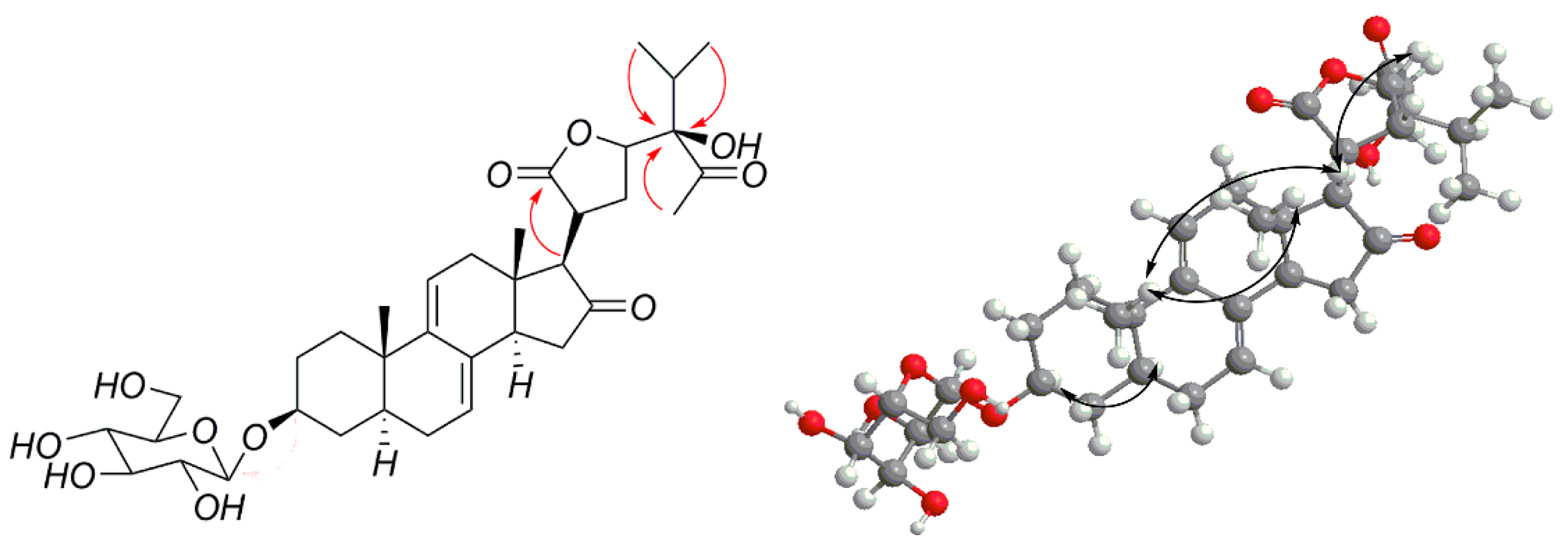
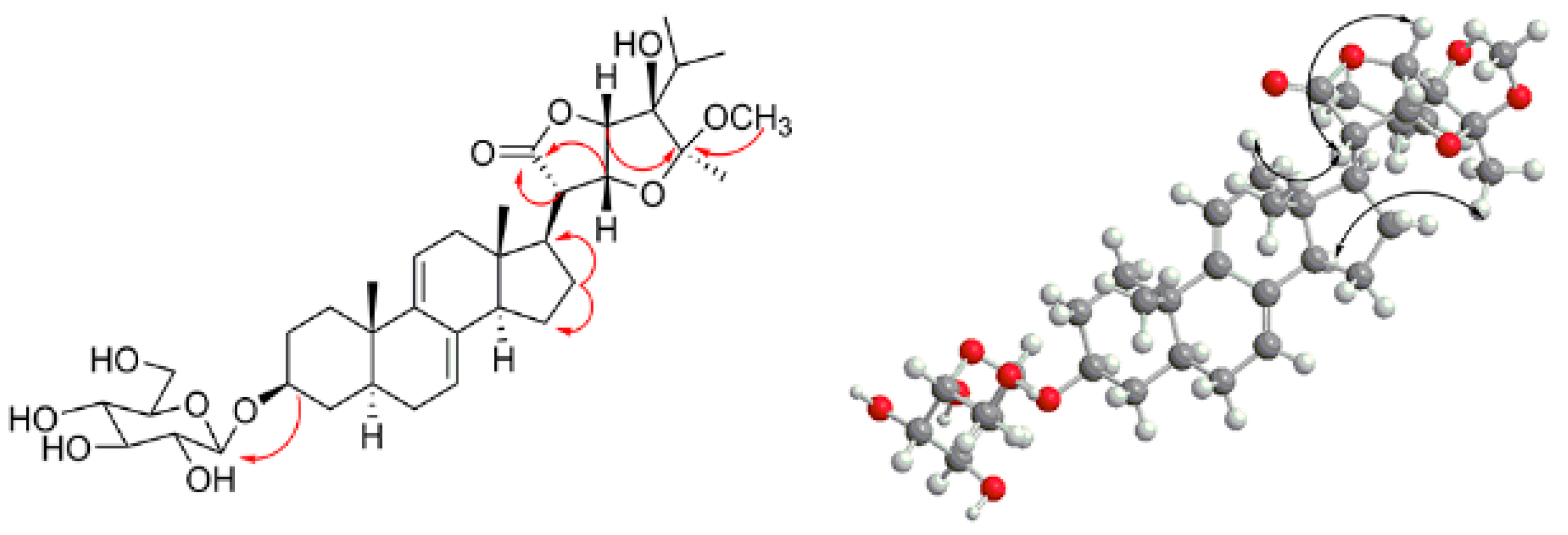
2.1. Isolation, Characterization, and Structure Elucidation of Compounds
2.2. Results of the Cytotoxicity Test
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Vernoniamyoside A (1)
3.3.2. Vernoniamyoside B (2)
3.3.3. Vernoniamyoside C (3)
3.3.4. Vernoniamyoside D (4)
3.3.5. Vernoamyoside D (5)
3.3.6. Vernonioside B2 (6)
3.4. Acid Hydrolysis of Compounds
3.5. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Ohigashi, H.; Jisaka, M.; Takagaki, T.; Nozaki, H.; Tada, T.; Huffman, M.A.; Nishida, T.; Kaji, M.; Koshimizu, K. Bitter principle and a related steroid glucoside from, a possible medicinal plant for wild chimpanzees. Agric. Biol. Chem. 1991, 55, 1201–1203. [Google Scholar] [CrossRef]
- Igile, G.O.; Oleszek, W.; Jurzysta, M.; Burda, S.; Fafunso, M.; Fasanmade, A.A. Flavonoids from vernonia amygdalina and their antioxidant activites. J. Agric. Food Chem. 1994, 42, 2445–2448. [Google Scholar] [CrossRef]
- Sinisi, A.; Millán, E.; Abay, S.M.; Habluetzel, A.; Appendino, G.; Muñoz, E.; Taglialatelascafati, O. Poly-electrophilic sesquiterpene lactones from vernonia amygdalina: New members and differences in their mechanism of thiol trapping and in bioactivity. J. Nat. Prod. 2015, 78, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Igile, G.O.; Oleszek, W.; Jurzysta, M.; Aquino, R.; Tommasi, N.; Pizza, C. Vernonioside d and e, two novel saponins from vernonia amygdalina del. (compositae). J. Nat. Prod. 1995, 589, 1438–1443. [Google Scholar] [CrossRef]
