Characterization of Condensed Tannins from Purple Prairie Clover (Dalea purpurea Vent.) Conserved as either Freeze-Dried Forage, Sun-Cured Hay or Silage
Abstract
:1. Introduction
2. Results
2.1. Characteristics of PPC Conserved as FD, HAY and SIL
2.2. CT Terminal and Extension Units
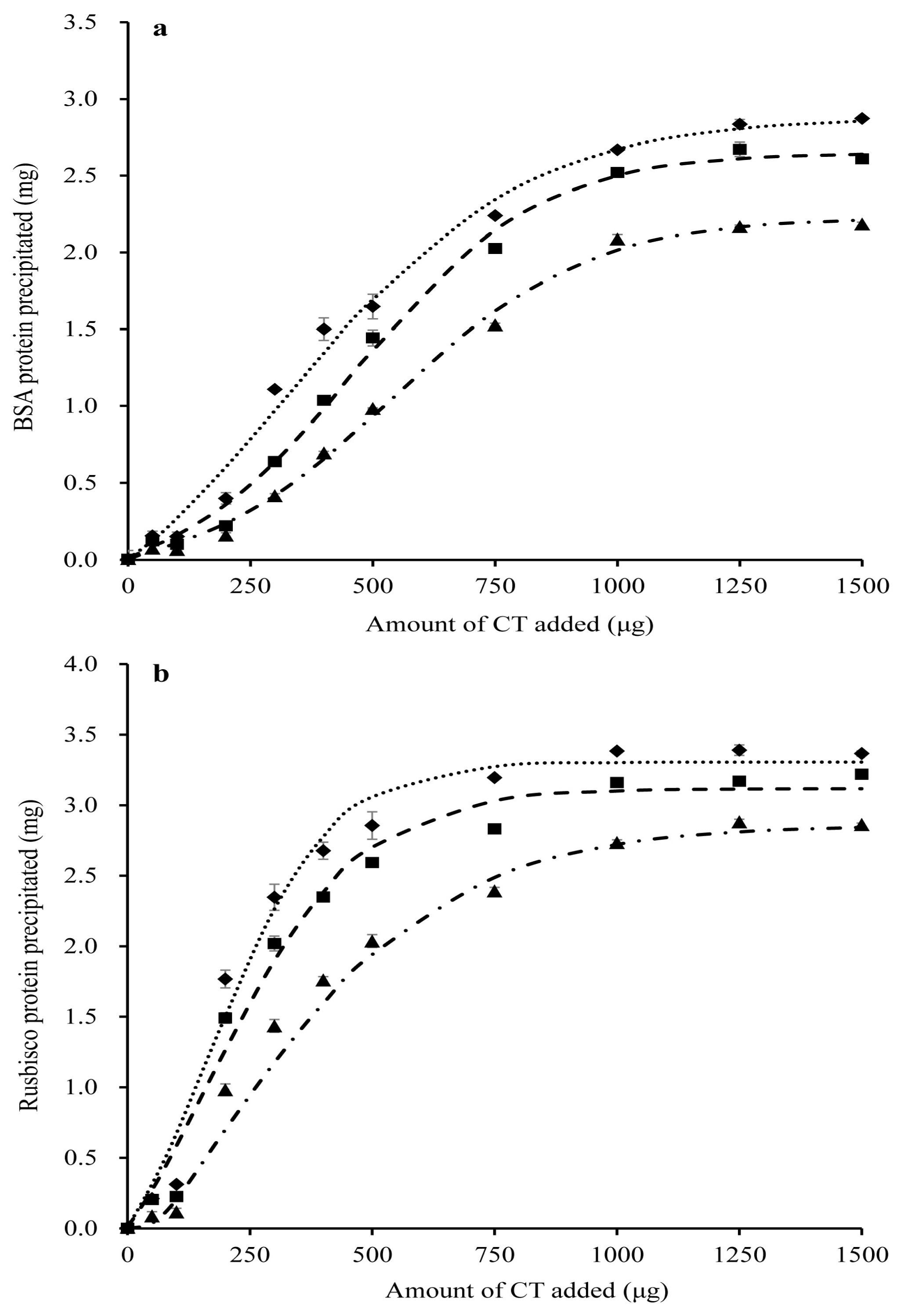
2.3. Protein-Precipitating Capacities of CT in PPC Conserved as FD, HAY and SIL
2.4. Growth of E. coli Affected by CT in PPC Conserved as FD, HAY and SIL
3. Discussion
3.1. Effect of Conservation Method on the Compositions of PPC
3.2. Effects of Forage Conservation Method on the Structure and Chemical Composition of PPC CT
3.3. Effect of Forage Conservation Method on the Protein-Precipitating Capacity of PPC CT
3.4. Effect of Forage Conservation Method on Anti-E. coli Activity of PPC CT
4. Materials and Methods
4.1. Conservation of PPC as Freeze-Dried, Hay or Silage
4.2. Chemical Analysis
4.3. In Situ Thiolysis of PPC Tannins and HPLC Analysis of Thiolysis Products
4.4. Determination of the Protein Precipitation Capacities of CT from Freeze-Dried, Silage or Hay PPC
4.5. Determination of CT from Freeze-Dried, Silage or Hay PPC on the Growth of E. coli
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, in press. [Google Scholar] [CrossRef]
- Azuhnwi, B.N.; Boller, B.; Dohme-Meier, F.; Hess, H.D.; Kreuzer, M.; Stringano, E.; Mueller-Harvey, I. Exploring variation in proanthocyanidin composition and content of sainfoin (Onobrychis viciifolia). J. Sci. Food Agric. 2013, 93, 2102–2109. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.; Alkhafadji, L.; Stringano, E.; Nilsson, S.; Mueller-Harvey, I.; Uden, P. Relationship between condensed tannin structures and their ability to precipitate feed proteins in the rumen. J. Sci. Food Agric. 2014, 94, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Hatew, B.; Stringano, E.; Mueller-Harvey, I.; Hendriks, W.H.; Cabonero, C.H.; Smith, L.M.J.; Pellikaan, W.F. Impact of variation in structure of condensed tannins from sainfoin (Onobrychis viciifolia) on in vitro ruminal methane production and fermentation characteristics. J. Anim. Physiol. Anim. Nutr. 2016, 100, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Hu, T.M.; Xu, Z.; Jin, L.; McAllister, T.A.; Acharya, S.; Zeller, W.; Hardcastle, E.; Drake, C.; Wang, Y. Structural composition and protein precipitation capacity of condensed tannins from purple prairie clover (Dalea purpurea Vent.). J. Anim. Sci. 2017, 95 (Suppl. 4), 141–142. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Jin, L.; Xu, Z.; Acharya, S.; McAllister, T.A.; Hu, T.M.; Peng, K.; Iwaasa, A.; Schellenberg, M.; Wang, Y. Effects of conservation method on condensed tannin content, ruminal degradation, and in vitro intestinal digestion of purple prairie clover (Dalea purpurea Vent.). Can. J. Anim. Sci. 2016, 96, 524–531. [Google Scholar] [CrossRef]
- Wang, Y.; Barbieri, L.R.; Berg, B.P.; McAllister, T.A. Effects of mixing sainfoin with alfalfa on ensiling, ruminal fermentation and total tract digestion of silage. Anim. Feed Sci. Technol. 2007, 135, 296–314. [Google Scholar] [CrossRef]
