[18F]fallypride-PET/CT Analysis of the Dopamine D2/D3 Receptor in the Hemiparkinsonian Rat Brain Following Intrastriatal Botulinum Neurotoxin A Injection
Abstract
:1. Introduction
2. Results
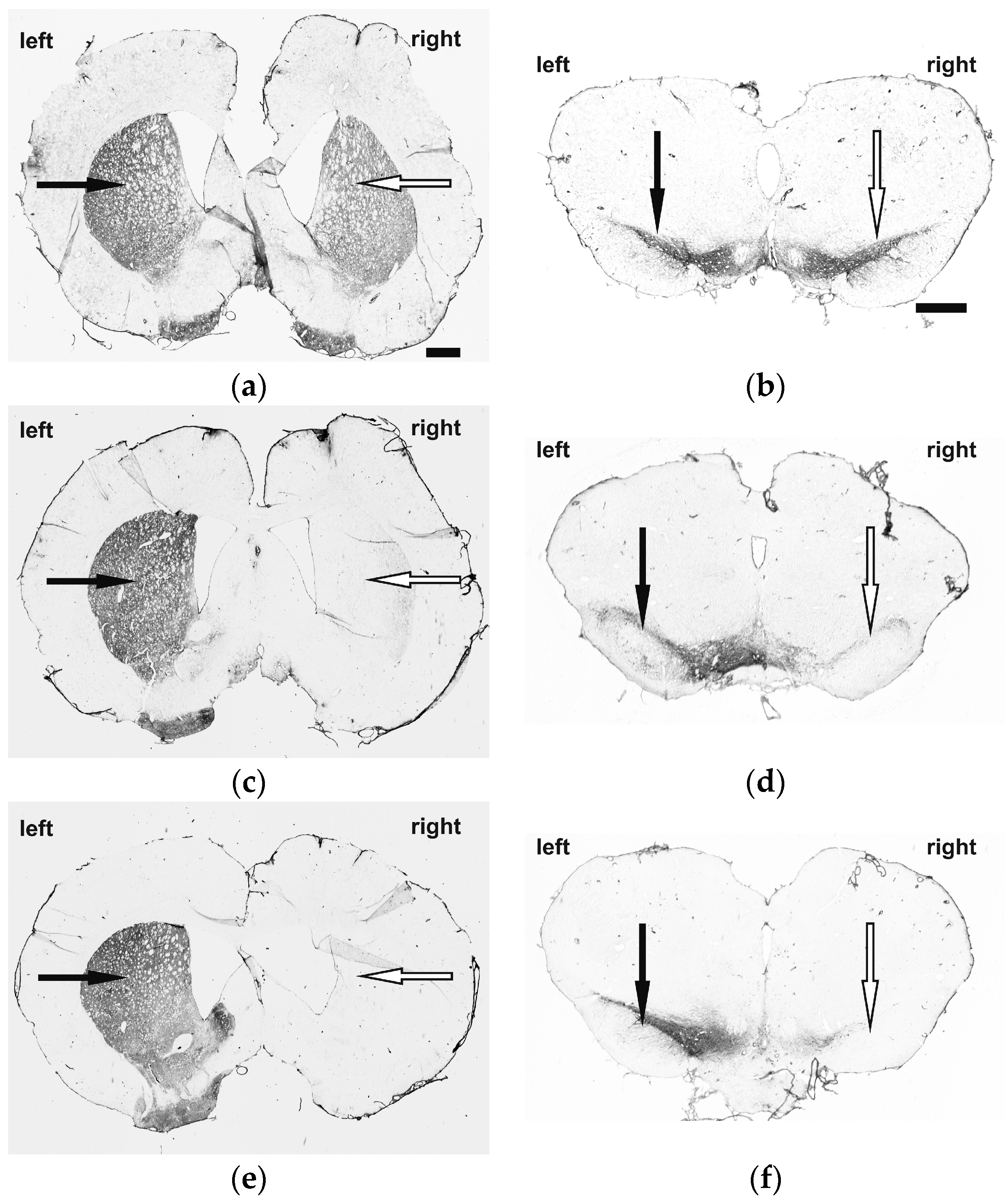
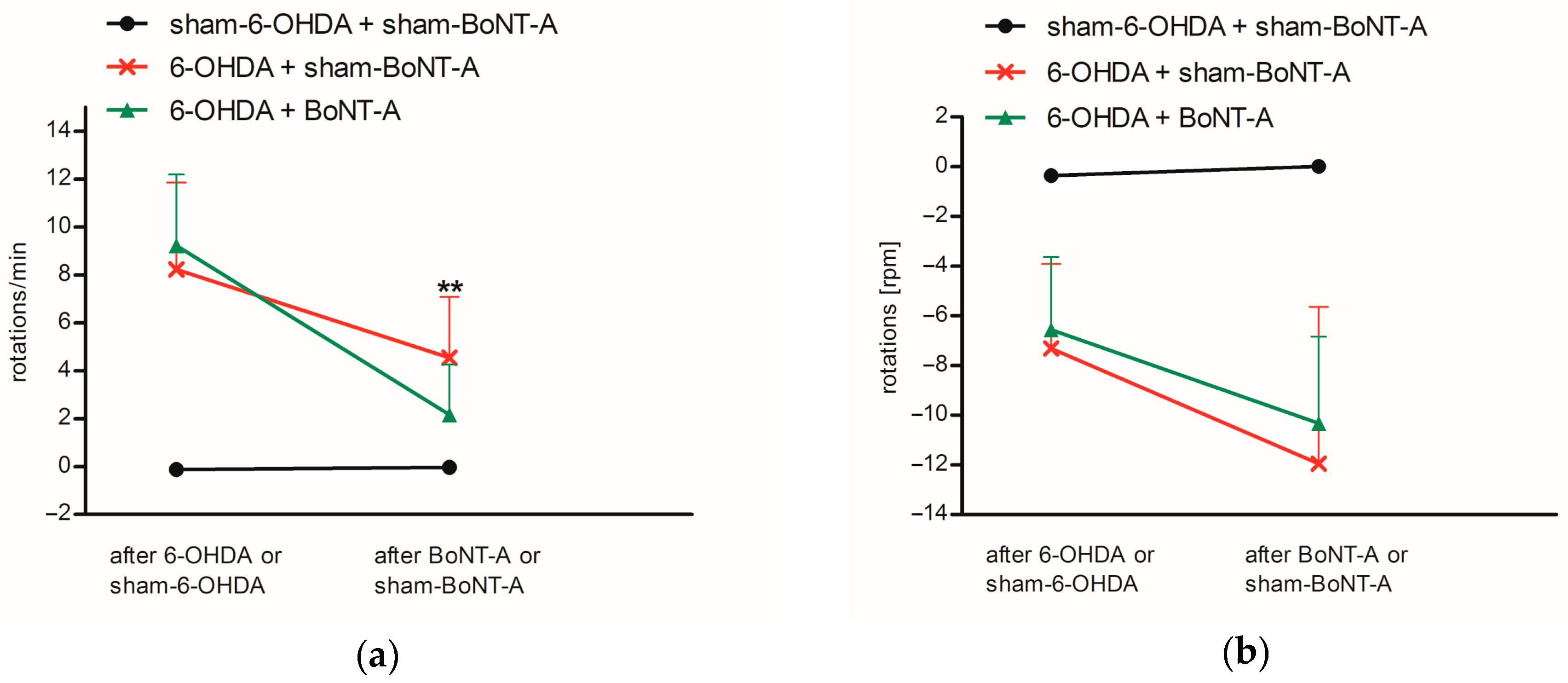
2.1. Immunohistochemistry and Behavioral Testing
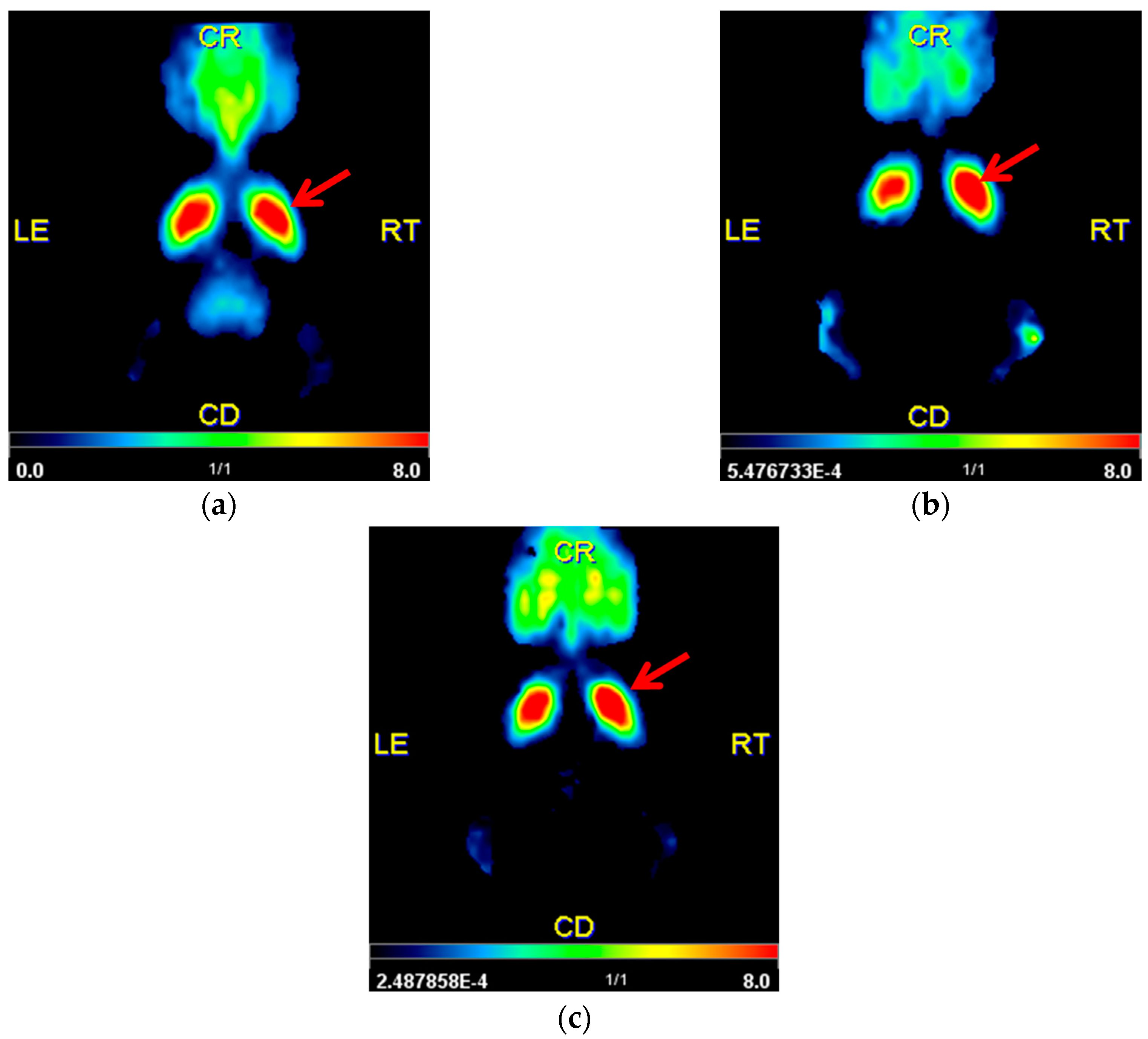
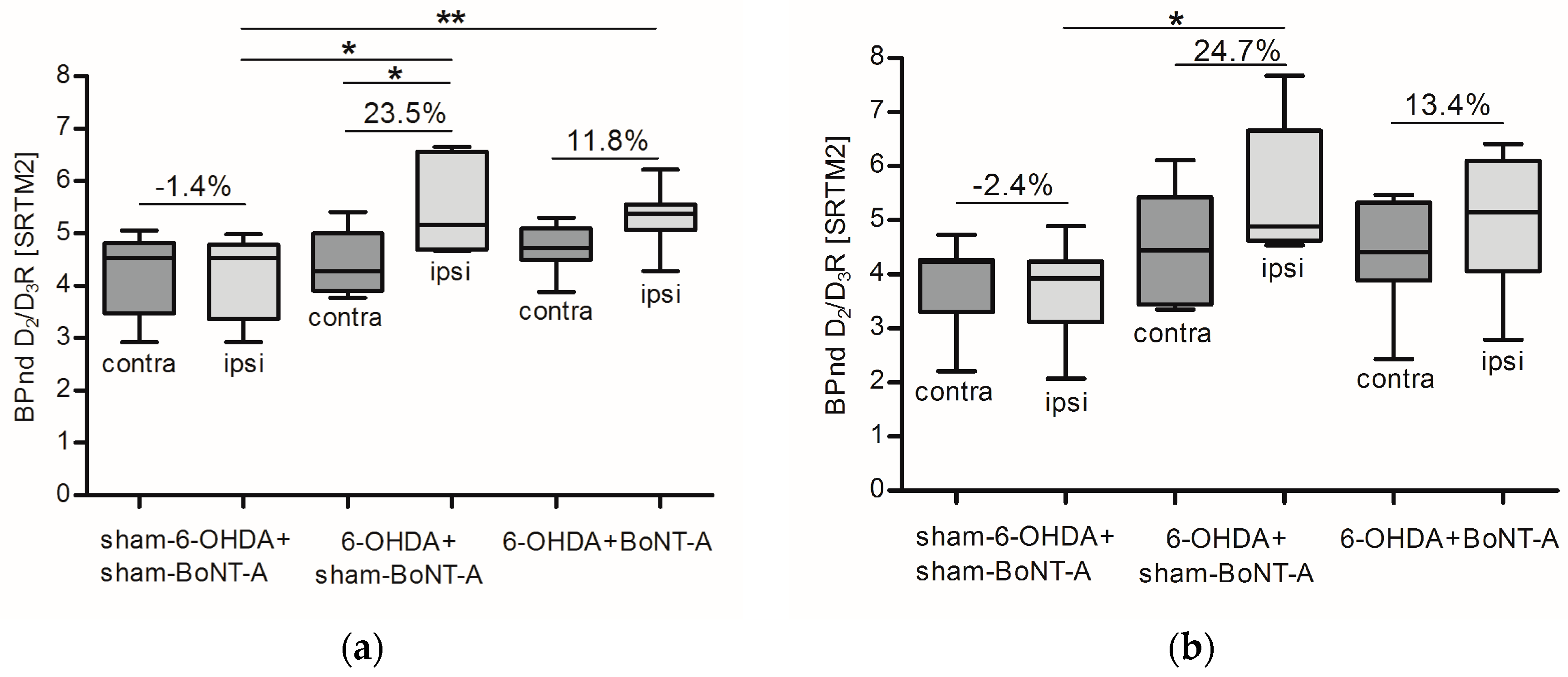
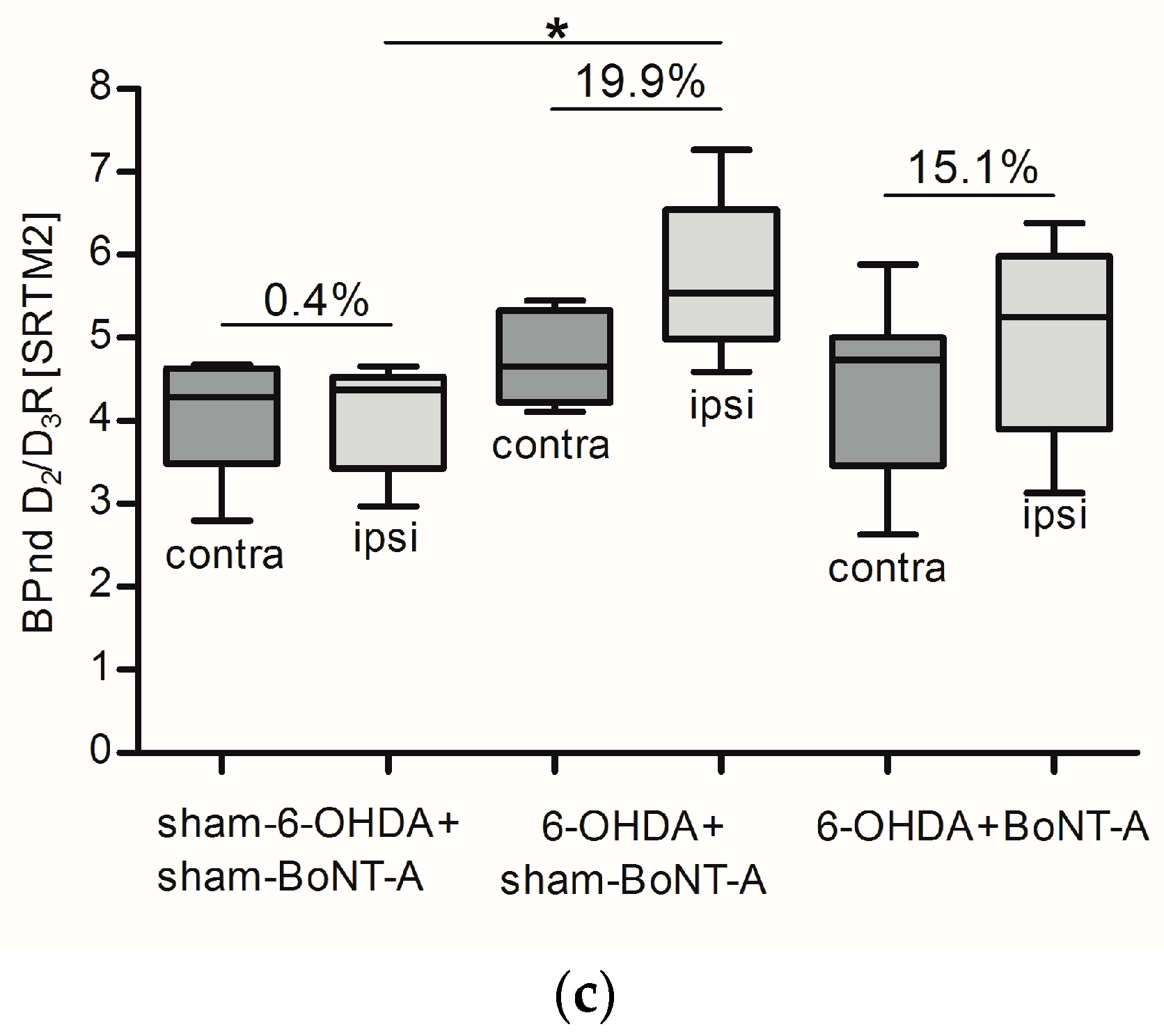
2.2. Striatal D2/D3R Availability
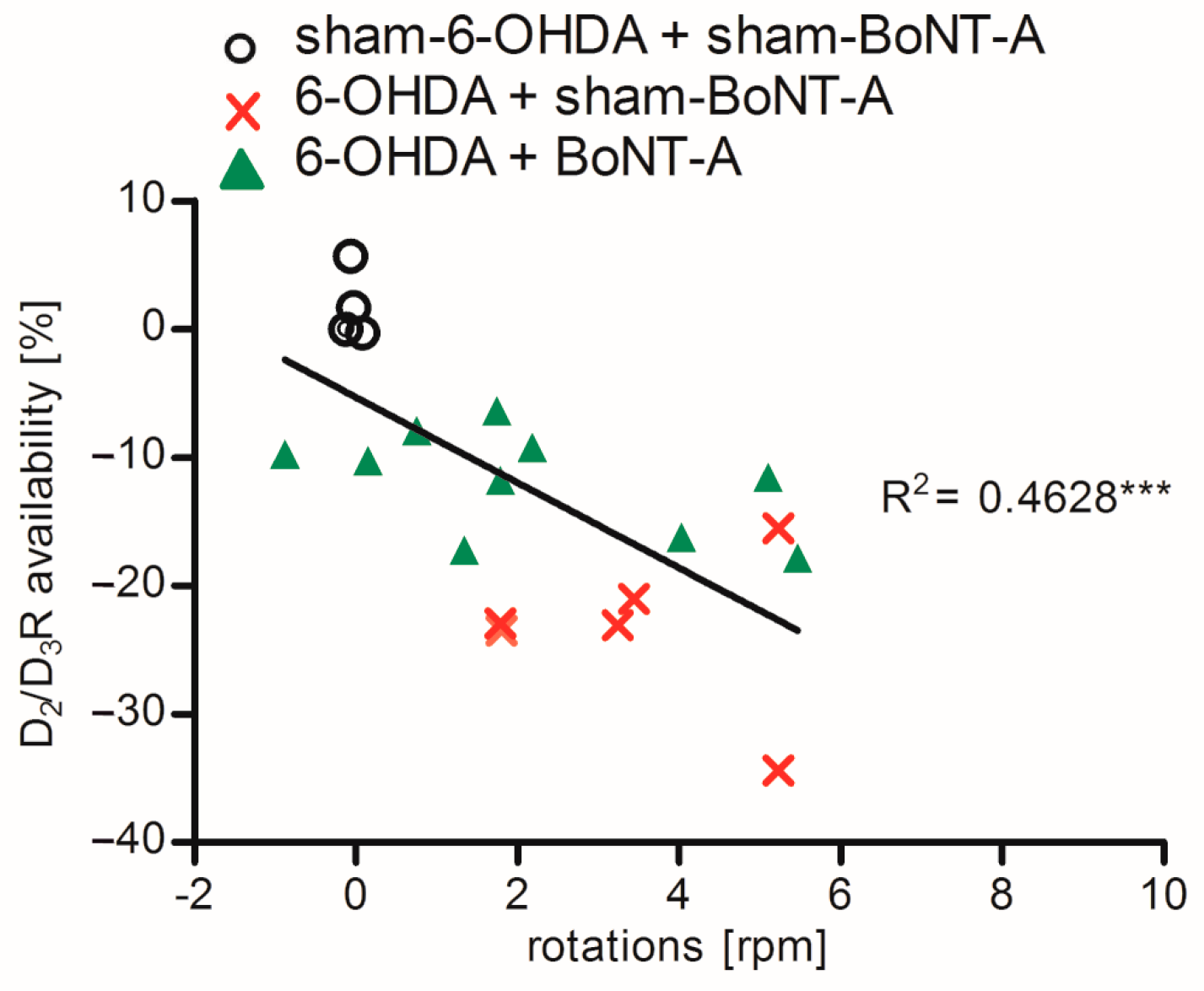
2.3. Correlation of D2/D3R Side Differences and Behavior
3. Discussion
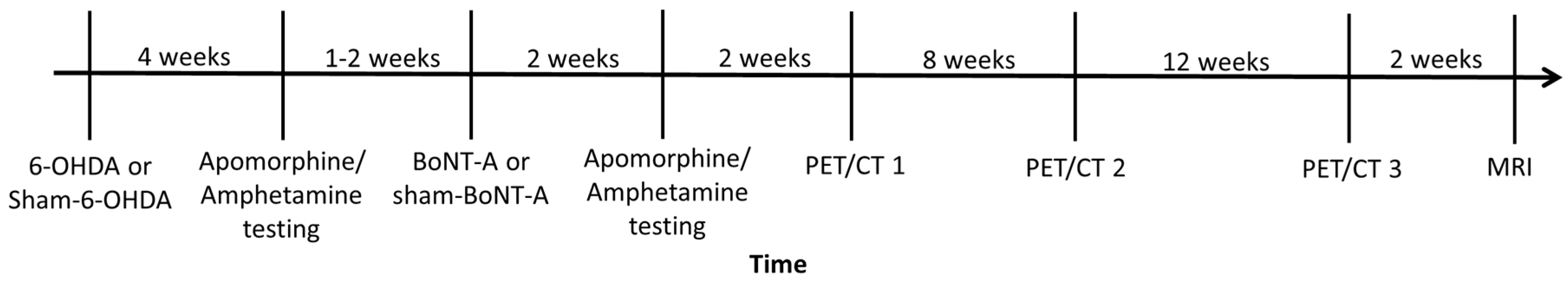
4. Materials and Methods
4.1. Animals
4.2. 6-OHDA and BoNT-A Injection
4.3. Immunohistochemistry
4.4. Behavioral Testing
4.5. Radioligand Preparation and PET/CT Imaging
4.6. MRI Imaging
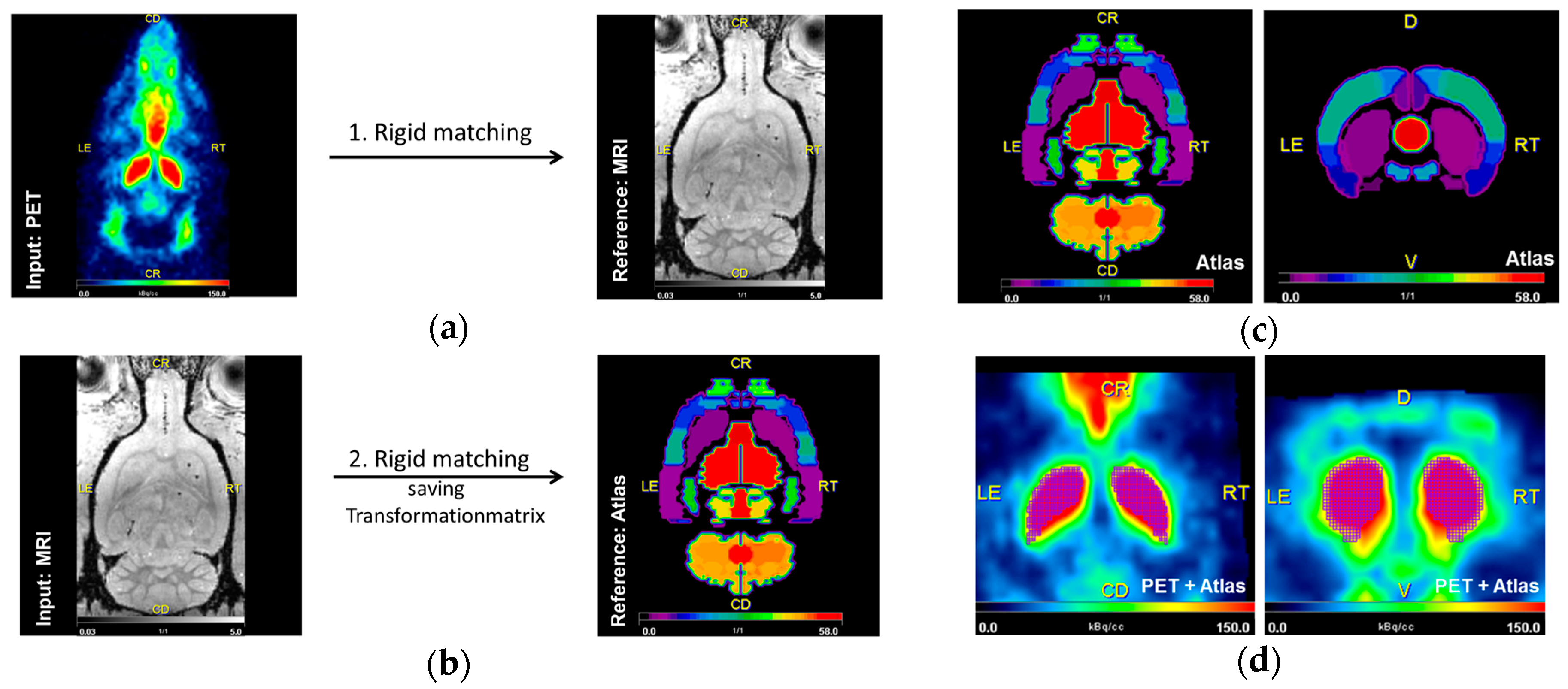
4.7. Image Analysis
4.8. Statistical Assessment
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BSA | Bovine serum albumin |
| BPnd | non-displaceable binding potential |
| BoNT-A | Botulinum neurotoxin A |
| CPu | caudate–putamen |
| D2/D3R | D2/D3 receptor |
| DA | dopamine |
| hemi-PD | hemiparkinsonian |
| MFB | medial forebrain bundle |
| MRI | magnetic resonance imaging |
| MSN | medium spiny neuron |
| PD | Parkinson’s disease |
| PET | positron emission tomography |
| PBS | phosphate-buffered saline |
| PVE | partial volume effect |
| SNAP25 | synaptosomal-associated protein of 25-kDa |
| SN(pc) | substantia nigra (pars compacta) |
| SRTM2 | simplified reference tissue model 2 |
| TAC | time-activity curve |
| TH | tyrosine hydroxylase |
| VOI | voxels of interest |
| 6-OHDA | 6-hydroxdopamine |
References
- Vučković, M.G.; Li, Q.; Fisher, B.; Nacca, A.; Leahy, R.M.; Walsh, J.P.; Mukherjee, J.; Williams, C.; Jakowec, M.W.; Petzinger, G.M. Exercise elevates dopamine D2 receptor in a mouse model of Parkinson’s disease: In vivo imaging with [18F]fallypride. Mov. Disord. 2010, 25, 2777–2784. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Kim, C.H.; Jeon, T.J.; Cho, W.G.; Lee, J.S.; Lee, S.J.; Choi, T.H.; Kim, B.S.; Yi, C.H.; Seo, Y.; et al. Evaluation of dopamine transporters and D2 receptors in hemiparkinsonian rat brains in vivo using consecutive PET scans of [18F]FPCIT and [18F]fallypride. Appl. Radiat. Isot. 2012, 70, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Wedekind, F.; Oskamp, A.; Lang, M.; Hawlitschka, A.; Zilles, K.; Wree, A.; Bauer, A. Intrastriatal administration of botulinum neurotoxin A normalizes striatal D2 R binding and reduces striatal D1 R binding in male hemiparkinsonian rats. J. Neurosci. Res. 2018, 96, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, J.; Christian, B.T.; Narayanan, T.K.; Shi, B.; Mantil, J. Evaluation of dopamine D-2 receptor occupancy by clozapine, risperidone, and haloperidol in vivo in the rodent and nonhuman primate brain using 18F-fallypride. Neuropsychopharmacology 2001, 25, 476–488. [Google Scholar] [CrossRef]