- Jisaka, M.; Ohigashi, H.; Takagaki, T.; Nozaki, H.; Tada, T.; Hirota, M.; Irie, R.; Huffman, M.A.; Nishida, T.; Kaji, M. Bitter steroid glucosides, vernoniosides a 1, a 2, and a 3, and related b 1 from a possible medicinal plant, vernonia amygdalina, used by wild chimpanzees. Tetrahedron 1992, 48, 625–632. [Google Scholar] [CrossRef]
- Quasie, O.; Zhang, Y.M.; Zhang, H.J.; Luo, J.; Kong, L.Y. Four new steroid saponins with highly oxidized side chains from the leaves of vernonia amygdalina. Phytochem. Lett. 2016, 15, 16–20. [Google Scholar] [CrossRef]
- Erasto, P.; Grierson, D.S.; Afolayan, A.J. Evaluation of antioxidant activity and the fatty acid profile of the leaves of vernonia amygdalina growing in south africa. Food Chem. 2007, 104, 636–642. [Google Scholar] [CrossRef]
- Eyong, E.U.; Atangwho, I.J.; David-Oku, E.; Agiang, M.A.; Ebong, P.E. Haematological and immunological effect of co- administration of extracts of vernonia amygdalina and azadirachta indica on normal and diabetic rats. Afr. J. Biotechnol. 2011, 10, 10258–10262. [Google Scholar] [CrossRef]
- Adedapo, A.A.; Otesile, A.T.; Soetan, K.O. Assessment of the anthelmintic efficacy of an aqueous crude extract of vernonia amygdalina. Pharm. Biol. 2008, 45, 564–568. [Google Scholar] [CrossRef]