- Waghorn, G. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production-Progress and challenges. Anim. Feed Sci. Technol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Liu, X.L.; Hao, Y.Q.; Jin, L.; Xu, Z.J.; McAllister, T.A.; Wang, Y. Anti-Escherichia coli O157:H7 properties of purple prairie clover and sainfoin condensed tannins. Molecules 2013, 18, 2183–2199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, L.; Ominski, K.H.; He, M.; Xu, Z.; Krause, D.O.; Acharya, S.N.; Wittenberg, K.M.; Liu, X.L.; Stanford, K.; et al. Screening of condensed tannins from Canadian prairie forages for anti-Escherichia coli O157:H7 with an emphasis on purple prairie clover (Dalea purpurea Vent.). J. Food Prot. 2013, 76, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Wang, Y.; Iwaasa, A.D.; Xu, Z.; Li, Y.; Schellenberg, M.P.; Liu, X.L.; McAllister, T.A.; Stanford, K. Purple prairie clover (Dalea purpurea Vent.) reduces fecal shedding of Escherichia coli in pastured cattle. J. Food Prot. 2015, 78, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.Q.; Jin, L.; Xu, Z.; Barbieria, L.R.; Acharyaa, S.; Hu, T.M.; Stanford, K.; McAllister, T.A.; Wang, Y. Effects of purple prairie clover (Dalea purpurea Vent.) on feed intake, nutrient digestibility and faecal shedding of Escherichia coli O157:H7 in lambs. Anim. Feed Sci. Technol. 2015, 207, 51–61. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Y.; Iwaasa, A.D.; Xu, Z.; Schellenberg, M.P.; Zhang, Y.G.; Liu, X.L.; McAllister, T.A. Effect of condensed tannins on ruminal degradability of purple prairie clover (Dalea purpurea Vent.) harvested at two growth stages. Anim. Feed Sci. Technol. 2012, 176, 17–25. [Google Scholar] [CrossRef]
- Li, Y.; Iwaasa, A.D.; Wang, Y.; Jin, L.; Han, G.; Zhao, M. Condensed tannins concentration of selected prairie legume forages as affected by phenological stages during two consecutive growth seasons in western Canada. Can. J. Plant Sci. 2014, 94, 817–826. [Google Scholar] [CrossRef]
- Broderick, G.A. Performance of lactating dairy cows fed either alfalfa silage or alfalfa hay as the sole forage. J. Dairy Sci. 1995, 78, 320–329. [Google Scholar] [CrossRef]
- Griffin, J.L.; Jung, G.A. Leaf and stem forage quality of big bluestem and switchgrass. Agron. J. 2003, 75, 723–726. [Google Scholar] [CrossRef]
- Wilkins, R.J. The Preservation of Forages. In Feed Science; Orskov, E.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 231–255. [Google Scholar]
- Enoh, M.B.; Kijora, C.; Peters, K.J.; Tanya, V.N.; Fonkem, D.; Mbanya, J. Investigation on change of forage quality at harvesting, during hay making and storage of hay harvested at different growth stages in the Adamawa plateau of Cameroon. Livest. Res. Rural Dev. 2005, 17, 1–6. [Google Scholar]