- Honer, M.; Brühlmeier, M.; Missimer, J.; Schubiger, A.P.; Ametamey, S.M. Dynamic imaging of striatal D2 receptors in mice using quad-HIDAC PET. J. Nucl. Med. 2004, 45, 464–470. [Google Scholar] [PubMed]
- Mukherjee, J.; Constantinescu, C.C.; Hoang, A.T.; Jerjian, T.; Majji, D.; Pan, M.-L. Dopamine D3 receptor binding of 18F-fallypride: Evaluation using in vitro and in vivo PET imaging studies. Synapse 2015, 69, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Guzman, J.N.; Tkatch, T.; Chen, S.; Goldberg, J.A.; Ebert, P.J.; Levitt, P.; Wilson, C.J.; Hamm, H.E.; Surmeier, D.J. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat. Neurosci. 2006, 9, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Bonsi, P.; Centonze, D.; Gubellini, P.; Bernardi, G.; Calabresi, P. Targeting striatal cholinergic interneurons in Parkinson’s disease: Focus on metabotropic glutamate receptors. Neuropharmacology 2003, 45, 45–56. [Google Scholar] [CrossRef]
- Goldberg, J.A.; Ding, J.B.; Surmeier, D.J. Muscarinic modulation of striatal function and circuitry. Handb. Exp. Pharmacol. 2012, 223–241. [Google Scholar] [CrossRef]
- Zhou, F.M.; Liang, Y.; Dani, J. A Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat. Neurosci. 2001, 4, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Threlfell, S.; Clements, M.A.; Khodai, T.; Pienaar, I.S.; Exley, R.; Wess, J.; Cragg, S.J. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J. Neurosci. 2010, 30, 3398–3408. [Google Scholar] [CrossRef] [PubMed]
- Duty, S.; Jenner, P. Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 2011, 164, 1357–1391. [Google Scholar] [CrossRef] [PubMed]
- Soto-Otero, R.; Méndez-Alvarez, E.; Hermida-Ameijeiras, A.; Muñoz-Patiño, A.M.; Labandeira-Garcia, J.L. Autoxidation and neurotoxicity of 6-hydroxydopamine in the presence of some antioxidants: Potential implication in relation to the pathogenesis of Parkinson’s disease. J. Neurochem. 2000, 74, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Bové, J.; Perier, C. Neurotoxin-based models of Parkinson’s disease. Neuroscience 2012, 211, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.; Lindqyist, M.; Magnusson, T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature 1957, 180, 1200. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R. Parkinson’s Disease: From Pathogenesis to Pharmacogenomics. Int. J. Mol. Sci. 2017, 18, 551. [Google Scholar] [CrossRef] [PubMed]
- Moritz, A.E.; Benjamin Free, R.; Sibley, D.R. Advances and challenges in the search for D2 and D3 dopamine receptor-selective compounds. Cell. Signal. 2018, 41, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W.; Stocchi, F. Levodopa: A new look at an old friend. Mov. Disord. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kühn, J.; Haumesser, J.K.; Beck, M.H.; Altschüler, J.; Kühn, A.A.; Nikulin, V.V.; van Riesen, C. Differential effects of levodopa and apomorphine on neuronal population oscillations in the cortico-basal ganglia loop circuit in vivo in experimental parkinsonism. Exp. Neurol. 2017, 298, 122–133. [Google Scholar] [CrossRef]