- Egedigwe, C.A.; Ejike, C.E.; Ijeh, I.I.; Onwuka, G.I.; Herbert, U.; Asumugha, A.V. Anti-obesity potentials of aqueous and methanol extracts of vernonia amygdalina del. Leaves in high-fat diet fed rats. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 86–93. [Google Scholar] [CrossRef]
- Atangwho, I.J.; Edet, E.E.; Uti, D.E.; Obi, A.U.; Asmawi, M.Z.; Ahmad, M. Biochemical and histological impact of vernonia amygdalina supplemented diet in obese rats. Saudi. J. Biol. Sci. 2012, 19, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Erasto, P.; Grierson, D.S.; Afolayan, A.J. Antioxidant constituents in vernonia amygdalina leaves. Pharm. Biol. 2008, 45, 195–199. [Google Scholar] [CrossRef]
- Adaramoye, O.; Ogungbenro, B.; Anyaegbu, O.; Fafunso, M. Protective effects of extracts of vernonia amygdalina, hibiscus sabdariffa and vitamin c against radiation-induced liver damage in rats. J. Radiat. Res. 2008, 49, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Oyugi, D.A.; Lin, C.W.; Izevbigie, E.B.; Lee, K.S. Isolation and characterization of the antibreast carcinoma cell growth components of vernonia amygdalina extracts. Exp. Biol. Med. 2010, 235, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Sonibare, M.A.; Moody, J.O.; Adesanya, E.O. Use of medicinal plants for the treatment of measles in nigeria. J. Ethnopharmacol. 2009, 122, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Izevbigie, E.B.; Bryant, J.L.; Walker, A. A novel natural inhibitor of extracellular signal-regulated kinases and human breast cancer cell growth. Exp. Biol. Med. 2004, 229, 163–169. [Google Scholar] [CrossRef]