- Scharenberg, A.; Arrigo, Y.; Gutzwiller, A.; Soliva, C.R.; Wyss, U.; Kreuzer, M.; Dohme, F. Palatability in sheep and in vitro nutritional value of dried and ensiled sainfoin (Onobrychis viciifolia) birdsfoot trefoil (Lotus corniculatus), and chicory (Cichorium intybus). Arch. Anim. Nutr. 2007, 61, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Scharenberg, A.; Arrigo, Y.; Gutzwiller, A.; Wyss, U.; Hess, H.D.; Kreuzer, M.; Dohme, F. Effect of feeding dehydrated and ensiled tanniferous sainfoin (Onobrychis viciifolia) on nitrogen and mineral digestion and metabolism of lambs. Arch. Anim. Nutr. 2007, 61, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.M.; Eriksson, T.; Uden, P. Effect of wilting, silage additive, PEG treatment and tannin content on the distribution of N between different fractions after ensiling of three different sainfoin (Onobrychis viciifolia) varieties. Grass Forage Sci. 2010, 65, 175–184. [Google Scholar] [CrossRef]
- Terrill, T.H.; Windham, W.R.; Evans, J.J.; Hoveland, C.S. Condensed tannins in sericea lespedeza: Effect of preservation method on tannin concentration. Crop Sci. 1990, 30, 219–224. [Google Scholar] [CrossRef]
- Aufrère, J.; Dudilieu, M.; Poncet, C. In vivo and in situ measurements of the digestive characteristics of sainfoin in comparison with lucerne fed to sheep as fresh forages at two growth stages and as hay. Animal 2008, 2, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; McAllister, T.A.; Acharya, S. Condensed tannins in sainfoin: Composition, concentration, and effects on nutritive and feeding value of sainfoin forage. Crop Sci. 2015, 55, 13–22. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Foo, L.Y.; Newman, R.; Waghorn, G.; McNabb, W.C.; Ulyatt, M.J. Proanthocyanidins from Lotus corniculatus. Phytochemistry 1996, 41, 617–624. [Google Scholar] [CrossRef]
- Foo, L.Y.; Lu, Y.; Mcnabb, W.C.; Waghorn, G.; Ulyatt, M.J. Proanthocyanidins from Lotus pedunculatus. Phytochemistry 1997, 45, 1689–1696. [Google Scholar] [CrossRef]
- Koupai-Abyazani, M.R.; Mccallum, J.; Muir, A.D.; Bohm, B.A.; Towers, G.H.N.; Gruber, M.Y. Developmental changes in the composition of proanthocyanidins from leaves of sainfoin (Onobrychis viciifolia Scop.) as determined by HPLC analysis. J. Agric. Food Chem. 1993, 41, 613–621. [Google Scholar] [CrossRef]
- Foo, L.Y.; Jones, W.T.; Porter, L.J.; Williams, V.M. Proanthocyanidin polymers of fodder legumes. Phytochemistry 1982, 21, 933–935. [Google Scholar]
- Marais, J.P.; Mueller-Harvey, I.; Brandt, E.V.; Ferreira, D. Polyphenols, condensed tannins, and other natural products in Onobrychis viciifolia (Sainfoin). J. Agric. Food Chem. 2000, 48, 3440–3447. [Google Scholar] [CrossRef] [PubMed]