- Horstink, M.; Tolosa, E.; Bonuccelli, U.; Deuschl, G.; Friedman, A.; Kanovsky, P.; Larsen, J.P.; Lees, A.; Oertel, W.; Poewe, W.; et al. Review of the therapeutic management of Parkinson’s disease. Report of a joint task force of the European Federation of Neurological Societies and the Movement Disorder Society-European Section. Part I: Early (uncomplicated) Parkinson’s disease. Eur. J. Neurol. 2006, 13, 1170–1185. [Google Scholar] [CrossRef] [PubMed]
- Horstink, M.; Tolosa, E.; Bonuccelli, U.; Deuschl, G.; Friedman, A.; Kanovsky, P.; Larsen, J.P.; Lees, A.; Oertel, W.; Poewe, W.; et al. Review of the therapeutic management of Parkinson’s disease. Report of a joint task force of the European Federation of Neurological Societies (EFNS) and the Movement Disorder Society-European Section (MDS-ES). Part II: Late (complicated) Parkinson’s dise. Eur. J. Neurol. 2006, 13, 1186–1202. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, H.H. Updates in the medical management of Parkinson disease. Clevel. Clin. J. Med. 2012, 79, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Wree, A.; Mix, E.; Hawlitschka, A.; Antipova, V.; Witt, M.; Schmitt, O.; Benecke, R. Intrastriatal botulinum toxin abolishes pathologic rotational behaviour and induces axonal varicosities in the 6-OHDA rat model of Parkinson’s disease. Neurobiol. Dis. 2011, 41, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, C.; Dräger, D.; Mix, E.; Hawlitschka, A.; Antipova, V.; Benecke, R.; Wree, A. Effects of intrastriatal botulinum neurotoxin A on the behavior of Wistar rats. Behav. Brain Res. 2012, 234, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Hawlitschka, A.; Antipova, V.; Schmitt, O.; Witt, M.; Benecke, R.; Mix, E.; Wree, A. Intracerebrally applied botulinum neurotoxin in experimental neuroscience. Curr. Pharm. Biotechnol. 2013, 14, 124–130. [Google Scholar] [PubMed]
- Antipova, V.; Hawlitschka, A.; Mix, E.; Schmitt, O.; Dräger, D.; Benecke, R.; Wree, A. Behavioral and structural effects of unilateral intrastriatal injections of botulinum neurotoxin a in the rat model of Parkinson’s disease. J. Neurosci. Res. 2013, 91, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Caleo, M.; Antonucci, F.; Restani, L.; Mazzocchio, R. A reappraisal of the central effects of botulinum neurotoxin type A: By what mechanism? J. Neurochem. 2009, 109, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Binz, T.; Sikorra, S.; Mahrhold, S. Clostridial neurotoxins: Mechanism of SNARE cleavage and outlook on potential substrate specificity reengineering. Toxins 2010, 2, 665–682. [Google Scholar] [CrossRef] [PubMed]
- Kroken, A.R.; Karalewitz, A.P.-A.; Fu, Z.; Kim, J.-J.P.; Barbieri, J.T. Novel ganglioside-mediated entry of botulinum neurotoxin serotype D into neurons. J. Biol. Chem. 2011, 286, 26828–26837. [Google Scholar] [CrossRef] [PubMed]