- Opata, M.M.; Izevbigie, E.B. Aqueous vernomia amygdalina extracts alter mcf-7 cell membrane permeability and efflux. Int. J. Environ. Res. Public Health 2006, 3, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Yedjou, C.; Izevbigie, E.; Tchounwou, P. Preclinical assessment of vernonia amygdalina leaf extracts as DNA damaging anti-cancer agent in the management of breast cancer. Int. J. Environ. Res. Public Health 2008, 5, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Gresham, L.J.; Ross, J.; Izevbigie, E.B. Vernonia amygdalina: Anticancer activity, authentication, and adulteration detection. Int. J. Environ. Res. Public Health 2008, 5, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Jisaka, M.; Ohigashi, H.; Takegawa, K.; Hirota, M.; Irie, R.; Huffman, M.A.; Koshimįzu, K. Steroid glucosides from vernonia amygdalina, a possible chimpanzee medicinal plant. Phytochemistry 1993, 34, 409–413. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds vernoniamyoside A–D, vernoamyoside D and vernonioside B2 are available from the authors. |
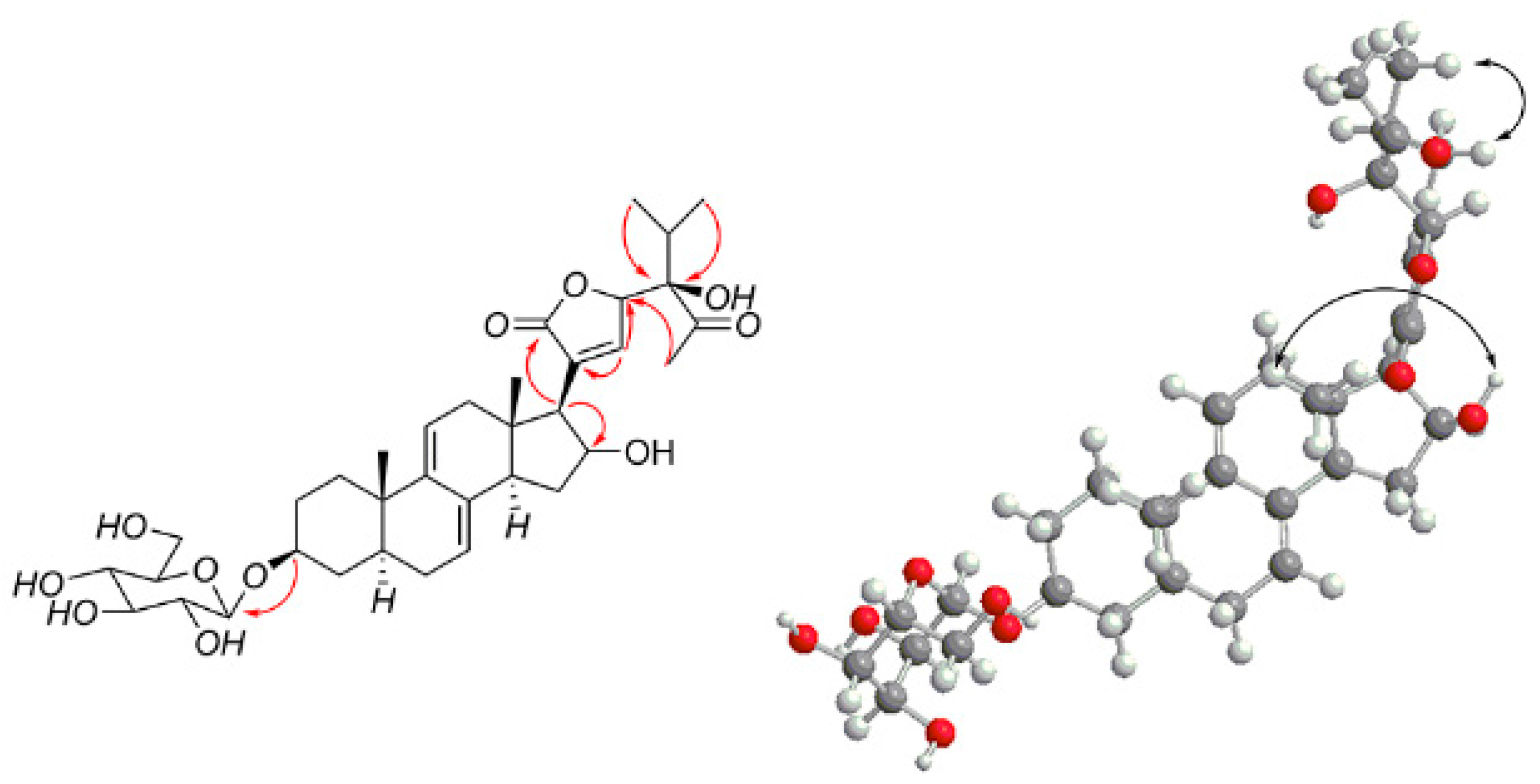
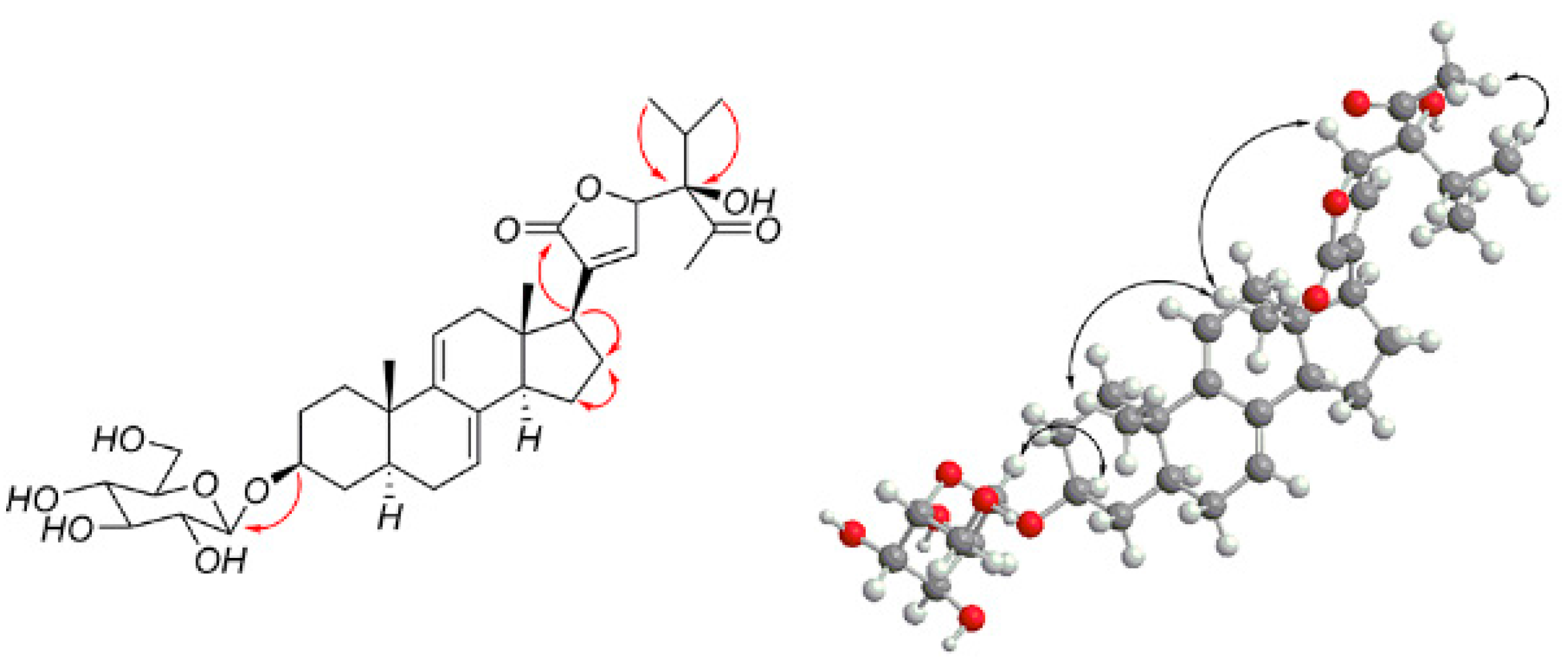
| No. | 1 a | 2 a | 3 a | 4 a | ||||
|---|---|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 34.1 | 1.27, 2.0, m | 34.3 | 1.23, 1.96, m | 34.3 | 1.24, 1.93, m | 34.3 | 1.24, 1.94, m |
| 2 | 29.2 | 1.48, 1.88, m | 29.3 | 1.45, 1.87, m | 29.2 | 1.44, 1.84,m | 29.2 | 1.46, 1.87, m |