- Gea, A.; Stringano, E.; Brown, R.H.; Mueller-Harvey, I. In situ analysis and structural elucidation of sainfoin (Onobrychis viciifolia) tannins for high-throughput germplasm screening. J. Agric. Food Chem. 2011, 59, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Kommuru, D.S.; Barker, T.; Desai, S.; Burke, J.M.; Ramsay, A.; Mueller-Harvey, I.; Miller, J.E.; Mosjidis, J.A.; Kamisetti, N.; Terrill, T.H. Use of pelleted sericea lespedeza (Lespedeza cuneata) for natural control of coccidia and gastrointestinal nematodes in weaned goats. Vet. Parasitol. 2014, 204, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, O.A.; Salminen, J.P.; Makila, L.; Kallio, H.P.; Yang, B. Proanthocyanidins and their contribution to sensory attributes of black currant juices. J. Agric. Food Chem. 2015, 63, 5373–5380. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Harvey, I.; Bee, G.; Dohme-Meier., F.; Hoste, H.; Karonen, M.; Kölliker, R.; Lüscher, A.; Niderkorn, V.; Pellikaan, W.; Salminen, J.P.; Skot, L.; Smith, L.; Thamsborg, S.; et al. Benefits of condensed tannins in forages fed to ruminants: Importance of structure, concentration and diet. Crop Sci. 2018, in press. [Google Scholar]
- Vidal, S.; Cartalade, D.; Souquet, J.M.; Fulcrand, H.; Cheynier, V. Changes in proanthocyanidin chain length in winelike model solutions. J. Agric. Food Chem. 2002, 50, 2261–2266. [Google Scholar] [CrossRef] [PubMed]
- Tharayil, N.; Suseela, V.; Triebwasser, D.J.; Preston, C.M.; Gerard, P.D.; Dukes, J.S. Changes in the structural composition and reactivity of Acer rubrum leaf litter tannins exposed to warming and altered precipitation: Climatic stress-induced tannins are more reactive. New Phytol. 2011, 191, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Jin, L.; Niu, D.; Huang, Q.; McAllister, T.A.; Yang, H.E.; Denise, H.; Xu, Z.; Acharya, S.; Wang, S.; et al. Condensed tannins affect bacterial and fungal microbiomes and mycotoxin production during ensiling and upon aerobic exposure. Appl. Environ. Microbiol. 2018, 84, e02274-17. [Google Scholar] [CrossRef] [PubMed]
- Haslam, E. Plant Polyphenols, Vegetable Tannins Revisited; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Hagerman, A.E.; Butler, L.G. The specificity of proanthocyanidin-protein interactions. J. Biol. Chem. 1981, 256, 4494–4497. [Google Scholar] [PubMed]
- Hagerman, A.E.; Rice, M.E.; Ritchard, N.T. Mechanisms of protein precipitation for two tannins, pentagalloyl glucose and epicatechin16 (4→8) catechin (procyanidin). J. Agric. Food Chem. 1998, 46, 2590–2595. [Google Scholar] [CrossRef]
- Horigome, T.; Kumar, R.; Okamoto, K. Effects of condensed tannins prepared from leaves of fodder plants on digestive enzymes in vitro and in the intestine of rats. Br. J. Nutr. 1988, 60, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Theodoridou, K.; Aufrère, J.; Andueza, D.; Pourrat, J.; Morvan, A.L.; Stringano, E.; Mueller-Harvey, I.; Baumont, R. Effects of condensed tannins in fresh sainfoin (Onobrychis viciifolia) on in vivo and in situ digestion in sheep. Anim. Feed Sci. Technol. 2010, 160, 23–38. [Google Scholar] [CrossRef]