- Mehlan, J.; Brosig, H.; Schmitt, O.; Mix, E.; Wree, A.; Hawlitschka, A. Intrastriatal injection of botulinum neurotoxin-A is not cytotoxic in rat brain—A histological and stereological analysis. Brain Res. 2016, 1630, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Zilles, K.; Dikow, H.; Hellfritsch, A.; Cremer, M.; Piel, M.; Rösch, F.; Hawlitschka, A.; Schmitt, O.; Wree, A. Dopamine, Noradrenaline and Serotonin Receptor Densities in the Striatum of Hemiparkinsonian Rats following Botulinum Neurotoxin-A Injection. Neuroscience 2018, 374, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Grossman, R.; Paden, C.M.; Fry, P.A.; Rhodes, R.S.; Biegon, A. Persistent region-dependent neuroinflammation, NMDA receptor loss and atrophy in an animal model of penetrating brain injury. Future Neurol. 2012, 7, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Qü, M.; Buchkremer-Ratzmann, I.; Schiene, K.; Schroeter, M.; Witte, O.W.; Zilles, K. Bihemispheric reduction of GABAA receptor binding following focal cortical photothrombotic lesions in the rat brain. Brain Res. 1998, 813, 374–380. [Google Scholar] [CrossRef]
- Jeon, B.S.; Jackson-Lewis, V.; Burke, R.E. 6-Hydroxydopamine lesion of the rat substantia nigra: Time course and morphology of cell death. Neurodegeneration 1995, 4, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Creese, I.; Burt, D.R.; Snyder, S.H. Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science 1977, 197, 596–598. [Google Scholar] [CrossRef] [PubMed]
- Przedbroski, S.; Leviver, M.; Jiang, H.; Ferreira, M.; Jackson-Lewis, V.; Donaldson, D.; Togasaki, D.M. Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by instrastriatal injection of 6-hydroxydopamine. Neuroscience 1995, 67, 631–647. [Google Scholar] [CrossRef]
- Sun, W.; Sugiyama, K.; Asakawa, T.; Yamaguchi, H.; Akamine, S.; Ouchi, Y.; Magata, Y.; Namba, H. Dynamic changes of striatal dopamine D2 receptor binding at later stages after unilateral lesions of the medial forebrain bundle in Parkinsonian rat models. Neurosci. Lett. 2011, 496, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Ungerstedt, U.; Butcher, L.L.; Butcher, S.G.; Andén, N.E.; Fuxe, K. Direct chemical stimulation of dopaminergic mechanisms in the neostriatum of the rat. Brain Res. 1969, 14, 461–471. [Google Scholar] [CrossRef]
- Ma, Y.; Zhan, M.; OuYang, L.; Li, Y.; Chen, S.; Wu, J.; Chen, J.; Luo, C.; Lei, W. The effects of unilateral 6-OHDA lesion in medial forebrain bundle on the motor, cognitive dysfunctions and vulnerability of different striatal interneuron types in rats. Behav. Brain Res. 2014, 266, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wei, X.; Gao, H.; Li, J.; Liao, J.; Liu, X.; Qin, B.; Yu, Y.; Deng, C.; Tang, B.; et al. Simvastatin reverses the downregulation of M1/4 receptor binding in 6-hydroxydopamine-induced parkinsonian rats: The association with improvements in long-term memory. Neuroscience 2014, 267, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ungerstedt, U.; Arbuthnott, G.W. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970, 24, 485–493. [Google Scholar] [CrossRef]
- Hudson, J.L.; van Horne, C.G.; Strömberg, I.; Brock, S.; Clayton, J.; Masserano, J.; Hoffer, B.J.; Gerhardt, G.A. Correlation of apomorphine- and amphetamine-induced turning with nigrostriatal dopamine content in unilateral 6-hydroxydopamine lesioned rats. Brain Res. 1993, 626, 167–174. [Google Scholar] [CrossRef]