| 3 | 76.3 | 3.57, m | 76.3 | 3.56, m | 76.2 | 3.56, m | 76.2 | 3.56, m |
| 4 | 33.7 | 1.22, d(11.6) | 33.7 | 1.19, d(11.6) | 33.7 | 1.18, d(11.7) | 33.7 | 1.18, d(11.7) |
| 1.83, m | 1.80, m | 1.79, m | 1.76, m | |||||
| 5 | 38.7 | 1.35 a | 38.7 | 1.29 a | 38.6 | 1.31 a | 38.6 | 1.31 a |
| 6 | 29.4 | 1.26, 1.88 a | 29.5 | 1.23, 1.87 a | 29.5 | 1.24, 1.84 a | 29.4 | 1.23, 1.87 a |
| 7 | 121.9 | 5.40, br s | 120.1 | 5.37, br s | 120.7 | 5.36, br s | 120.7 | 5.39, br s |
| 8 | 133.1 | 135.8 | 135.1 | 135.7 | ||||
| 9 | 143.9 | 143.3 | 143.6 | 143.7 | ||||
| 10 | 35.8 | 35.5 | 35.6 | 35.9 | ||||
| 11 | 117.6 | 5.62, d(5.8) | 119.0 | 5.53, d(6.2) | 117.9 | 5.47 a | 118.3 | 5.51 d(6.2) |
| 12 | 39.0 | 2.27, m | 40.8 | 2.01, d(7.4) | 39.6 | 1.83, d(6.6) | 39.4 | 1.94, d(6.6) |
| 2.84, m | 2.17, m | 2.16, m | ||||||
| 13 | 40.3 | 41.8 | 43.6 | 43.4 | ||||
| 14 | 45.0 | 2.65 a | 45.1 | 1.94 a | 48.7 | 2.54 a | 46.0 | 2.52 a |
| 15 | 37.0 | 2.02, 2.34 a | 28.0 | 1.47, 2.09 a | 34.5 | 1.66, 1.92 a | 26.5 | 1.91 a |
| 16 | 214.2 | 22.8 | 1.39, 1.76 a | 74.3 | 4.40 a | 23.1 | 1.49, 1.85 a | |
| 17 | 61.2 | 2.63, d(2.9) | 50.9 | 2.19 a | 56.6 | 2.41, d(7.5) | 50.8 | 2.27 a |
| 18 | 13.8 | 0.52, s, 3H | 11.8 | 0.54, s, 3H | 13.2 | 0.30, s, 3H | 12.1 | 0.32, s, 3H |
| 19 | 19.4 | 0.87, s, 3H | 19.4 | 0.85, s, 3H | 19.3 | 0.81, s, 3H | 19.3 | 0.82, s, 3H |
| 20 | 36.8 | 2.98, ddd, (12.2,8.7,3.2) | 47.6 | 2.76, dd, | 133.9 | 134.9 | ||