- Theodoridou, K.; Aufrère, J.; Niderkorn, V.; Andueza, D.; Morvan, A.L.; Picard, F.; Baumont, R. Effect of plant development during first and second growth cycle on chemical composition, condensed tannins and nutritive value of three sainfoin (Onobrychis viciifolia) varieties and lucerne. Grass Forage Sci. 2011, 66, 402–414. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Kilmister, R.L.; Kelm, M.A.; Downey, M.O. Impact of condensed tannin size as individual and mixed polymers on bovine serum albumin precipitation. Food Chem. 2014, 160, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Ropiak, H.M.; Lachmann, P.; Ramsay, A.; Green, R.J.; Mueller-Harvey, I. Identification of structural features of condensed tannins that affect protein aggregation. PLoS ONE 2017, 12, e0170768. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.T.; Broadhurst, R.B.; Lyttleton, J.W. The condensed tannins of pasture legume species. Phytochemistry 1976, 15, 1407–1409. [Google Scholar] [CrossRef]
- Mangan, J.L. Nutritional effects of tannins in animal feeds. Nutr. Res. Rev. 1988, 1, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Guimarães-Beelen, P.M.; Berchielli, T.T.; Beelen, R.; Filho, J.A.; Oliveira, S.G. Characterization of condensed tannins from native legumes of the Brazilian northeastern semi-arid. Sci. Agric. 2006, 63, 150–153. [Google Scholar]
- De Freitas, V.; Mateus, N. Structural features of procyanidin interactions with salivary proteins. J. Agric. Food Chem. 2001, 49, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Rosales, R.B. Condensed Tannins in Tropical Forage Legumes: Their Characterization and Study of Their Nutritional Impact from the Standpoint of Structure-Activity Relationships. Ph.D Thesis, University of Reading, Reading, UK, 1999. [Google Scholar]
- Kumar, R.; Horigome, T. Fractionation, characterization and protein precipitating capacity of the condensed tannins from Robinia pseudoacacia (L.) leaves. J. Agric. Food Chem. 1986, 34, 487–489. [Google Scholar] [CrossRef]
- Osborne, N.J.T.; McNeill, D.M. Characterisation of Leucaena condensed tannins by size and protein precipitation capacity. J. Sci. Food Agric. 2001, 81, 1113–1119. [Google Scholar] [CrossRef]
- Huang, X.D.; Liang, J.B.; Tan, H.Y.; Yahya, R.; Khamseekhiew, B.; Ho, Y.W. Molecular weight and protein binding affinity of Leucaena condensed tannins and their effects on in vitro fermentation parameters. Anim. Feed Sci. Technol. 2010, 159, 81–87. [Google Scholar] [CrossRef]
- Huang, X.D.; Liang, J.B.; Tan, H.Y.; Yahya, R.; Long, R.; Ho, Y.W. Protein-binding affinity of Leucaena condensed tannins of differing molecular weights. J. Agric. Food Chem. 2011, 59, 10677–10682. [Google Scholar] [CrossRef] [PubMed]
- Bacon, J.R.; Rhodes, M.J.C. Binding affinity of hydrolyzable tannins to parotid saliva and to proline-rich proteins derived from it. J. Agric. Food Chem. 2000, 48, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; McAllister, T.A.; Iwaasa, A. Effects of condensed tannins from purple prairie clover on the growth of E. coli O157:H7. J. Anim. Sci. 2009, 87, 31. [Google Scholar]