- Ginovart, N.; Farde, L.; Halldin, C.; Swahn, C.G. Effect of reserpine-induced depletion of synaptic dopamine on [11C]raclopride binding to D2-dopamine receptors in the monkey brain. Synapse 1997, 25, 321–325. [Google Scholar] [CrossRef]
- Seeman, P.; Guan, H.C.; Niznik, H.B. Endogenous dopamine lowers the dopamine D2 receptor density as measured by [3H]raclopride: Implications for positron emission tomography of the human brain. Synapse 1989, 3, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Young, L.T.; Wong, D.F.; Goldman, S.; Minkin, E.; Chen, C.; Matsumura, K.; Scheffel, U.; Wagner, H.N. Effects of endogenous dopamine on kinetics of [3H]N-methylspiperone and [3H]raclopride binding in the rat brain. Synapse 1991, 9, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Staunton, D.A.; Wolfe, B.B.; Groves, P.M.; Molinoff, P.B. Dopamine receptor changes following destruction of the nigrostriatal pathway: Lack of a relationship to rotational behavior. Brain Res. 1981, 211, 315–327. [Google Scholar] [CrossRef]
- Fornaretto, M.G.; Caccia, C.; Caron, M.G.; Fariello, R.G. Dopamine Receptors Status After Unilateral Nigral 6-OHDA Lesion Hybridization Study in the Rat Brain. Mol. Chem. Neuropathol. 1993, 19, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Yanai, K.; Zhao, X.L.; Watanabe, T. The effect of dopamine D1 receptor stimulation on the up-regulation of histamine H3-receptors following destruction of the ascending dopaminergic neurones. Br. J. Pharmacol. 1996, 118, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Tanji, H.; Kato, H.; Imai, Y.; Mizugaki, M.; Itoyama, Y. Temporal changes of dopaminergic and glutamatergic receptors in 6-hydroxydopamine-treated rat brain. Eur. Neuropsychopharmacol. 2000, 10, 365–375. [Google Scholar] [CrossRef]
- Xu, Z.C.; Ling, G.; Sahr, R.N.; Neal-Beliveau, B.S. Asymmetrical changes of dopamine receptors in the striatum after unilateral dopamine depletion. Brain Res. 2005, 1038, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Inaji, M.; Okauchi, T.; Ando, K.; Maeda, J.; Nagai, Y.; Yoshizaki, T.; Okano, H.; Nariai, T.; Ohno, K.; Obayashi, S.; et al. Correlation between quantitative imaging and behavior in unilaterally 6-OHDA-lesioned rats. Brain Res. 2005, 1064, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Hume, S.P.; Opacka-Juffry, J.; Myers, R.; Ahier, R.G.; Ashworth, S.; Brooks, D.J.; Lammertsma, A.A. Effect of L-dopa and 6-hydroxydopamine lesioning on [11C]raclopride binding in rat striatum, quantified using PET. Synapse 1995, 21, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Doorduin, J.; Elsinga, P.H.; Dierckx, R.A.J.O.; de Vries, E.F.J.; Casteels, C. Altered adenosine 2A and dopamine D2 receptor availability in the 6-hydroxydopamine-treated rats with and without levodopa-induced dyskinesia. Neuroimage 2017, 157, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ariano, M.A.; Stromski, C.J.; Smyk-Randall, E.M.; Sibley, D.R. D2 dopamine receptor localization on striatonigral neurons. Neurosci. Lett. 1992, 144, 215–220. [Google Scholar] [CrossRef]