| (10.8, 6.6) | ||||||||
| 21 | 177.3 | 175.4 | 173.3 | 172.9 | ||||
| 22 | 26.0 | 1.88, 2.16 a | 79.1 | 4.58, t(6.0*2) | 146.8 | 7.49, br s | 146.9 | 7.44, br s |
| 23 | 80.6 | 4.63, d(5.5) | 78.7 | 4.62, d(5.1), | 82.8 | 5.24, m | 82.7 | 5.22, m |
| 24 | 83.0 | 82.6 | 83.3 | 83.3 | ||||
| 25 | 33.5 | 2.07, m | 30.8 | 1.91, m | 32.9 | 2.16, m | 32.8 | 2.16, m |
| 26 | 17.1 | 0.83, d(7.0) | 16.5 | 0.87, d(6.8) | 16.9 | 0.82, d(6.9) | 16.9 | 0.81, d(6.8) |
| 27 | 17.0 | 0.91, d(7.0) | 17.6 | 1.03, d(6.8) | 16.7 | 0.97, d(6.9) | 16.6 | 0.96, d(6.8) |
| 28 | 213.0 | 106.9 | 211.2 | 211.1 | ||||
| 29 | 29.2 | 2.15, s, 3H | 15.0 | 1.26, s, 3H | 28.1 | 2.09, s, 3H | 28.1 | 2.09, s, 3H |
| Glu | ||||||||
| 1′ | 100.9 | 4.24, d(8.1) | 100.9 | 4.23, d(8.1) | 100.9 | 4.22, d(8.0) | 100.9 | 4.22, d(8.1) |
| 2′ | 73.5 | 2.90 a | 73.5 | 2.90 a | 73.5 | 2.89 a | 73.5 | 2.89 a |
| 3′ | 76.7 | 3.10 a | 76.7 | 3.10 a | 76.8 | 3.10 a | 76.8 | 3.11 a |
| 4′ | 70.1 | 3.03 a | 70.1 | 3.02 a | 70.1 | 3.02 a | 70.1 | 3.02 a |
| 5′ | 76.8 | 3.10 a | 76.8 | 3.10 a | 76.8 | 3.10 a | 76.8 | 3.11 a |
| 6′ | 61.1 | 3.42, 3.65 a | 61.1 | 3.41, 3.65 a | 61.2 | 3.41, 3.65 a | 61.1 | 3.41, 3.65 a |
| OCH3 | 49.5 | 3.15, s, 3H | ||||||
| Sample | BT-549 | MDA-MB-231 | MCF-7 | Hela | ||||
|---|---|---|---|---|---|---|---|---|
| Average Absorbance | Inhibition (%) | Average Absorbance | Inhibition (%) | Average Absorbance | Inhibition (%) | Average Absorbance | Inhibition (%) | |