- Foo, L.Y.; Yinrong, L.; Howell, A.B.; Vorsa, N. A-type proamthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbricated Escherichia coli. J. Nat. Prod. 2000, 63, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Voravuthikunchai, S.; Lortheeranuwat, A.; Jeeju, W.; Sririrak, T.; Phongpaichit, S.; Supawita, T. Effective medicinal plants against enterohaemorrhagic Escherichia coli O157:H7. J. Ethnopharm. 2004, 94, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Vattem, D.A.; Lin, Y.T.; Labbe, R.G.; Shetty, K. Antimicrobial activity against select food-borne pathogens by phenolic antioxidants enriched in cranberry pomace by solid-state bioprocessing using the food grade fungus Rhizopus oligosporus. Process Biochem. 2004, 39, 1939–1946. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Nohynek, L.; Hartmann-Schmidlin, S.; Kahkonen, M.; Heinonen, M.; Maatta-Riihinen, M.; Oksman-Caldentey, K.M. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microbiol. 2005, 98, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Min, B.R.; Pinchak, W.E.; Anderson, R.C.; Callaway, T.R. Effect of tannins on the in vitro growth of Escherichia coli O157:H7 and in vivo growth of generic Escherichia coli excreted from steers. J. Food Prot. 2007, 70, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Weng, W.L.; Stojanovic, J.; Lu, Y.; Jung, Y.S.; Silva, J.L. Antimicrobial effect of water-soluble muscadine seed extracts on Escherichia coli O157:H7. J. Food Prot. 2008, 71, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, T.; Zhao, W.H.; Hu, Z.Q. Mechanism of action and potential for use of tea catechin as an anti-infective agent. Anti-Infect. Agents Med. Chem. 2007, 6, 57–62. [Google Scholar] [CrossRef]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochem. Biophys. Acta 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Tsuchiya, H. Stereospecificity in membrane effects of catechins. Chem.-Biol. Interact. 2001, 134, 41–54. [Google Scholar] [CrossRef]
- Ayres, K.W.; Acton, D.F.; Ellis, J.G. The Soils of Swift Current Map Area 725 Saskatchewan, Publ. 56; University of Saskatchewan: Saskatoon, SK, Canada, 1985; p. 226. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Agricultural Chemists, 16th ed.; AOAC International: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Zahiroddini, H.; Baah, J.; Absalom, W.; McAllister, T.A. Effect of an inoculant and hydrolytic enzymes on fermentation and nutritive value of whole crop barley silage. Anim. Feed Sci. Technol. 2004, 117, 317–330. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Blummel, M.; Borowy, N.K.; Becker, K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agric. 1993, 61, 161–165. [Google Scholar] [CrossRef]
- Terrill, T.; Rowan, A.; Douglas, G.; Barry, T. Determination of extractable and bound condensed tannin concentrations in forage plants, protein concentrate meals and cereal grains. J. Sci. Food Agric. 1992, 58, 321–329. [Google Scholar] [CrossRef]