- Brock, J.W.; Farooqui, S.; Ross, K.; Prasad, C. Localization of dopamine D2 receptor protein in rat brain using polyclonal antibody. Brain Res 1992, 578, 244–250. [Google Scholar] [CrossRef]
- Yung, K.K.L.; Bolam, J.P.; Smith, A.D.; Hersch, S.M.; Ciliax, B.J.; Levey, A.I. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: Light and electron microscopy. Neuroscience 1995, 65, 709–730. [Google Scholar] [CrossRef]
- Wu, Y.; Carson, R.E. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J. CerebR. Blood Flow Metab. 2002, 22, 1440–1452. [Google Scholar] [CrossRef] [PubMed]
- Lammertsma, A.A.; Bench, C.J.; Hume, S.P.; Osman, S.; Gunn, K.; Brooks, D.J.; Frackowiak, R.S. Comparison of methods for analysis of clinical [11C]raclopride studies. J. Cerebr. Blood Flow Metab. 1996, 16, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Martres, M.P.; Sales, N.; Bouthenet, M.L.; Schwartz, J.C. Localisation and pharmacological characterisation of D-2 dopamine receptors in rat cerebral neocortex and cerebellum using [125I]iodosulpride. Eur. J. Pharmacol. 1985, 118, 211–219. [Google Scholar] [CrossRef]
- Seneca, N.; Finnema, S.J.; Farde, L.; Gulyás, B.; Wikström, H.V.; Halldin, C.; Innis, R.B. Effect of amphetamine on dopamine D2 receptor binding in nonhuman primate brain: A comparison of the agonist radioligand [11C]MNPA and antagonist [11C]raclopride. Synapse 2006, 59, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, C.C.; Coleman, R.A.; Pan, M.-L.; Mukherjee, J. Striatal and extrastriatal microPET imaging of D2/D3 dopamine receptors in rat brain with [18F]fallypride and [18F]desmethoxyfallypride. Synapse 2011, 65, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Yoder, K.K.; Albrecht, D.S.; Kareken, D.A.; Federici, L.M.; Perry, K.M.; Patton, E.A.; Zheng, Q.-H.; Mock, B.H.; O’Connor, S.; Herring, C.M. Test-retest variability of [11C]raclopride-binding potential in nontreatment-seeking alcoholics. Synapse 2011, 65, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Prieto, E.; Collantes, M.; Delgado, M.; Juri, C.; García-García, L.; Molinet, F.; Fernández-Valle, M.E.; Pozo, M.A.; Gago, B.; Martí-Climent, J.M.; et al. Statistical parametric maps of 18F-FDG PET and 3-D autoradiography in the rat brain: A cross-validation study. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.J.; Huang, S.C.; Phelps, M.E. Quantitation in positron emission computed tomography: 1. Effect of object size. J. Comput. Assist. Tomogr. 1979, 3, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.J.; Huang, S.C.; Plummer, D.; Phelps, M.E. Quantitation in positron emission computed tomography: 6. effect of nonuniform resolution. J. Comput. Assist. Tomogr. 1982, 6, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Hamer, R.M.; Lawler, C.P.; Mailman, R.B.; Lieberman, J.A. Striatal volume changes in the rat following long-term administration of typical and atypical antipsychotic drugs. Neuropsychopharmacology 2002, 27, 143–151. [Google Scholar] [CrossRef]