| Compound 1 | 0.2748 | 63.61 | 0.6102 | 28.97% | 0.4217 | 46.54 | 0.5212 | 42.05 |
| Compound 2 | 0.2836 | 62.17 | 0.6196 | 27.78% | 0.4893 | 37.07 | 0.6063 | 31.64 |
| Compound 3 | 0.4546 | 34.18 | 0.5803 | 32.74% | 0.4728 | 39.38 | 0.6464 | 26.73 |
| Compound 4 | 0.3946 | 44.00 | 0.5899 | 31.53% | 0.5301 | 31.36 | 0.5957 | 32.93 |
| Compound 5 | 0.4410 | 36.41 | 0.5734 | 33.61% | 0.3990 | 49.72 | 0.6893 | 21.48 |
| Compound 6 | 0.3510 | 51.14 | 0.5961 | 30.75% | 0.4750 | 39.08 | 0.5737 | 35.63 |
| Positive control | 0.1515 | 83.79 | 0.1789 | 83.39% | 0.0734 | 95.32 | 0.0996 | 92.70 |
| Negative control | 0.6634 | 0.8399 | 0.7540 | 0.8648 | ||||
| Blank | 0.0525 | 0.0470 | 0.0400 | 0.0477 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Song, H.; Wu, X.; Zhang, S.; Gao, X.; Li, F.; Zhu, X.; Chen, Q. Steroidal Saponins from Vernonia amygdalina Del. and Their Biological Activity. Molecules 2018, 23, 579. https://doi.org/10.3390/molecules23030579
Wang J, Song H, Wu X, Zhang S, Gao X, Li F, Zhu X, Chen Q. Steroidal Saponins from Vernonia amygdalina Del. and Their Biological Activity. Molecules. 2018; 23(3):579. https://doi.org/10.3390/molecules23030579
Chicago/Turabian StyleWang, Jing, Hua Song, Xiaoxue Wu, Shuyi Zhang, Xuemin Gao, Funan Li, Xuan Zhu, and Qing Chen. 2018. "Steroidal Saponins from Vernonia amygdalina Del. and Their Biological Activity" Molecules 23, no. 3: 579. https://doi.org/10.3390/molecules23030579