- Williams, A.R.; Fryganas, C.; Ramsay, A.; Mueller-Harvey, I.; Thamsborg, S.M. Direct anthelmintic effects of condensed tannins from diverse plant sources against Ascaris suum. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Desrues, O.; Mueller-Harvey, I.; Pellikaan, W.F.; Enemark, H.L.; Thamsborg, S.M. Condensed tannins in the gastrointestinal tract of cattle after sainfoin (Onobrychis viciifolia) intake and their possible relationship with anthelmintic effects. J. Agric. Food Chem. 2017, 65, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Ropiak, H.M.; Ramsay, A.; Mueller-Harvey, I. Condensed tannins in extracts from European medicinal plants and herbal products. J. Pharm. Biomed. Anal. 2016, 121, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Z.; Bach, S.J.; McAllister, T.A. Sensitivity of Escherichia coli to seaweed (Ascophyllum nodosum) phlorotannins and terrestrial tannins. Asian-Aust. J. Anim. Sci. 2009, 22, 238–245. [Google Scholar] [CrossRef]
- McAllister, T.A.; Martinez, T.; Bae, H.D.; Muir, A.D.; Yanke, L.J.; Jones, G.A. Characterization of condensed tannins purified from legume forages: Chromophore production, protein precipitation, and inhibitory effects on cellulose digestion. J. Chem. Ecol. 2005, 31, 2049–2068. [Google Scholar] [CrossRef] [PubMed]
- Dalgaard, P.; Koutsoumanis, K. Comparison of maximum specific growth rates and lag times estimated from absorbance and viable count data by different mathematical models. J. Microbiol. Methods 2001, 43, 183–196. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS Online Doc® 9.1.3; SAS Institute Inc.: Cary, NC, USA, 2012. [Google Scholar]
Sample Availability: Samples of the PPC CT are available from the authors. |


| Item | FD | HAY | SIL | SEM | p-Value |
|---|---|---|---|---|---|
| Organic matter | 924 | 923 | 913 | 3.96 | 0.174 |
| Crude protein (N × 6.25) | 158 | 157 | 165 | 2.20 | 0.058 |
| Neutral detergent fibre | 439 b | 497 a | 480 a | 5.74 | 0.001 |
| Acid detergent fibre | 405 b | 422 a | 417 a | 2.38 | 0.006 |
| Acid detergent lignin | 79.8 b | 98.3 a | 90.1 a,b | 2.79 | 0.009 |
| Water-soluble carbohydrate | 25.4 a | 11.5 b | 2.4 c | 0.34 | <0.001 |
| Total phenols 1 | 66.2 a | 51.4 b | 28.6 c | 0.95 | <0.001 |
| Condensed tannins (CT) | |||||
| Extractable CT | 70.2 a | 64.1 a | 27.4 b | 1.53 | <0.001 |
| Fibre-bound CT | 5.2 b | 5.6 b | 7.1 a | 0.17 | <0.001 |
| Protein-bound CT | 9.0 c | 12.4 b | 44.3 a | 0.17 | <0.001 |
| Total CT | 84.5 | 82.2 | 78.9 | 1.45 | 0.083 |
| Terminal Units (%) | Extension Units (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| GC 1 | EGC | C | EC | GC-BM 2 | EGC-BM | C-BM | EC-BM | |
| FD | 0 | 0 | 1.56 a | 4.26 b | 0 | 23.50 b | 2.42 b | 68.26 a |
| HAY | 0 | 0 | 1.26 b | 4.13 b | 0 | 27.97 a | 2.19 b | 64.45 b |
| SIL | 0 | 0 | 1.29 b | 6.94 a | 0 | 19.17 c | 3.78 a | 68.82 a |
| SEM | - | - | 0.063 | 0.282 | - | 0.928 | 0.164 | 0.743 |
| p-value | - | - | 0.008 | <0.001 | - | <0.001 | <0.001 | 0.002 |
| mDP 1 | PC (%) 2 | PD (%) | Cis (%) 3 | Trans (%) | |
|---|---|---|---|---|---|
| FD | 17.3 a | 76.5 b | 23.5 b | 96.0 a | 4.0 b |
| HAY | 18.6 a | 72.0 c | 28.0 a | 96.6 a | 3.4 b |
| SIL | 12.4 b | 80.8 a | 19.2 c | 94.9 b | 5.1 a |