- Garcia, D.V.; Casteels, C.; Schwarz, A.J.; Dierckx, R.A.J.O.; Koole, M.; Doorduin, J. Correction: A Standardized Method for the Construction of Tracer Specific PET and SPECT Rat Brain Templates: Validation and Implementation of a Toolbox. PLoS ONE 2015, 10, e0143900. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic press: San Diego, CA, USA, 2007; ISBN 9780125476126. [Google Scholar]
- Hawlitschka, A.; Holzmann, C.; Witt, S.; Spiewok, J.; Neumann, A.-M.; Schmitt, O.; Wree, A.; Antipova, V. Intrastriatally injected botulinum neurotoxin-A differently effects cholinergic and dopaminergic fibers in C57BL/6 mice. Brain Res. 2017, 1676, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, M.; Mock, B.H.; Glick-Wilson, B.E.; Yoder, K.K.; Hutchins, G.D.; Zheng, Q.-H. An improved synthesis of dopamine D2/D3 receptor radioligands [11C]fallypride and [18F]fallypride. Appl. Radiat. Isot. 2010, 68, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Newport, D.; Chen, M.; Stout, D.B.; Chatziioannou, A.F. Performance evaluation of the inveon dedicated PET preclinical tomograph based on the NEMA NU-4 standards. J. Nucl. Med. 2009, 50, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Kemp, B.J.; Hruska, C.B.; McFarland, A.R.; Lenox, M.W.; Lowe, V.J. NEMA NU 2-2007 performance measurements of the Siemens Inveon preclinical small animal PET system. Phys. Med. Biol. 2009, 54, 2359–2376. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, W.K.; Mirrione, M.M.; Biegon, A.; Alexoff, D.L.; Patel, V.; Dewey, S.L. Serial microPET measures of the metabolic reaction to a microdialysis probe implant. J. Neurosci. Methods 2006, 155, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Mintun, M.A.; Raichle, M.E.; Kilbourn, M.R.; Wooten, G.F.; Welch, M.J. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann. Neurol. 1984, 15, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, J.; Yang, Z.-Y.; Lew, R.; Brown, T.; Kronmal, S.; Cooper, M.D.; Seiden, L.S. Evaluation of d-Amphetamine Effects on the Binding of Dopamine D-2 Receptor Radioligand, 18F-Fallypride in Nonhuman Primates Using Positron Emission Tomography. Synapse 1997, 27, 1–13. [Google Scholar] [CrossRef]
- Cropley, V.L.; Innis, R.B.; Nathan, P.J.; Brown, A.K.; Sangare, J.L.; Lerner, A.; Ryu, Y.H.; Sprague, K.E.; Pike, V.W.; Fujita, M. Small effect of dopamine release and no effect of dopamine depletion on [18F]fallypride binding in healthy humans. Synapse 2008, 62, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Rominger, A.; Wagner, E.; Mille, E.; Böning, G.; Esmaeilzadeh, M.; Wängler, B.; Gildehaus, F.-J.; Nowak, S.; Bruche, A.; Tatsch, K.; et al. Endogenous competition against binding of [18F]DMFP and [18F]fallypride to dopamine D(2/3) receptors in brain of living mouse. Synapse 2010, 64, 313–322. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Group | PET/CT 1 | PET/CT 2 | PET/CT 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BPnd Left | BPnd Right | (%) | BPnd Left | BPnd Right | (%) | BPnd Left | BPnd Right | (%) | |