| SEM | 0.66 | 0.93 | 0.93 | 0.20 | 0.20 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Protein 1 | Parameters 2 | FD | HAY | SIL | SEM | p-Value |
|---|---|---|---|---|---|---|
| BSA | a0 + a | 3.1 a | 2.9 b | 2.4 c | 0.01 | <0.001 |
| b | 323 b | 467 a | 541 a | 0.01 | <0.001 | |
| c | 244 a | 185 b | 200 b | 7.72 | 0.004 | |
| PP | 124 c | 172 b | 223 a | 2.57 | <0.001 | |
| Rubisco | a0 + a | 3.4 a | 3.3 b | 3.0 c | 0.02 | <0.001 |
| b | 173 | 166 | 152 | 11.10 | 0.428 | |
| c | 120 b | 152 b | 242 a | 17.14 | 0.006 | |
| PP | 62 c | 70 b | 97 a | 1.08 | <0.001 |
| Strain | Parameter | Conservation Method | Tannin Concentration (µg/mL) | SEM | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 100 | |||||
| E. coli 25922 | µ | FD | 1.24 a | 1.06 B,b | 1.06 b | 0.91 c | 0.032 | <0.001 |
| HAY | 1.23 a | 1.12 A,b | 1.02 c | 0.98 c | 0.018 | <0.001 | ||
| SIL | 1.25 a | 1.15 A,b | 1.00 c | 0.94 c | 0.016 | <0.001 | ||
| SEM | 0.014 | 0.014 | 0.03 | 0.03 | ||||
| p-value | 0.598 | 0.008 | 0.368 | 0.250 | ||||
| L | FD | 0.52 b | 0.59 A,b | 0.85 a | 0.82 a | 0.028 | <0.001 | |
| HAY | 0.53 b | 0.49 B,b | 0.86 a | 0.89 a | 0.011 | <0.001 | ||
| SIL | 0.52 b | 0.51 B,b | 0.82 a,b | 0.95 a | 0.073 | 0.006 | ||
| SEM | 0.004 | 0.012 | 0.030 | 0.085 | ||||
| p-value | 0.422 | 0.003 | 0.659 | 0.570 | ||||
| E. coli 35281 | µ | FD | 1.04 a | 0.92 b | 0.92 b | 0.91 b | 0.014 | <0.001 |
| HAY | 1.06 a | 0.91 b | 0.90 b | 0.86 b | 0.015 | <0.001 | ||
| SIL | 1.05 a | 0.89 b | 0.91 b | 0.91 b | 0.016 | <0.001 | ||
| SEM | 0.011 | 0.018 | 0.015 | 0.016 | ||||
| p-value | 0.572 | 0.583 | 0.747 | 0.133 | ||||
| L | FD | 0.32 b | 0.32 b | 0.33 b | 0.41 A,a | 0.016 | 0.011 | |
| HAY | 0.32 b | 0.30 b | 0.32 b | 0.39 B,a | 0.007 | <0.001 | ||
| SIL | 0.31 c | 0.34 b,c | 0.35 a,b | 0.38 B,a | 0.006 | <0.001 | ||
| SEM | 0.008 | 0.016 | 0.011 | 0.005 | ||||
| p-value | 0.530 | 0.310 | 0.158 | 0.032 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, K.; Huang, Q.; Xu, Z.; McAllister, T.A.; Acharya, S.; Mueller-Harvey, I.; Drake, C.; Cao, J.; Huang, Y.; Sun, Y.; et al. Characterization of Condensed Tannins from Purple Prairie Clover (Dalea purpurea Vent.) Conserved as either Freeze-Dried Forage, Sun-Cured Hay or Silage. Molecules 2018, 23, 586. https://doi.org/10.3390/molecules23030586
Peng K, Huang Q, Xu Z, McAllister TA, Acharya S, Mueller-Harvey I, Drake C, Cao J, Huang Y, Sun Y, et al. Characterization of Condensed Tannins from Purple Prairie Clover (Dalea purpurea Vent.) Conserved as either Freeze-Dried Forage, Sun-Cured Hay or Silage. Molecules. 2018; 23(3):586. https://doi.org/10.3390/molecules23030586
Chicago/Turabian StylePeng, Kai, Qianqian Huang, Zhongjun Xu, Tim A. McAllister, Surya Acharya, Irene Mueller-Harvey, Christopher Drake, Junming Cao, Yanhua Huang, Yuping Sun, and et al. 2018. "Characterization of Condensed Tannins from Purple Prairie Clover (Dalea purpurea Vent.) Conserved as either Freeze-Dried Forage, Sun-Cured Hay or Silage" Molecules 23, no. 3: 586. https://doi.org/10.3390/molecules23030586