| sham-6-OHDA + sham-BoNT-A sham-6-OHDA + sham-BoNT-A sham-6-OHDA + sham-BoNT-A sham-6-OHDA + sham-BoNT-A sham-6-OHDA + sham-BoNT-A sham-6-OHDA + sham-BoNT-A sham-6-OHDA + sham-BoNT-A sham-6-OHDA + sham-BoNT-A sham-6-OHDA + sham-BoNT-A | 5.06 2.92 4.53 4.58 4.03 * * * * | 4.98 2.92 4.53 4.59 3.80 * * * * | −1.66 −0.01 −0.04 0.28 −5.71 * * * * | 2.21 3.74 2.87 4.25 4.24 3.81 4.73 4.27 4.23 | 2.07 3.93 2.78 4.15 3.93 3.46 4.89 4.32 4.13 | −6.35 5.21 −3.37 −2.28 −7.22 −9.32 3.34 1.04 −2.48 | * 4.56 3.29 4.65 4.07 4.68 2.80 4.50 4.07 | * 4.44 3.23 4.53 4.02 4.52 2.97 4.66 4.30 | * −2.66 −1.84 −2.46 −1.36 −3.47 6.23 3.60 5.66 |
| |Mean| ± SD | 4.2 ± 0.8 | 4.2 ± 0.8 | 1.4 ± 2.5 | 3.8 ± 0.8 | 3.7 ± 0.9 | 2.4 ± 4.9 | 4.1 ± 0.7 | 4.1 ± 0.7 | 0.4 ± 4.0 |
| 6-OHDA + sham-BoNT-A 6-OHDA + sham-BoNT-A 6-OHDA + sham-BoNT-A 6-OHDA + sham-BoNT-A 6-OHDA + sham-BoNT-A 6-OHDA + sham-BoNT-A 6-OHDA + sham-BoNT-A | 4.86 5.41 3.77 4.08 3.96 4.49 * | 6.53 6.65 4.67 4.71 4.79 5.54 * | 34.37 22.90 23.84 15.53 21.02 23.50 * | 6.11 * 3.35 4.44 * 4.74 3.53 | 7.67 * 4.54 4.88 * 5.64 4.71 | 25.53 * 35.56 10.04 * 18.80 33.46 | 4.11 4.66 5.45 5.21 * 4.33 * | 4.59 5.54 7.26 5.83 * 5.37 * | 11.53 19.07 33.13 11.98 * 23.95 * |
| |Mean| ± SD | 4.4 ± 0.6 | 5.5 ± 0.9 | 23.5 ± 6.1 | 4.4 ± 1.1 | 5.5 ± 1.3 | 24.7 ± 14.8 | 4.8 ± 1.0 | 5.7 ± 1.0 | 19.9 ± 11.4 |
| 6-OHDA + BoNT-A 6-OHDA + BoNT-A 6-OHDA + BoNT-A 6-OHDA + BoNT-A 6-OHDA + BoNT-A 6-OHDA + BoNT-A 6-OHDA + BoNT-A 6-OHDA + BoNT-A 6-OHDA + BoNT-A 6-OHDA + BoNT-A | 4.58 4.54 5.07 5.09 4.85 4.37 4.59 3.88 5.30 5.10 | 5.33 4.90 5.54 5.42 5.43 5.15 5.12 4.28 6.22 5.59 | 16.19 7.94 9.23 6.39 11.80 17.86 11.55 10.26 17.22 9.74 | * 5.47 4.41 2.43 * * 3.88 4.33 4.94 5.32 | * 6.10 5.15 2.79 * * 4.06 5.13 5.26 6.41 | * 11.59 16.68 14.93 * * 4.77 18.59 6.49 20.38 | 3.30 * 2.63 4.74 5.88 4.86 3.63 5.00 4.58 5.01 | 3.92 * 3.13 5.25 6.38 6.03 3.87 5.93 5.23 5.78 | 18.62 * 19.11 10.77 8.56 24.05 6.45 18.53 14.22 15.38 |
| |Mean| ± SD | 4.7 ± 0.4 | 5.3 ± 0.5 | 11.8 ± 4.0 | 4.4 ± 1.0 | 5.0 ± 1.2 | 13.4 ± 6.0 | 4.4 ± 1.0 | 5.1 ± 1.1 | 15.1 ± 5.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mann, T.; Kurth, J.; Hawlitschka, A.; Stenzel, J.; Lindner, T.; Polei, S.; Hohn, A.; Krause, B.J.; Wree, A. [18F]fallypride-PET/CT Analysis of the Dopamine D2/D3 Receptor in the Hemiparkinsonian Rat Brain Following Intrastriatal Botulinum Neurotoxin A Injection. Molecules 2018, 23, 587. https://doi.org/10.3390/molecules23030587
Mann T, Kurth J, Hawlitschka A, Stenzel J, Lindner T, Polei S, Hohn A, Krause BJ, Wree A. [18F]fallypride-PET/CT Analysis of the Dopamine D2/D3 Receptor in the Hemiparkinsonian Rat Brain Following Intrastriatal Botulinum Neurotoxin A Injection. Molecules. 2018; 23(3):587. https://doi.org/10.3390/molecules23030587
Chicago/Turabian StyleMann, Teresa, Jens Kurth, Alexander Hawlitschka, Jan Stenzel, Tobias Lindner, Stefan Polei, Alexander Hohn, Bernd J. Krause, and Andreas Wree. 2018. "[18F]fallypride-PET/CT Analysis of the Dopamine D2/D3 Receptor in the Hemiparkinsonian Rat Brain Following Intrastriatal Botulinum Neurotoxin A Injection" Molecules 23, no. 3: 587. https://doi.org/10.3390/molecules23030587





